Recent advances in peptide-based nanovaccines for re-emerging and emerging infectious diseases
Abstract
Peptide-based nanovaccines have emerged as promising strategies for combating re-emerging and emerging infectious diseases. They exhibit excellent immunogenicity and therapeutic potential. They have shown the ability to elicit robust immune responses, such as activation of antigen-presenting cells, induction of antibodies and T-cell responses, and generation of memory immune cells. This comprehensive review article aims to provide a thorough overview of recent advances in the field, including immunological mechanisms, structural design approaches, and utilization of various nanomaterials. Overall, peptide-based nanovaccines hold great promise in combating infectious diseases. Precise design and assembly of targeted and tailored immune responses will enable effective prevention, treatment, and long-term protection. Further research is needed to optimize their efficacy, safety, and clinical translation. The knowledge gained from these studies will pave the way for a future with more effective immunotherapeutic interventions against infectious diseases.
INTRODUCTION
Peptide-based vaccines have shown promise for personalized immunotherapy because of their ability to target specific amino acid sequences of pathogens or protein antigens [1]. However, these subunit vaccines suffer from issues such as poor stability, weak immunogenicity, and limited duration of immune response [2]. To address these challenges, incorporating antigenic peptides into nanostructures has emerged as a crucial strategy for the development of nanovaccines [3].
Nanotechnology has advanced to the point that researchers are now exploring the use of "nanovaccines," which combine pathogen-specific antigens coupled with nanomaterials to stimulate strong immune responses. These nanovaccines exploit the use of subunits of the pathogen to enhance their tunability and safety. The inclusion of these subunits leads to the development of vaccines that are safer, have controlled immune responses, and provide protection against multiple pathogens [4].
Nanoparticles provide reduced degradation of subunits, increased antigen loading, and improved stability. Smaller, more specific subunits may lack immunogenicity, which can be addressed with adjuvants that generate immunomodulatory signals. Conventional adjuvants may cause side effects, immunotolerance to the target antigen, individual-specific responses, and undesired reactions to self-antigens, thereby limiting their utility [4]. Thus, nanoparticles can serve as adjuvants, potentially reducing the reliance on strong conventional adjuvants such as alum.
Various types of nanoparticles have found widespread applications as antigen delivery vehicles, immunogens, and adjuvants [5]. Synthetic nanoparticles predominantly enhance internalization by interacting structurally with antigen-presenting cells (APCs), which lack specific cell receptor binding sites. Conversely, protein/peptide-based nanoparticles exhibit dual structural and functional interactions since it can carry antigens and engage pattern-associated receptors on antigen-presenting cells [6].
The creation of peptide-based nanovaccines involves two primary approaches such as a) mechanical assembly of nanomaterials and antigenic peptides into nanostructures, and b) construction of nanostructures through self-assembly using basic segments, achieved through protein engineering technology and intermolecular forces [7,8]. Notably, rational design of nanovaccines offers several advantages compared to conventional subunit vaccines, including efficient delivery to APCs, enhanced in vivo stability and half-life of antigenic peptides, and multivalent antigen presentation [9].
Peptide-based nanovaccines can elicit both cellular and humoral immunity, leading to the induction of memory responses [3]. The sustained release of antigens from nanoparticle depots enables prolonged stimulation, reducing the need for more boosters. This versatility allows nanovaccines to serve as both therapeutic and prophylactic agents administered either before or after disease occurrence [10]. However, to achieve desirable immune responses, careful optimization of nanoparticle safety, biodistribution, and residence time is crucial for nanoparticle-based vaccines.
This review focuses on the current advances in the utilization of peptide-based nanovaccines for re-emerging and emerging infectious diseases. The immunological mechanisms, structural design approaches, and nanomaterials used for peptide-based nanovaccines have also been summarized. Recent challenges, opportunities, research gaps, and future perspectives in this field have also been provided to gain more attention from nanotechnology and vaccinology researchers.
METHODS
Three databases were used to search for relevant articles including PubMed, Google Scholar, and Scopus published from 2013-2023. The following keywords were used: “peptide-based nanovaccines”, “peptide nanovaccines”, and “peptide-based nanovaccines for infectious diseases”.
MECHANISMS OF NANOVACCINES
The use of nanoparticle-based vaccines has become an attractive strategy for augmenting primary and secondary immune responses [11]. Nanovaccines achieve this enhancement through various mechanisms, including activation of innate, cellular, and humoral immunity (Figure 1), as discussed in this section.
Activation of innate immunity
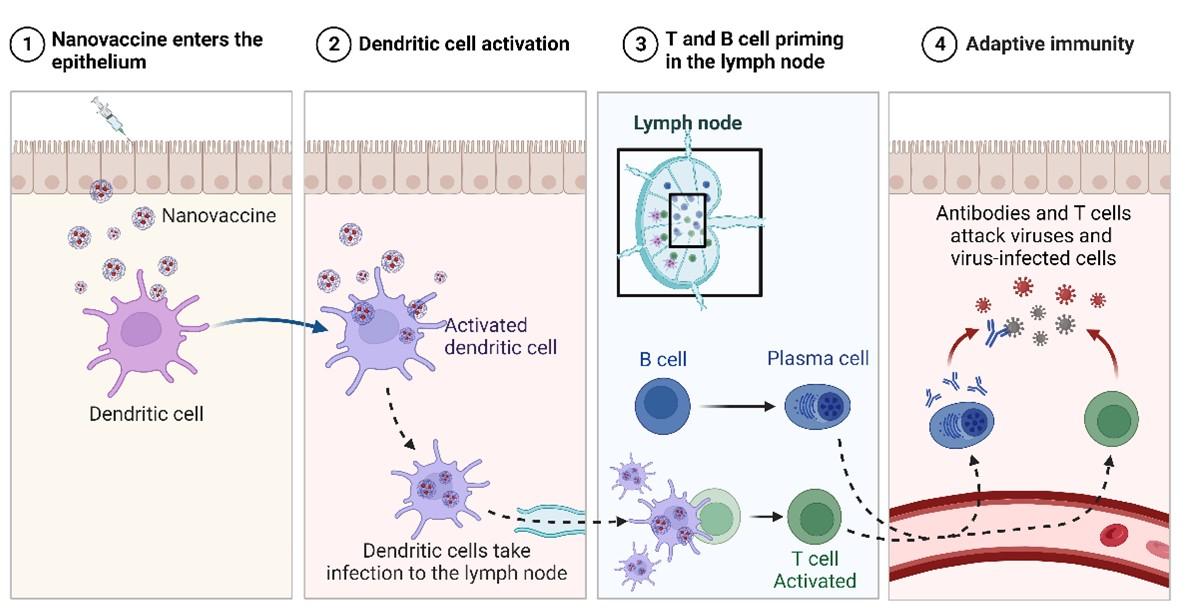
Upon immunization, nanovaccines interact with immune cells by recognizing pathogen-associated molecular patterns (PAMPs) on the nanovaccine surface [12]. PAMPs act as ligands for pattern recognition receptors (PRRs) abundantly present in these immune cells [13]. Consequently, PAMP-PRR interactions trigger endocytosis, with larger particles being engulfed by macrophages while smaller particles are engulfed by dendritic cells (DCs). To facilitate direct delivery to APCs and evade macrophage degradation, nanovaccine particles were modified to enhance their survival [14]. Moreover, the type of PAMPs present influences cytokine and chemokine secretion by neutrophils and macrophages, further stimulating APC activation. Eventually, cytokines and chemokines lead to the activation and maturation of APCs, thereby initiating robust cellular and humoral immune responses.
Activation of cellular immunity
After vaccination, cellular immunity is stimulated leading to pathogen neutralization and immunological memory development [15]. This is initiated by the migration of activated dendritic cells to the lymphatic organs after capturing nanovaccine particles. Activated DCs display antigens to CD8+ CTLs via MHC class I receptors, leading to strong cell mediated immune responses and target cell elimination via apoptosis [16].
Activated DCs also present antigens to CD4+ T-helper helper (Th) cells via the MHC-II receptor. Th cells are categorized into Th1 and Th2 subsets based on the type of cytokines secreted [17]. The Th1 subset primarily produces proinflammatory cytokines, stimulating CTL proliferation and reinforcing cell-mediated immunity. The Th2 cell subset secretes another class of cytokines, which promote B-cell production in humoral immunity [18]. The delicate balance between Th1/Th2 activity significantly influences the overall prophylactic or therapeutic potential of candidate nanovaccines [19]. In some cases, nanovaccines may act by suppressing T-regulatory (Treg) cells, which naturally inhibit the activation and proliferation of effector T cells in the body [20].
Activation of humoral immunity
Nanovaccines can induce robust Th2 cytokine responses that stimulate B cells in the lymph nodes and spleen. B cells recognize soluble antigens via their BCRs and undergo proliferation in the germinal center. B cells become specific to antigen epitopes, leading to proliferation of specific B cells through clonal selection. Activated B cells become either antibody-secreting plasma cells producing soluble antibodies or memory cells providing immunity for future encounters with the same antigen [21].
Plasma cells have a limited lifespan, leading to a gradual decline in antibody titers over time [22]. In such instances, memory cells stored in lymphatic organs or the bone marrow become active and provide protection against reinfection with the same antigen. Memory B cells rapidly multiply and transform into antibody-secreting cells, primarily producing IgG antibodies to counter the antigen. Similarly, memory T cells, including CD4+ and CD8+ cells, contribute to the production of additional cytokine and chemokine signals, enhancing both cellular and humoral immune responses. However, significant structural changes to the antigen (epitope) may render memory B and T cells unable to provide sufficient immunity [23]. Although primary vaccination can provide ~90% protection, the remaining 10% may still have detrimental effects, necessitating booster doses to achieve full 100% protection [24].
In a related context, nanoparticles play a crucial role in enhancing immunological memory [22]. Nanoparticles enable sustained antigen release, leading to increased B-cell proliferation and subsequent generation of more memory cells [25]. Additionally, their small size allows nanoparticles to efficiently migrate the lymphatic system, further promoting the production of additional memory B and T cells [26].
NANOVACCINE DESIGN APPROACHES
Formation of nanostructures
Incorporating an antigenic peptide into a nanostructure is crucial for activating immune responses, as peptides are generally non-immunogenic [27]. Nanostructures are commonly formed through the conjugation of antigens (Figure 2) with molecules using self-assembly [8]. Notably, nanoparticles (NPs) are commonly employed owing to their small size, customizable surface, enhanced solubility, and multifunctionality, thereby offering new prospects for vaccine design [28]. Utilizing NPs in vaccine formulations promotes immunogenicity, antigen stability, sustained release, and targeted delivery [29].
To achieve efficient delivery of vaccine antigens, nanoparticles are used either by encapsulating the antigens within or by decorating them on the nanoparticle surface (Figure 2). Encapsulating free antigens within nanoparticles prevents degradation, ensuring controlled and sustained release at the target site eliminating the need for booster doses [30]. In addition to encapsulation, various other techniques have been explored for co-delivering antigens with nanoparticles (Figure 2).
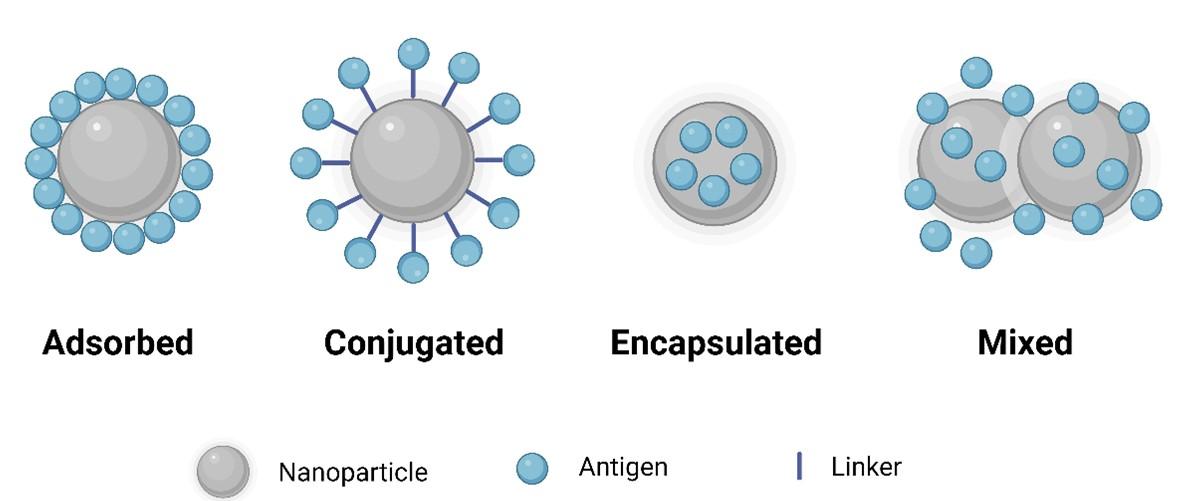
Formation of nanoparticle-based delivery systems
Nanoparticle-based delivery systems offer the advantage of reducing side effects correlated with peptide antigens [31]. Constructing nanovaccines with minimal side effects but with high immunogenicity involves assembling peptide antigens from pathogens to form nanoparticles with stable structures [27]. Directly combining antigenic peptides with nanomaterials is a common and straightforward approach for creating nanovaccines. Several prophylactic nanovaccines, including Mosquirix [32], Novavax [33], and Norwalk [34], have already been approved for human applications.
Formation of self-assembled peptide nanoparticles
The use of self-assembled peptide NPs represents a unique and advantageous approach for designing organized NPs with tailored functions. These NPs offer smaller size, biocompatibility, multivalent nanomaterials with reduced toxicity, and well-defined physical and chemical properties [9]. Peptide NPs form through connections of antigens and carrier-antigen conjugates, which then self-assemble into nanostructures (Figure 2) [8]. These self-assembled nanostructures can elicit both cellular and humoral responses [3]. The development of peptide-based nanovaccines involves designing self-assembly strategies, where a peptide carrier is linked to a peptide epitope. This results in a conjugate that retains the self-assembly properties and forms nanostructures [8].
Formation of nanoparticle-based adjuvants
Nanoparticles have demonstrated potential as adjuvants owing to their immunomodulatory activities, including immune cell activation, complement system activation, and inflammasome activation [35]. Compared to conventional adjuvants, nanoparticles serve as more effective adjuvants by eliciting higher and more robust immune responses [36]. Their small size enables their internalization by DCs to promote cellular and humoral responses. Lipid nanoparticles (LNP)-adjuvant formulations showed improved immunogenic responses against OVA and HBsAg in mice, leading to better outcomes compared to using HBsAg alone [37].
NANOMATERIALS FOR NANOVACCINE DESIGN
Polymer-based nanoparticles
Polymers are widely investigated and utilized for vaccine delivery, particularly NPs. These polymer-based systems offer numerous advantages such as the capacity to conjugate various antigenic peptides, mimicking infection in diverse manners, flexibility in the design process, and acting as depots (Figure 3), thereby prolonging the activation of both cellular and humoral responses [38].
Poly-lactic-co-glycolic acid (PLGA) is a fully biodegradable polymer [39], and commonly employed in peptide/protein-based nanovaccines [30]. Peptide antigens can be adsorbed or encapsulated on the surface of PLGA nanoparticles to safeguard them from proteasomal degradation prior to uptake by APCs [30]. PLGA NPs have a dual function, acting as both adjuvants and antigen delivery vehicles, increasing immune responses by initiating antigen uptake by DCs, DC activation and maturation, and specific immune response induction [40]. Other polymers utilized include chitosan, polyacrylates, and their derivatives [41].
Lipid-based nanoparticles
Liposomes are widely studied as immune stimulation and vaccine delivery systems, offering advantages such as targeted delivery, high loading capacity, versatile structure modification, biocompatibility, adjustable properties, and agent protection [42]. Liposomes are quickly emerging as a multifunctional vaccine adjuvant delivery system (VADS), with immunomodulatory effects and adjuvant properties, promoting the induction of adaptive immunity [43,44]. Lipidated peptides, also known as lipopeptides, form self-assembled nanostructures [45] and being formulated by exploiting the amphiphilic properties of lipid-peptide structures [42]. Lipopeptide structures are employed as nanoparticles for several vaccines against various diseases, including GAS [46], hookworms [47], and malaria [48].
Lipopeptides, specifically lipid core peptides (LCPs), are well known for their ability to stimulate immune responses and form self-adjuvanting nanoparticles [49]. LCPs consist of lipids covalently linked together, a branching moiety, and peptide epitopes, enabling the delivery of peptide epitopes for various applications [49].
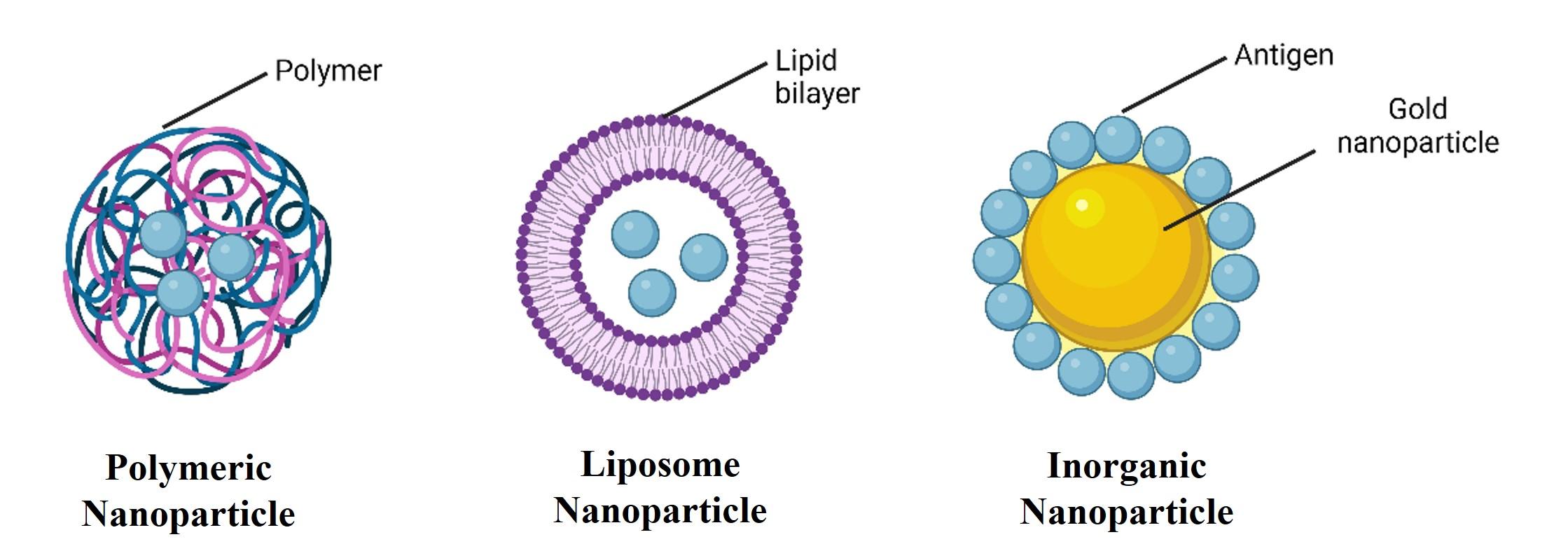
Inorganic nanoparticles
Inorganic nanoparticles are widely studied in nanovaccine formulations, offering advantages such as rigid and porous structures, surface functionalization with various ligands, and controlled synthesis [50]. Antigen peptides play a multifunctional role in the production of inorganic nanoparticles by interacting with inorganic materials to assemble them into functional complexes (Figure 3). As a result, inorganic nanoparticles can be produced cost-effectively and easily modified with antigens, and their surfaces can be modified further to elicit strong and robust immune responses [51].
Various inorganic nanoparticles are extensively studied for delivering peptide/protein-based nanovaccines [52]. Among them, gold nanoparticles (AuNPs) are particularly popular for vaccine delivery. AuNPs are efficiently taken up by cells, enhancing antigen delivery and increasing the antigenic peptide load delivered to APCs [53]. AuNPs serve as effective carriers and adjuvants for peptide antigens. Additionally, they were previously utilized for peptide delivery against influenza [54] and malaria [55].
APPLICATIONS OF PEPTIDE-BASED NANOVACCINES IN INFECTIOUS DISEASES
Tuberculosis
Tuberculosis (TB), the most lethal infectious disease, remains a leading cause of global mortality. Approximately 10% of affected patients show signs and symptoms within 1-2 years of infection, while the rest develop the disease after being immunocompromised. HIV-positive individuals who are also infected with Mycobacterium tuberculosis (Mtb) further aggravate the situation, resulting in an annual mortality rate of 33% [56].
There is a critical need for booster doses that enhance T-cell responses in BCG-vaccinated individuals. Thus, archaeosomes encapsulating the T cell antigen Rv3619c were developed, which induced a type-1 cytokine response upon immunization [57]. It also increased the production of antigen-specific T lymphocytes and IgG2a antibodies. These archaeosomes, containing the antigen, effectively reduce mycobacterial burden in the spleen and lungs of animals during pathogen challenge [57].
Nanoparticle-encapsulated antigen preparations demonstrate higher potency owing to their depot-forming ability with a controlled and slow release leading to increased processing of antigens by APCs [58]. Moreover, liposome-encapsulated antigens can induce stronger and more robust cellular and humoral immune responses than free antigens. Upon vaccination with liposome-encapsulated antigen, it showed significantly lower Mtb burden in the spleen and lungs after 16 weeks post-immunization with Mtb [59].
Preclinical and clinical studies evaluating diverse antigen-loaded NPs have demonstrated the ability of nanovaccines to elicit a strong and durable cell-mediated immune response against Mtb, offering promise as a novel and effective prophylactic approach against TB. While nanotechnology has mitigated issues related to adjuvants and delivery vehicles, challenges persist owing to the influence of the host environment which remains a significant obstacle in developing efficient TB vaccines.
Malaria
Malaria affects nearly 200 million individuals every year, leading to ~500,000 global mortality [60]. The complex life cycle of this pathogen is a major challenge in combating the disease. Vaccines targeting both the pre-erythrocytic and blood stages of malaria have been developed [61]. Nanovaccines have also been used to target multiple malaria stages. For instance, vaccination with rMSP-1-loaded NPs increased the levels of parasite-inhibitory antibodies. Moreover, IO nanoparticles were effectively internalized by macrophages and dendritic cells, leading to the enhanced production of chemokines and cytokines [62].
Immune responses against malaria sexual stage antigens play a crucial role in reducing disease transmission [55]. Pfs25 is an important transmission-blocking vaccine antigen; however, its immunogenicity in humans needs further investigation. To enhance immune responses, nanoformulations have been employed in conjunction with Pfs25. For instance, codon-harmonized Pfs25 (CHrPfs25) has been combined with AuNPs as an adjuvant to achieve stronger transmission-blocking antibodies. The co-delivery of CHrPfs25 with AuNPs likely facilitates immune cell uptake, contributing to an enhanced immune response [55].
Carboxylated polystyrene nanoparticles loaded with ovalbumin demonstrated adjuvant properties, inducing IL-10 and granulocyte colony-stimulating factor, and affecting dendritic cell migration and homing. Immunization with these nanoparticles led to the production of antimalarial antibodies, conferring immune readiness against subsequent infectious challenges [63]. Additionally, stable nanomimics, namely polymersomes linked to parasite attachment receptors, were developed. These nanomimics blocked parasite re-invasion after release from host cells, offering potential for modulating immune responses against malaria and optimizing vaccine design [64]. The increasing importance of peptide-based nanovaccines in inhibiting various life cycle stages presents promising prospects for developing an effective vaccination strategy against malaria.
Influenza
Influenza viruses display distinct matrix proteins and viral nucleoproteins, which lead to antigenic variability. Influenza viruses A and B cause annual epidemics that affect millions of individuals [65]. Despite vaccination efforts, ongoing antigenic changes and environmental selection contribute to the persistence of influenza epidemics [66]. Therefore, there is a pressing need for more effective vaccines that can induce both humoral and cellular immune responses against diverse influenza variants [67]. Encouragingly, the use of nanoparticles to associate with various influenza antigens has shown promising initial outcomes in enhancing immunity against diverse influenza virus antigens.
Given the antigenic variability of influenza A virus (IAV), researchers are exploring nanovaccines that target multiple serotypes using the conserved ectodomain of influenza matrix protein 2 (M2e). For instance, NPs covalently linked to three sequential repeats of the M2e demonstrated increased titers of mucosal secretory IgA antibodies and M2e-specific IgG. Additionally, this nanovaccine induces an increased cellular immune response, offering protection against lethal IAV infections in mice when administered intranasally [68].
Hemagglutinin trimers have been explored as potential antigens for stimulating strong immune response against the IAV. BALB/C mice were immunized with trimeric H7 conjugated to nanodiamond and showed significantly increased H7-specific IgG secretion [69]. Polyanhydride nanoparticles have been found to provide equivalent immune responses at doses 64-fold lower than those of free antigens [70]. Furthermore, antigen encapsulation in polyanhydride-conjugated nanovaccines remained stable for one year at room temperature, offering significant advantage in stockpiling pandemic vaccines [71].
In response to multiple strains of influenza virus and antigenic variability, the development of a universal nanovaccine that can provide broad cross-protection against different strains is essential to mitigate public health threats [72]. Researchers have explored the structural features of peptides or antigens to design rational broad-spectrum nanovaccines that contain multiple antigens. Double-layered protein nanoparticles incorporating four types of M2e from different viral consensus sequences were developed. This nanovaccine elicited strong humoral immunity and the M2e-specific antibodies showed strong cross-reactivity with different influenza virus antigens. Additionally, Uni4C13 induced strong cell-mediated immunity, as demonstrated by increased IFN-γ-secreting splenocytes. Sera from Uni4C13 immunized mice provided protection against viral infection for an extended period, indicating the potential for long-lasting immunity [72,73]. Thus, peptide-based vaccines can be used to protect public health against different strains of infectious pathogens.
HIV
Human immunodeficiency virus (HIV), a major cause of global mortality, leads to CD4+ T cell depletion and AIDS development, highlighting the critical requirement for an HIV vaccine. PLGA-based NPs were employed to co-deliver antigens and TLR agonists. Vaccination with HIV-1P24-Nef/FLiC/PLGA nanovaccines resulted in increased IgG production, even at a lower antigen dose. Nanovaccines promote Th1 polarization and enhance Th1 cytokine patterns [74].
Amantadine-coated AgNPs stimulated the secretion of HIV-specific CTLs, leading to a significant increase in TNF-α secretion. These HIV-specific CTLs were able to enhance the elimination of HIV-infected cells and reduce HIV production in vitro [75]. Additionally, AuNPs loaded with HIV-1 peptide improved antigen presentation. Immunization with the AuNP-based nanovaccine resulted in enhanced production of HIV-specific CD8+ and CD4+ T cells, along with elevated production of pro-inflammatory and pro-Th1 cytokines [76,77]. The protective capacity of intravaginally delivered nanogold formulations has been previously demonstrated. The combination of EFV and GNPs in thermogels showed superior inhibition of pre-interaction viral dissemination compared to individual treatments, providing strong anti-HIV prophylactic effects [78].
Nano-lipid complexes (NLC) have been shown to stimulate p24-specific immune responses for HIV. Intradermal vaccination with NLC-loaded p24 antigen resulted in significantly higher levels of p24-specific antibodies [79]. Thus, the development of peptide-based nanovaccines for HIV holds promise in preventing the spread of the disease.
CONCLUSIONS
Peptide-based nanovaccines have emerged as promising strategies for combating re-emerging and emerging infectious diseases. These nanovaccines offer several advantages including precise antigen targeting, improved stability, controlled release, and enhanced immunogenicity. The use of peptides as carriers has shown great potential for delivering peptide antigens, promoting antigen uptake by APCs, and eliciting robust immune responses.
Despite the significant progress, some research gaps remain. First, further investigation is required to optimize the design and formulation of peptide-based nanovaccines to achieve maximum immune stimulation and long-term memory. Additionally, more preclinical and clinical studies are required to validate the efficacy and safety of these nanovaccines in various disease models and in human subjects. The long-term stability of these vaccines, their manufacturing scalability, and the optimization of immune responses are areas that require further investigation.
The future of peptide-based nanovaccines is promising, with potential applications for a wide range of infectious diseases. Future research should focus on expanding the repertoire of antigen targets, exploring novel nanomaterials, and fine-tuning vaccine formulations for personalized immunotherapeutic strategies. Furthermore, the integration of systems biology and artificial intelligence may enable the rational design of highly efficient peptide-based nanovaccines tailored to individual patients, thereby revolutionizing the field of precision immunotherapy.
ACKNOWLEDGMENTS
The authors would like to thank the DOST S&T Fellows Program, the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) for funding this research project, and the Industrial Technology Development Institute (DOST-ITDI) for hosting this research project.
AUTHOR CONTRIBUTIONS
FLO: Conception and design of the study, wrote the first draft of the manuscript, critically revised the manuscript and funding acquisition. FLO also has approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Heitmann JS, Bilich T, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature. 2022;601:617–622.
- [2]Rasheed M, Saleem M, et al. Effect of caffeine-loaded silver nanoparticles on minerals concentration and antibacterial activity in rats. J Adv Biotechnol Exp Ther. 2023;6:495.
- [3]Koirala P, Bashiri S, et al. Current prospects in peptide-based subunit nanovaccines. In: Thomas S, editor. Vaccine Des. Methods Protoc. Vol. 3 Resour. Vaccine Dev. New York, NY: Springer US; 2022. p. 309–338.
- [4]Bagwe PV, Bagwe PV, et al. Peptide-based vaccines and therapeutics for COVID-19. Int J Pept Res Ther. 2022;28:94.
- [5]Akash S, Hossain M, et al. Devising a multi epitope vaccine toward the dengue virus using the computational method in Bangladesh. J Adv Biotechnol Exp Ther. 2023;6:44.
- [6]Tsoras AN, Champion JA. Protein and peptide biomaterials for engineered subunit vaccines and immunotherapeutic applications. Annu Rev Chem Biomol Eng. 2019;10:337–359.
- [7]Orosco FL. Current progress in diagnostics, therapeutics, and vaccines for African swine fever virus: Vet Integr Sci. 2023;21:751–781.
- [8]Zhao G, Chandrudu S, et al. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur Polym J. 2017;93:670–681.
- [9]Zottig X, Al-Halifa S, et al. Self-assembled peptide nanorod vaccine confers protection against influenza A virus. Biomaterials. 2021;269:120672.
- [10]Bhardwaj P, Bhatia E, et al. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020;108:1–21.
- [11]Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: An immunological and materials perspective. Adv Healthc Mater. 2013;2:72–94.
- [12]Blok BA, Arts RJW, et al. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98:347–356.
- [13]Demento SL, Siefert AL, et al. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29:294–306.
- [14]Toy R, Roy K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng Transl Med. 2016;1:47–62.
- [15]Hussein WM, Liu T-Y, et al. Multiantigenic peptide–polymer conjugates as therapeutic vaccines against cervical cancer. Bioorg Med Chem. 2016;24:4372–4380.
- [16]Gross BP, Wongrakpanich A, et al. A therapeutic microparticle-based tumor lysate vaccine reduces spontaneous metastases in murine breast cancer. AAPS J. 2014;16:1194–1203.
- [17]Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517.
- [18]Walsh KP, Mills KHG. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530.
- [19]Tran E, Turcotte S, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645.
- [20]Vignali DAA, Collison LW, et al. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532.
- [21]Berkowska MA, Driessen GJA, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158.
- [22]de Titta A, Ballester M, et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci. 2013;110:19902–19907.
- [23]Levine TP, Chain BM, et al. The cell biology of antigen processing. Crit Rev Biochem Mol Biol. 1991;26:439–473.
- [24]Seidman JC, Richard SA, et al. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses. 2012;6:52–62.
- [25]Demento SL, Cui W, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964.
- [26]Reddy ST, van der Vlies AJ, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164.
- [27]Skwarczynski M, Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomed. 2014;9:2657–2669.
- [28]Davis ME, Chen Z, et al. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782.
- [29]Li J, Huang D, et al. Multifunctional biomimetic nanovaccines based on photothermal and weak-immunostimulatory nanoparticulate cores for the immunotherapy of solid tumors. Adv Mater. 2022;34:2108012.
- [30]Gutjahr A, Phelip C, et al. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines. 2016;4:34.
- [31]Wen R, Umeano AC, et al. Nanoparticle systems for cancer vaccine. Nanomed. 2019;14:627–648.
- [32]Didierlaurent AM, Laupèze B, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63.
- [33]Roldão A, Mellado MCM, et al. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176.
- [34]Correia-Pinto JF, Csaba N, et al. Vaccine delivery carriers: Insights and future perspectives. Int J Pharm. 2013;440:27–38.
- [35]Zhu M, Wang R, et al. Applications of nanomaterials as vaccine adjuvants. Hum Vaccines Immunother. 2014;10:2761–2774.
- [36]Zaman M, Good MF, et al. Nanovaccines and their mode of action. Methods. 2013;60:226–231.
- [37]Swaminathan G, Thoryk EA, et al. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine. 2016;34:110–119.
- [38]Pippa N, Gazouli M, et al. Recent advances and future perspectives in polymer-based nanovaccines. Vaccines. 2021;9:558.
- [39]Jin H, Chong H, et al. Preparation and evaluation of amphipathic lipopeptide-loaded PLGA microspheres as sustained-release system for AIDS prevention. Eng Life Sci. 2020;20:476–484.
- [40]Han S, Ma W, et al. Intracellular signaling pathway in dendritic cells and antigen transport pathway in vivo mediated by an OVA@DDAB/PLGA nano-vaccine. J Nanobiotechnology. 2021;19:394.
- [41]Nevagi RJ, Khalil ZG, et al. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018;80:278–287.
- [42]Wang N, Chen M, et al. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J Controlled Release. 2019;303:130–150.
- [43]Negahdaripour M, Golkar N, et al. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol Adv. 2017;35:575–596.
- [44]Orosco FL. Immune evasion mechanisms of porcine epidemic diarrhea virus: A comprehensive review: Vet Integr Sci. 2024;22:171–192.
- [45]Hamley IW. Lipopeptides for vaccine development. Bioconjug Chem. 2021;32:1472–1490.
- [46]Huang W, Madge HYR, et al. Structure-activity relationship of lipid, cyclic peptide and antigen rearrangement of physically mixed vaccines. Int J Pharm. 2022;617:121614.
- [47]Bartlett S, Eichenberger RM, et al. Lipopeptide-based oral vaccine against hookworm infection. J Infect Dis. 2020;221:934–942.
- [48]Alving CR, Peachman KK, et al. Army liposome formulation (ALF) family of vaccine adjuvants. Expert Rev Vaccines. 2020;19:279–292.
- [49]Marasini N, Khalil ZG, et al. Lipid core peptide/poly(lactic-co-glycolic acid) as a highly potent intranasal vaccine delivery system against Group A streptococcus. Int J Pharm. 2016;513:410–420.
- [50]Pigliacelli C, Sánchez-Fernández R, et al. Self-assembled peptide–inorganic nanoparticle superstructures: from component design to applications. Chem Commun. 2020;56:8000–8014.
- [51]Al-Halifa S, Gauthier L, et al. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019;10.
- [52]Bai S, Jiang H, et al. Aluminum nanoparticles deliver a dual-epitope peptide for enhanced anti-tumor immunotherapy. J Controlled Release. 2022;344:134–146.
- [53]Staroverov SA, Volkov AA, et al. Prospects for the use of spherical gold nanoparticles in immunization. Appl Microbiol Biotechnol. 2019;103:437–447.
- [54]Tao W, Gill HS. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307–2315.
- [55]Kumar R, Ray PC, et al. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine. 2015;33:5064–5071.
- [56]Floyd K, Glaziou P, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6:299–314.
- [57]Dhanasooraj D, Kumar RA, et al. Vaccine delivery system for tuberculosis based on nano-sized hepatitis B virus core protein particles. Int J Nanomedicine. 2013;8:835–843.
- [58]Das I, Padhi A, et al. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of γδ T cells in mice. Nanotechnology. 2017;28:165101.
- [59]Diogo GR, Hart P, et al. Immunization with Mycobacterium tuberculosis antigens encapsulated in phosphatidylserine liposomes improves protection afforded by BCG. Front Immunol. 2019;10.
- [60]Cohen JM, Okumu F, et al. The fight against malaria: Diminishing gains and growing challenges. Sci Transl Med. 2022;14:eabn3256.
- [61]Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines. 2016;15:765–781.
- [62]Pusic K, Aguilar Z, et al. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J. 2013;27:1153–1166.
- [63]Xiang SD, Kong YY, et al. Nanoparticles modify dendritic cell homeostasis and induce non-specific effects on immunity to malaria. Trans R Soc Trop Med Hyg. 2015;109:70–76.
- [64]Najer A, Wu D, et al. Nanomimics of host cell membranes block invasion and expose invasive malaria parasites. ACS Nano. 2014;8:12560–12571.
- [65]Houser K, Subbarao K. Influenza vaccines: Challenges and solutions. Cell Host Microbe. 2015;17:295–300.
- [66]Blackburne BP, Hay AJ, et al. Changing selective pressure during antigenic changes in human influenza H3. PLOS Pathog. 2008;4:e1000058.
- [67]Woodland DL, Hogan RJ, et al. Cellular immunity and memory to respiratory virus infections. Immunol Res. 2001;24:53–67.
- [68]Qi M, Zhang X-E, et al. Intranasal nanovaccine confers homo- and hetero-subtypic influenza protection. Small. 2018;14:1703207.
- [69]Pham NB, Ho TT, et al. Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J Nanobiotechnology. 2017;15:69.
- [70]Huntimer L, Wilson Welder JH, et al. Single immunization with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. J Biomed Mater Res B Appl Biomater. 2013;101B:91–98.
- [71]Petersen LK, Phanse Y, et al, Narasimhan B. Amphiphilic Polyanhydride Nanoparticles stabilize Bacillus anthracis protective antigen. Mol Pharm. 2012;9:874–882.
- [72]Deng L, Mohan T, et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun. 2018;9:359.
- [73]Mahedi MRA, Rawat A, et al. Understanding the global transmission and demographic distribution of Nipah virus (NiV). Res J Pharm Technol. 2023;16:3588–3594.
- [74]Rostami H, Ebtekar M, et al. Co-utilization of a TLR5 agonist and nano-formulation of HIV-1 vaccine candidate leads to increased vaccine immunogenicity and decreased immunogenic dose: A preliminary study. Immunol Lett. 2017;187:19–26.
- [75]Li W, Balachandran YL, et al. Amantadine surface-modified silver nanorods improves immunotherapy of HIV vaccine against HIV-infected cells. ACS Appl Mater Interfaces. 2018;10:28494–28501.
- [76]Climent N, García I, et al. Loading dendritic cells with gold nanoparticles (GNPs) bearing HIV-peptides and mannosides enhance HIV-specific T cell responses. Nanomedicine Nanotechnol Biol Med. 2018;14:339–351.
- [77]Orosco F. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Vet J. 2023;13:1056–1056.
- [78]Malik T, Chauhan G, et al. Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif Cells Nanomedicine Biotechnol. 2018;46:79–90.
- [79]Bayon E, Morlieras J, et al. Overcoming immunogenicity issues of HIV p24 antigen by the use of innovative nanostructured lipid carriers as delivery systems: evidences in mice and non-human primates. Npj Vaccines. 2018;3:1–14.