Expression patterns of the phosphoproteins and total proteins in TLQP-21 (a VGF derived peptide) treated SH-SY5Y cells
Abstract
VGF (non-acronymic), belonging to a large granin family, gives rise to a number of bioactive peptides by proteolysis and exert an extensive array of biological effects on energy metabolism, pain modulation, gastric secretion function, reproduction, mood regulation, and, diabetes. Among VGF-derived peptides, TLQP-21 (The first four amino acids, in short TLQP (Thr-Leu-Gln-Pro) generalizes the nomenclature of the peptide by its length) is the most studied although little is known yet about downstream molecular mechanisms of action of VGF-derived peptides like TLQP-21. So here as a preliminary analysis, total protein expression was carried out in addition to the phosphoproteomic study of SH-SY5Y cells treated with TLQP-21, using the same cell extracts. Comparison of simple 1D SDS-PAGE gels stained with SYPRO® Ruby protein gel stain was carried out to assess whether changes in protein expression could be seen even at such low separation resolution. Expression of several proteins most likely Microtubule-associated protein 1B (MW 271 kDa), Tubulin beta chain (MW 57), Tubulin beta-4B chain (MW 50), Alpha-2-macroglobulin (MW 163), etc. in TLQP-21 treated and control samples was found significantly different, indicating that the peptide TLQP-21 exerts biological effects on SH-SY5Y cells. Further studies are required to validate the identity of the modulated proteins, obtained from mass spectrometry. Identification of modulated proteins after TLQP-21 treatment would open new avenues to discover the molecular mechanisms of its physiological and pharmacological state.
INTRODUCTION
Protein phosphorylation provides key informations within signal transduction cascades and protein function modulations. It plays a key role in the regulation of most of the aspects of cellular biology [1]. To detect protein phosphorylation directly in polyacrylamide gels, fluorescence-based detection technology, known as phosphoprotein gel stain, has been introduced [2, 3] without using any antibody or radioisotope, compatible with mass spectrometry [1]. For phosphoproteomic study as well as for identification of kinase targets in signaling pathways, phosphoprotein gel stain is a suitable method [1].
SYPRO® Ruby dye is a quantitative, total-protein stain. Determining the ratio of Pro-Q® Diamond dye to SYPRO® Ruby dye signal intensities for each band or spot provides a measure of the phosphorylation level normalized to the total amount of protein. Using both stains in combination, it is possible to distinguish a lightly phosphorylated, high-abundance protein from a heavily phosphorylated, low-abundance protein. Thus, analysis of (phosphor and/or total) proteins by Pro-Q® Diamond phosphoprotein gel stain becomes most useful as well as most effective when used in combination with SYPRO® Ruby protein gel stain.
VGF (a non-acronymic name) is not to be confused with VEGF (vascular endothelial growth factor). The VGF gene was first recognized as a nerve growth factor (NGF) responsive gene. NGF33.1, a nervous system-specific mRNA was cloned by treatment of PC12 cells with NGF. For the first time, in 1985 Levi et al. [4] designated the clone corresponding to the NGF-inducible mRNA as VGF, after successful clarification of the nucleic acid as well as amino acid sequences of the NGF33.1 cDNA clone. The term ‘VGF’ was coined very interestingly as the selection of this clone was from plate V of the nerve Growth Factor induced PC12 cell cDNA library [4, 5].
From VGF, several peptides are derived like APPG-40, APPG-37, GRPE-37, NERP-1, NERP-2, NAPP-129 (VGF 20), VGF 18, HFHH-51 (VGF 6), HHPD-1, AQEE-30 (Peptide V), LQEQ-19, TLQP-21 and TLQP-62 (VGF 10). Out of these peptides, TLQP-21 is of great importance because of its several physiological roles. TLQP-21 plays vital roles in the following physiological actions: Energy expenditure [6, 7, 8] metabolic functions [6, 15], glucose-stimulated insulin secretion (GSIS) [9], nociception [10, 11, 12], blood pressure/hypertension regulation [13], gastric contractility [14, 15], regulation of gastric acid secretion [16, 17, 18], reproduction [19, 20], stress [21, 22], neuroprotective agent [23], anorexia [6, 7]. To the best of our knowledge, no proteomic or phosphoproteomic studies have yet focused on the effect of VGF derived bioactive peptide TLQP-21 on signaling pathways in SH-SY5Y cells.
Taken together all these in considerations, a study on the expression of phosphoproteins and total proteins in TLQP-21 treated SH-SY5Y cells was conducted to conclude whether modulation in protein expression could be found using Pro-Q® Diamond phosphoprotein gel stain, in conjunction with SYPRO® Ruby protein gel stain.
MATERIALS AND METHODS
TLQP-21
TLQP-21 (human, molecular weight 2490.88 Da) and modified TLQP-21 (human) containing biotin at N-terminus and a cysteine residue at the C-terminus with total molecular weight 2820.32 Da, were purchased from ChinaPeptides Co. Ltd., Shanghai. The purity >95% of the both peptides, TLQP-21 and biotinlyted TLQP-21 were confirmed by HPLC and MS analysis. It was in the form of white lyophilized powder and stored at -21˚C immediately upon arrival for short time storage and at -80˚C for long term storage, as per instruction of the supplier. The peptide sequence is – TLQPPSALRRRHYHHALPPSR
Thr – Leu – Gln – Pro – Pro – Ser – Ala – Leu – Arg – Arg – Arg – His – Tyr -His – His – Ala – Leu – Pro – Pro – Ser – Arg
TLQP-21 solutions were constituted by dissolving the lyophilized powder in filtered (0.22 μm, Millex, Merck Millipore Ltd.) PBS, and were used instantly or was kept at -80 ˚C for long term storage.
SH-SY5Y cell culture
SH-SY5Y (European Collection of Cell Cultures, ECACC; catalog number-94030304) is a thrice cloned (SK-N-SH → SH-SY → SH-SY5 → SH-SY5Y) subline of the neuroblastoma cell line SK-N-SH which was established in 1970 from a metastatic bone tumor of a four year-old female with neuroblastoma [24, 25]. As per standard protocol, the cells were always used at less than 20 passages and were grown in 100×20 mm Falcon Petri dishes (Life Sciences) on a culture medium composed of 1:1 F12 HAM (Sigma Aldrich) and Earle’s Balanced Salt Solution (EBSS) (Sigma Aldrich), which was supplemented with 1% Penicilin-Streptomicin (P/S) (Invitrogen), 15% fetal bovine serum (FBS) (Gibco), 1% Non-Essential Amino Acids (NEAA) (Sigma Aldrich) and 1% Glutamine (Gln) (Sigma Aldrich). And for optimum growth, 5% CO2-humidified incubator was kept at 37 ˚C.
Phosphoprotein gel staining
SH-SY5Y cells in Petri dishes with confluent growth were incubated for 6 hours with the peptide TLQP-21 at a concentration of 1μg/ml. Then the cell homogenates were prepared as discussed above. As control, cells without peptide incubation were grown, then homogenized under the same conditions as treated cells.
Cell homogenate samples (20 μl) were boiled in 2X Laemmli buffer (Bio-Rad) for 10 minutes at 100 ˚C, spun, and loaded to SDS-PAGE gel. After electrophoresis (200V, 01h), the gels were washed in dH2O for 10 minutes. Then the gels were kept with fixing solution composed of 50% methanol and 10% acetic acid in an orbital shaker step at 50 rpm for half an hour at room temperature. The fixation step was repeated once. Followed by washing the gel with water three times each for ten minutes, the gels were incubated for one and half hour with Pro-QR Diamond (Invitrogen) gel stain in the dark with agitation at 50 rpm. The gels were then destained with Pro-QR Diamond phosphoprotein gel destaining solution (20 % acetonitrile, 50 mM sodium acetate, pH 4) for 30 minutes with shaking. The destaining procedure was repeated two times more, followed by washing the gels two times each for 5 minutes with water. And then the gel was imaged using a Typhoon FLA9500 scanner at a 100 μm resolution at 532nm green laser.
For SYPRO® Ruby protein gel staining
Samples (25 μl) from cell homogenates of the cells treated with the peptide and not treated were heated in 2X Laemmli buffer (Bio-Rad) for 10 minutes at 100 °C, spun, and loaded to SDS-PAGE gel. After electrophoresis (200V, 01h), the gel was taken out from cassette, followed by continuous washing for 10 minutes in dH2O. Then the gel was treated for 60 minutes at room temperature with fixing solution, consists of 10% methanol and 7% acetic acid in an orbital shaker step at 50 rpm, followed by overnight incubation with SYPRO® Ruby Protein Gel Stain at room temperature with shaking. The gel was then placed into a staining container covering with a lid to protect it from the light, in addition, the container was wrapped in aluminum foil to further shield the stain from light during the staining process, as per instruction of the supplier. The gel was shifted to a clean staining dish followed by staining for overnight. Then the gel was washed with the fixing solution in the same condition of staining followed by 5 minutes washing in dH2O. Finally, the gel was taken from the container to take the image in a MolecularImager® Gel DocTM system (Bio Rad, Hercules). To capture the best image, the highest sensitivity of the CCD camera was used at a resolution of 1392 x 1040 pixels with 12 bit gray scale levels per pixel.
Protein detection using mass spectrometric analysis
From the gels, spots were chosen for mass spectrometric analysis and were excised with a pipette tip and then in-gel digestion was performed manually with trypsin following the standard protocol described by Shevchenko et al., 2006. The excised spots were washed three times with 100 μl of ammonium bicarbonate 50 mM in 50% methanol (grade HPLC Scharlau) and reduced with 10 mM DTT (SERVA Electrophoresis GmbH). After this, the gel pieces were washed three times with ammonium bicarbonate, dried in a SpeedVac (Thermo Scientific) and alkylated with 55 mM iodoacetamide (IAA) (Sigma- Aldrich). The gel pieces were washed again with ammonium bicarbonate, dehydrated with acetonitrile and dried again in a SpeedVac (Thermo Scientific). Trypsin, modified with porcine (Promega) was added in 20 mM ammonium bicarbonate at the final concentration of 20 ng/mL and digestion let proceed overnight at 37ºC. Peptide extraction from the gel pieces was carried out three times with 40 μL of 60% acetonitrile in 0.5% of formic acid. Then the extracts were collected followed by drying in the SpeedVac (Thermo Scientific) and kept it at -20ºC. After digestion the spots were identified using a model 4800 MALDITOF/TOF mass spectrometer (ABSciex, Framingham, MA, EEUU) at the Proteomics Lab of the Foundation IDICHUS (University Clinical Hospital, University of Santiago de Compostela, Santiago de Compostela, Spain), as per protocol described above. Programmed laboratory analysis of mass data was conducted by using the 4000 Series. It is to be noted that to get the best results, all of the MS/MS spectra were performed taking the considerations of metastable suppression as well as by selecting the precursor ions with a relative resolution of 300 (FWHM).
For convenience, through the GPS Explorer Software v3.6 both of the MS/MS spectra data and Explorer Software V3.5. MS data were pooled together; followed by the database search which was performed with the Mascot v2.1 search tool (Matrix Science, London, UK) screening SwissProt (release 56.0). It is noteworthy that carbamidomethyl cysteine was set as a fixed modification and oxidized methionine was set as potential variable modification, following by searches which were restricted to human taxonomy. The precursor mass tolerance was fixed at 30 ppm whereas the MS/MS tolerance was set at 0.35 Da. And 1 missed tryptic cleavage site was allowed. All spectra and database findings were manually inspected and afterwards all these were detailed using the softwares, as mentioned above [27, 28, 29].
RESULTS
As supplementary confirmation that TLQP-21exerts biological effects in the model system, SH-SY5Y cells; homogenates from TLQP-21 treated or not treated (control) cells were used to conduct 1D SDS-PAGE, then it was stained with the dye Pro-Q Diamond, followed by SYPRO® Ruby dye. As seen in the Figure 1, at position 3, 4 and 5; band intensity becomes higher in SYPRO® Ruby dye staining indicate the nonphosphorylated proteins. Differences in band intensity were seen at position 1 to 5 of the gels. The bands’ intensity at 1 (A, Pro Q® Diamond) and 3 (both in A, Pro Q® Diamond and B, SYPRO® Ruby dye) were less in the samples treated with the peptide in comparison to not treated (control) ones suggesting that the peptide on SH-SY5Ycells might provoke dephosphorylation of specific phosphoproteins. The bands at position 1 and 3 from A, Pro Q® Diamond stained gel was cut and analysed by mass spectrometry, results are detailed in the Table 1.
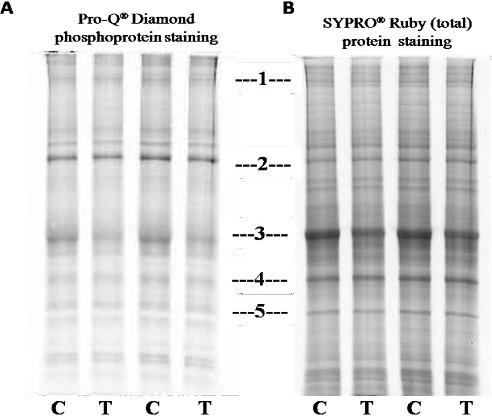
A comparison was made among Pro Q® Diamond staining, SYPRO® Ruby protein gel stain and simple 1D SDS-PAGE gels: Several bands with altered intensity in the SYPRO® Ruby experiment were of the same positions in the gels as those in the Pro Q® Diamond, suggesting that both techniques were detecting, to some extent, similar changes. This also suggested that the proteins with altered phosphorylation status were probably abundant proteins, hence their changes were “picked up” by SYPRO® Ruby staining.
Out of the proteins listed in the table 1, several proteins like Microtubule-associated protein 1B (MW 271 kDa), Tubulin beta chain (MW 57), Tubulin beta-4B chain (MW 50), Alpha-2-macroglobulin (MW 163) were of interests, as these are related with neurological and other functions of VGF derived peptide TLQP-21 [27, 29]. Further studies are required to validate the results obtained from mass spectrometry and to illustrate the further downstream consequences with reference to clinical studies.
Table 1. List of proteins with altered expression levels in Pro Q® Diamond stained gel.
DISCUSSION
In TLQP-21 treated rat pituitary tumor cell lines (GH3), no difference was found in pERK and pAMPK though there was difference in expression of pAKT and p-p38 [26]. In another study, in rat cerebellar granule cells (CGCs) TLQP-21 was shown to activate ERK½ significantly. Akt phosphorylation was found to be increased after 15 minute of treatment with TLQP-21, while using insulin-like growth factor-1 (IGF-1) phosphorylation of Akt increased further after the same time interval. IGF-1 treatment augmented Akt phosphorylation after 24 and 48 h of incubation whereas TLQP-21 did not modify the amount of phosphorylated Akt, although it is to mentioned here that total Akt and α-tubulin were found to be expressed more than before in both cases in rat CGCs [22].
In another study of TLQP-21 induced signal transduction pathway in mice 3T3-L1 adipocytes, TLQP-21 did not bring any change in expression of Akt (Ser473), PKC (protein kinase C; pan Ser660), p38 (Thr180/Tyr182)and JNK (c-Jun N-terminal kinase; Thr183/Tyr185, PKA and HSL , whereas TLQP-21 increased phosphorylation of AMPK and ERK [6].
From literature review it is evident that this is the first study in human cell line, to observe the effect of the peptide, TLQP-21 whether it modulates the total proteins or the phosphoproteins or not. The significance of the study lies into the fact that it highlights a vital opening point for more advanced exploration into cell signaling by TLQP-21. Further proteomic analysis, followed by 2D Gel- or LC-MALDI TOF/TOF will help us for better understanding in this regard.
ACKNOWLEDGMENTS
Md. Shamim Akhter is the recipient of an Erasmus Mundus EXPERTS – II scholarship for doctoral program.
CONFLICT OF INTEREST
The author declares that no conflict of interest exists.
References
- [1]Schulenberg B, Goodman TN, Aggeler R, Capaldi RA, Patton WF. Characterization of dynamic and steady-state protein phosphorylation using a fluorescent phosphoprotein gel stain and mass spectrometry. Electrophoresis 2004; 25 : 2526-2532.
- [2]Steinberg T, Agnew B, Gee K, Leung W, Goodman T, Schulenberg B, et al. Proteomics 2003; 3: 1128–1144.
- [3]Martin K, Steinberg T, Cooley L, Gee K, Beechem J, Patton W. Proteomics 2003; 3: 1244–1255.
- [4]Levi A, Eldridge JD., Paterson BM. Molecular cloning of a gene sequence regulated by nerve growth factor. Science 1985; 229 (4711):393-395.
- [5]Possenti R, Eldridge JD., Paterson BM., Grasso A, Levi A. A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. EMBO J 1989; 8(8): 2217-2223.
- [6]Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L. et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J 2012; 441 (1): 511-22.
- [7]Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JW, Carter, WG et al. VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology 2007; 148: 4044-4055.
- [8]Bartolomucci A, Corte GL, Possenti R, Locatelli V, Rigamonti AE, Torsello A, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2006; 103 : 14584–14589.
- [9]Stephens SB, Schisler JC, Hohmeier HE, An J, Sun AY, Pitt GS, et al. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab 2012; 16 (1): 33-43. doi: 10.1016/j.cmet.2012.05.011.
- [10]Fairbanks CA, Peterson CD, Speltz RH, Riedl, MS, Kitto, KF, Dykstra, JA, et al. The VGF-derived peptide TLQP-21 contributes to inflammatory and nerve injury induced hypersensitivity. Pain 2014; 155: 1229–1237.
- [11]Chen YC, Pristerá A, Ayub M, Swanwick RS., Karu K, Hamada Y, et al. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J Biol Chem 2013; 288 (48): 34638-46.
- [12]Rizzi R, Bartolomucci A, Moles A, D’Amato F, Sacerdote P, Levi A, et al. The VGF-derived peptide TLQP-21: a new modulatorypeptide for inflammatory pain. Neurosci Lett 2008; 441: 129–133.
- [13]Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin WJ, et al. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J 2014; 28: 2120–2133.
- [14]Severini C, La Corte G, Improta G, Broccardo M, Agostini S, Petrella C, et al. In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. British journal of pharmacology 2009; 157: 984-993.
- [15]Bartolomucci A, Moles A, Levi A, Possenti R. Pathophysiological role of TLQP-21: gastrointestinal and metabolic functions. Eat Weight Disord. 2008; 13(3): e49-54. PMID: 19011364.
- [16]Sibilia V, Pagani F, Bulgarelli I, Tulipano G, Possenti R, Guidobono F. Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a vgf-derived peptide, in rats. Amino Acids 2012; 42 (4): 1261-8. doi: 10.1007/s00726-010-0818-6. Epub 2010 Dec 4.
- [17]Sibilia V, Pagani F, Bulgarelli I, Mrak E, Broccardo M, Improta G, et al. TLQP-21, a VGF-derived peptide, prevents ethanol-induced gastric lesions: insights into its mode of action. Neuroendocrinology 2010a; 92 (3): 189-97. doi: 10.1159/000319791.
- [18]Sibilia V, Pagani F, Bulgarelli I, Tulipano G, Possenti R, Guidobono F. Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a VGF-derived peptide, in rats. Amino Acid 2010b. 10.1007/s00726-010-0818-6.
- [19]Aguilar E, Pineda R, Gayta´ n F, Sa´nchez-Garrido MA, Romero M, Romero-Ruiz A, et al. Characterization of the reproductive effects of the VGF-derived peptide TLQP-21 in female rats: in vivo and in vitro studies. Neuroendocrinology 2013; 98: 38–50.
- [20]Pinilla L, Pineda R, Gaytan F, Romero M, Garcia-Galiano D, Sanchez-Garrido M.A, et al. Characterization of the reproductive effects of the anorexigenic VGF-derived peptide TLQP-21: in vivo and in vitro studies in male rats. American Journal of Physiology Endocrinology and Metabolism 2011; 300: 837-847.
- [21]Razzoli M, Bo E, Pascucci T, Pavone F, D’Amato FR, Cero, et al. Implication of the VGF-derived peptide TLQP-21 in mouse acute and chronicstress responses. Behav. Brain Res 2012; 229: 333–339.
- [22]Bartolomucci A, Possenti R, Mahata SK., Fischer-Colbrie R, Loh YP, Salton SR. The extended graninfamily: structure, function, and biomedical implications. Endocr Rev 2011; 32 (6): 755-97.
- [23]Severini C, Ciotti MT, Biondini L, Quaresima S, Rinaldi AM, Levi A, Frank C, Possenti R. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem 2008; 104: 534-544.
- [24]Biedler JL., Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973; 33 (11): 2643–52.
- [25]Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 1978; 38 : 3751–7.
- [26]Passeri PP, Biondini L, Mongiardi MP, Mordini N, Quaresima S, Frank C, et al. Neuropeptide TLQP-21, a VGF internal fragment, modulates hormonal gene expression and secretion in GH3 cell line. Neuroendocrinology 2013; 97: 212–224. DOI: 10.1159/000339855.
- [27]Akhter S, Chakraborty S, Moutinho,D, AÂlvarez-Coiradas E, Rosa I, Viñuela J, et al. The human VGF-derived bioactive peptide TLQP-21 binds heat shock 71 kDa protein 8 (HSPA8) on the surface of SH-SY5Y cells. PLoS ONE 2017; 12(9): e0185176. https://doi.org/10.1371/journal. pone.0185176.
- [28]Ayub M. Investigating the mechanisms of action of VGF-derived peptides in the nervous system. Ph D Thesis. 2012; Imperial College, London.
- [29]Akhter S. Isolation of VGF derived neuropeptide receptor. Ph. D. Thesis. 2015; University of Santiago de Compostela, Spain.