Analysis of telomere length in the coronary artery to determine the pathogenesis of coronary artery disease
Abstract
Endothelial dysfunction is one of the earliest pathological features in atherosclerosis. The current study designed to analyse the telomere length of human coronary artery derived endothelial cells to identify the relationship between telomere shortening and coronary artery disease (CAD)s. Coronary artery endothelial cells obtained from 30 patients with CAD and cell line were analysed by real time PCR method. Relative telomere and single-copy 36B4 gene (T/S) ratio which corresponds to the telomere length in coronary artery was significantly smaller in coronary artery disease patients than compared with control cell line. Flow cytometry analysis resulted endothelial cell count ranged from 0.55 x 106 to 0.96 x 106. Findings of this study suggest that telomere shortening in coronary artery endothelial cells play important role in the coronary artery disease.
INTRODUCTION
There are many unanswered questions about what causes the adverse effects associated with aging, and even what aging actually is, including the potential for continuing creativity and contributions to society. Answers to these questions will give us powerful insights into the mechanisms of aging and the causes and treatment of age-related diseases. A continuing debate has been whether a pattern of aging free of disease can be dissected away from the overlapping development of age-related diseases such as cancer, cardiovascular disease, osteoporosis, osteoarthritis, diabetes, and a large variety of neurodegenerative diseases, including Alzheimer’s disease. It is clear that some age-related changes are risk factors for disease and that these changes can be both intrinsic and extrinsic, and either random or programmed (genetic). Intrinsic stochastic factors in aging includes oxidative damage to proteins, DNA, and lipids; glycation; changes in protein conformation; and induction of mutations. Genetic factors in aging includes genes implicated in regulating longevity and aging includes genes for proteins with antioxidant activities, e.g., superoxide dismutase, catalase and thioredoxin; and signal transduction proteins, e.g., insulin receptor, phosphatidyl inositol-3-kinase, ras and GTP-binding protein (the methuselah gene product) and telomerase deficiency/ telomere length.
In spite of maintenance of systolic function during rest, there may be changes takes place in the diastolic phase of the cardiac cycle that occurs along with ageing. During ageing diastolic dysfunction is widely recognized and their association with cellular senescence is clearly not understood. Cardiovascular risk factors such as atherosclerosis, heart failure and hypertension, are highly associated with leukocyte telomere shortening, but it remains undetermined. Telomere length alone cannot satisfy the criteria as biomarker for ageing, but this adds the predictive to that of chronological age and can be considered as marker for cardiovascular ageing [1]. The telomeres become critically short, the cell is no longer able to replicate and enters cellular senescence [2,3,4]. Cardiac tissue from animal models shows substantial loss of telomere length with age and telomerase knockout in mice lead to heart failure, suggesting a possible role for telomere shortening in the development of heart ageing. The association of leukocyte telomere length (LTL) with the other tissues was also consistently high [5]. The average LTL is inversely correlated with age and is associated with age-related disorders, including CVD [6].
Ischemic heart disease or coronary artery disease (CAD) is a representative atherosclerosis associated disorder. Cellular senescence and endothelial dysfunction were suggested to be important in its pathological mechanism [7,8,9]. Even though the telomere shortening in leukocytes of CAD patients has been reported, the telomere length of coronary artery endothelial cells of such CAD patients has not been directly measured. Because of the small size of coronary arteries, it is very difficult to extract optimum quantity of DNA from coronary endothelial cells for telomere length measurement using southern hybridization analysis. In the current study, we analysed the telomere shortening in coronary artery endothelial cells of patients with CAD using a PCR method, by which the relative telomere length could be evaluated in a small amount of DNA.
MATERIALS AND METHODS
Study Population
An observational study of 30 CAD patients as confirmed by angiography were enrolled in this study. CAD with 1-3 vessel disease were included. Cardiovascular risk factors and other factors were evaluated. Clinical characteristics of all patients with CAD, including age, gender, types of CAD, and presence of risk factors (cigarette smoking, hypertension as defined by the Joint National Committee V, diabetes mellitus as defined by the WHO Study Group and hypercholesterolemia [8]. Study subjects were categorized according to age into 2 groups, of 32 to 55 years (group 1, n = 18) and of 56 to 68 years (group 2, n = 12).
Tissue samples and isolation of endothelial cells
In this study, resected coronary arteries after Coronary Artery Bypass Grafting (CABG) were obtained in sterile containers with DMEM medium. The study carried out by collecting the patient’s samples, those will be disposed after the surgery. All these samples were processed within 3 hours after collection. The collected artery measurement ranged from 15 to 35 mm in length. In the patients with CAD, coronary segments containing the culprit lesion responsible for acute or old myocardial infarction were excluded. Isolation of the coronary endothelial cells was performed according to techniques previously reported [9,10,11]. Briefly, each of the frozen coronary artery samples was cut longitudinally, and its luminal surface with endothelial cells was scraped carefully with a scalpel. The isolated endothelial cells were processed directly for flow cytometry.
Analysis of endothelial cells-population using flow cytometry
Cells were resuspended in the buffer solution. Cell suspensions were analyzed by FACScanto II system (Becton Dickinson, US) as previously reported [11-14]. CD146, is a well-described adhesion marker of endothelial cells, has also been identified on a limited number of other cell types and in tissues on both cellular components of vessel wall. Briefly, cells staining with 10 µl of FITC-conjugated anti-human CD146mAb (BD Biosciences, US) and kept for incubation at 4°C for 30 minutes in dark. Cells were washed twice with FACS buffer. The percentage of positivity against CD146 antibody was determined by side scatter fluorescence dot plot analysis after appropriate gating. Data processed using the FACS Diva software (BD Biosciences, San Jose, CA). For each analysis, a corresponding negative control with IgG–FITC antibody was used. The number of endothelial cells as a percentage of the total live cells was calculated for each sample and was further normalized by subtracting the percentage of the relevant isotype control.
Measurement of telomere length and activity
Real time quantitative polymerase chain reaction (RT-q-PCR) used to determine relative telomere lengths from samples of patients and cell line DNA. Telomere length was determined according to the method described by Cawthon [15]. DNA extraction from coronary artery intimal material was analysed using DNeasy tissue kit (Qiagen, Germany) according to the manufacturer’s recommendations. SYBR Green RT-qPCR Core Reagent Kit (Bio-Rad Laboratories, India) was used in this assay. Two plates of unknowns were run; one for the telomere reaction and one for the 36B4 reaction. All DNA samples, including the Jurkat cell line DNA control tubes, were run in triplicates. One tube of no template control (NTC), consisting of only PCR master mix and distilled water, was also run on each plate to assess the presence of primer dimer. A standard curve using Jurkat DNA was run on each plate as duplicates of five serially diluted wells ranging in 20 final well concentrations from 12.6 to 100ng, as described by Cawthon.
The ratio of the telomere (T) and single-copy 36B4 gene (S) matrices reflect the length of telomeres.
Simultaneously stock mix 1,25 x (1x mixture: PCR buffer 1x (Fermentas 10X PCR Hotstartbuf + KCl), MgCl2 2 mM, dNTP 0.2 mM, 0.5 μM of each primer, 0.05 units / μl of Taq polymerase Maxima (Fermentas), SYBR Green I 0.2x) have been prepared. Thermal profiling modifications for the 36B4 primer occurred at Step 2, Stage 2: 58.0o C for 1 min 30s. Thermocycler is set to read SYBR green fluorescence during Stage 2, at which SYBR Green is bound to double stranded DNA (dsDNA)
The primer sequences were:
Tel1-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT;
Tel2-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA;
36B4u-CAAGTGGGAAGGTGTAATCC;
36B4d- CCCATTCTATCATCAACGGGTACAA.
Samples were amplified in a thermocycler CFX96 (BIORAD). We calculated the difference between cycle thresholds of amplification of the telomere and single copy of the gene (∆Ct), and based on these results appreciated relative telomere lengths. The genomic DNA of the endothelial cell line and control cell sample was used as a reference point.
Calculating T/S and Relative T/S Ratio
The relative T/S ratio is used to report relative telomere lengths among samples and is proportional to the average telomere length. Basically, by using a single gene of known sequence number, sample amplification can be normalized against single gene amplification. This normalization of unknown sample telomere repeat content vs. known single gene repeat content establishes a scale for comparison, a ratio of value. The telomere length is reported in relation to the 36B4. 36B4, encodes the acidic ribosomal phosphoprotein P0; also termed as b-globin, located on chromosome 12. We chose the 36B4 gene, it has been validated for gene dosage studies. The relative ratio of 36B4 gene copies to β-globin gene copies in the experimental DNAs versus the reference DNA. Cawthon [15] gives the following formula to determine a T/S ratio, which must be established for the experimental samples, reference DNA, and for the 35ng Jurkat control:
[2Ct (telomeres)/2Ct(36B4)]-1 = 2-ΔCt
Cawthon gives the following formula to calculate the relative T/S ratio for the experimental samples:
2-(ΔCt1-ΔCt2) = 2-ΔΔ Ct
However, these same values can be obtained by means of less complicated equations. For this study, the T/S ratio was established using this formula:
ΔCt = Ct (telomere) – Ct (36B4)
The relative T/S ratio was then calculated using this formula:
ΔΔCt = ΔCt (unknown) – ΔCt (control)
Once T/S ratio values are established for unknowns, controls, and standards, they can be entered into Microsoft Excel, with the necessary formulas, to calculate the relative T/S ratio. The greater the relative T/S ratio is, the longer the telomere.
Statistical analysis
All values were expressed as means ± SD. The intergroup comparisons were performed using Student’s t-test or ANOVA when appropriate. The association endothelial cell telomere length with CAD and other parameters were evaluated using Spearman’s and Pearson coefficients, linear and multivariate regression analysis. Differences were considered statistically significant at p < 0.05. Statistical analysis was performed with a commercially available statistics package (IBM SPSS Statistics 20.0; Chicago, IL, USA).
RESULTS
Population study
Overall the mean age of the cases were 42.5±7.3 years (range, 32-68). Of the study group, 10 subjects was overweight (mean BMI 26.5±5.8 kg/m2) and 20 were smokers. Study subjects were categorized according to age into 2 groups, of 32 to 55 years (group 1, n = 18) and of 56 to 68 years (group 2, n = 12). The mean age was 39±7.9 year and 62±6.4 year, respectively (p<0.001). There were no significant differences in the sex, BMI and the levels of BP between the groups. Septal wall thickness (SWT) and posterior wall thickness (PWT) were larger in older individuals (p<0.01), but there was no difference between groups in LV mass index (LVMI). Older (60-68 yrs) subjects had lower values of LV diameter at the end of diastole (LVDD), LV end-diastolic volume (LVDV) and end-systolic volume (LVSV).
Endothelial cell isolation and flow cytometry
Endothelial cells were removed from the coronary artery by scraping, and its scraped luminal surface material contained linear endothelial cells. All coronary artery specimens after separation of the luminal surface by scraping shows no endothelial cell lining at the luminal surface site (Figure 1). After flow cytometry with anti-human CD146mAb, endothelial cell count was observed ranged from 0.55 x 106 to 0.96 x 106.
PCR data analysis
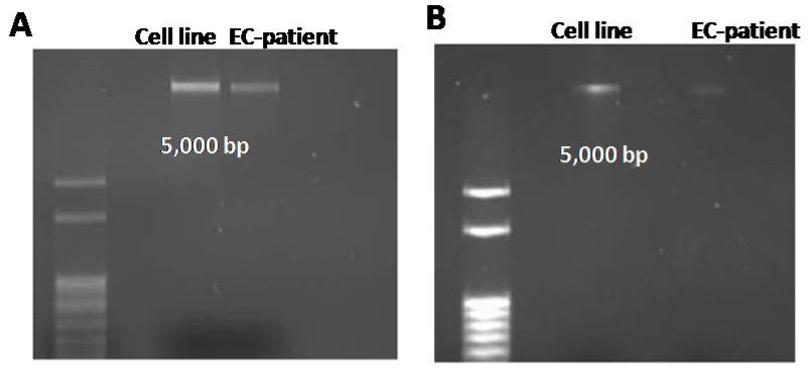
DNA isolation was followed by the employment of 1.5% agarose gel electrophoresis to confirm intact human genomic DNA before use in qPCR, as shown in Figure 2. Genomic DNA is present when DNA samples do not migrate to bottom of agarose gel, as the gel has pores in it and DNA is a large molecule that will not move through the pores. Both samples migration, after both DNA isolations, was significantly higher than the 5,000 base pair band, indicating samples consisted of intact, whole genomic DNA.
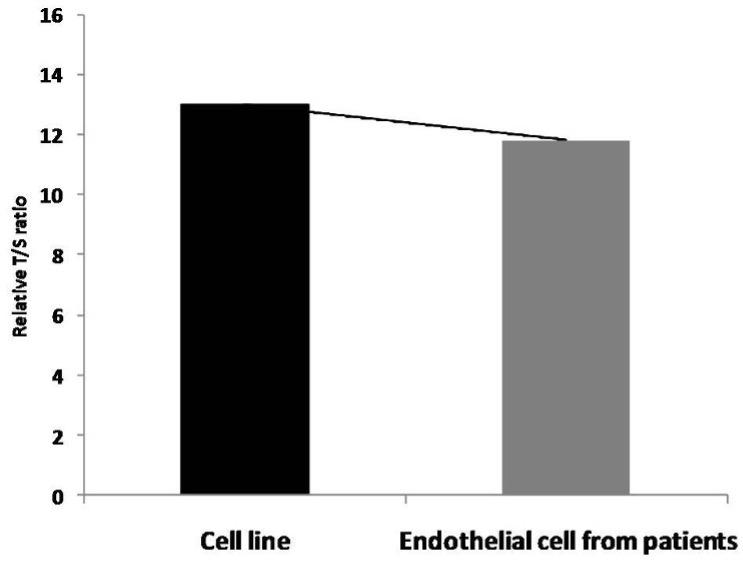
Relative T/S ratio which corresponds to the telomere length was less compared with cell line (Figure 3). Analysis by two-way ANOVA of telomere length from previously isolated DNA from endothelial cells of cell line and patients along with DNA isolated from sub-cultured cells showed significant differences (p < 0.01) between age, extent of sub-culturing, and interaction effects.
Results showed that telomere length in endothelial cells from Cell line was longer compared to telomere length in endothelial cells from patients (p < 0.01; Students t – test).
DISCUSSION
The results of this study demonstrate that Cawthon’s primers and RT-PCR protocol can be applied to samples of patient’s and cell line to successfully assess telomere length in recently isolated or cells sequentially “aged” in the laboratory. In congruence with Cawthon’s results, there was no occurrence of primer dimer-derived products, as evidenced by the absence of detectable fluorescence in the reaction tubes containing only master mix [15]. As expected, cell line endothelial cells demonstrated longer telomeres than patient’s endothelial cells.
Despite increasing evidence of cellular senescence in patients with CAD no comprehensive study examining telomere length of coronary endothelial cells has been reported [16]. Only a few studies have dealt with the measurement of telomere length in the iliac artery or abdominal aorta [10,17,18]. These previous studies revealed that the telomere length of endothelial cells was shortened with aging. The present results that the telomere length of coronary endothelial cells were reduced as a patient’s age are compatible with these previous findings. However, several studies have suggested that aging induces endothelial dysfunction, which is recognized to be a factor in cardiovascular diseases [18-22]. Because telomere shortening and endothelial dysfunction are age related phenomena, telomere shortening in coronary endothelial cells may be related directly to coronary endothelial dysfunction. Recently, our study examined coronary arteries obtained from patients with CAD who underwent CABG found that the circulatory coronary endothelial progenitor cells were senescent and functionally deteriorated [4].The present findings of the direct measurement of telomere contents in coronary endothelial cells complement previous findings and support the concept that telomere shortening in coronary endothelial cells with aging may contribute to coronary endothelial dysfunction and the development of CAD in humans. The present analysis showed that telomeres of coronary endothelial cells were shorter in patients with CAD than in the cell line, suggesting that other factors in telomere shortening other than aging had been acting on patients with CAD. Two mechanisms of telomere reduction have been proposed: (1) human telomeres undergo progressive shortening with cell division through replication dependent sequence loss at DNA termini [21-25] and (2) persistent mild oxidative stress leads to telomere shortening. Free radicals generated by hypertension [25,26], diabetes mellitus, and cigarette smoking can injure endothelial cells [27-32].
In conclusion, we demonstrated that the telomeres of endothelial cells implicated in coronary artery atherosclerosis were markedly shortened. This finding suggests that telomere shortening and cellular senescence in coronary artery endothelial cells are focal phenomena and may play pathogenisis of CAD.
ACKNOWLEDGEMENT
The authors extend their appreciation to management of Narayana Medical College & hospital for funding the research work.
AUTHOR CONTRIBUTIONS
MVS designed and performed the experiment. MVS and SG analyzed the data; MVS wrote the draft, MVS and SG critically revised the manuscript; MRI supervised the study.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
References
- [1]Akasheva DU, Plokhova EV, Tkacheva ON, Strazhesko ID, Dudinskaya EN, Kruglikova AS, Pykhtina VS, Brailova NV, Pokshubina IA, Sharashkina NV, Agaltsov MV. Age-related left ventricular changes and their association with leukocyte telomere length in healthy people. PloS one. 2015;10(8):e0135883.
- [2]Butler RN, Warner HR, Williams TF. The aging factor in health and disease: The promise of basic research on aging. Aging Clin Exp Res. 2004;16(2):104-12.
- [3]Nilsson PM, Tufvesson H, Leosdottir M, Melander O. Telomeres and cardiovascular disease risk: an update 2013. Transl Res. 2013;162(6):371-80.
- [4]Shaik MV, Gangapatnam S, Edwin R. A study of Analysis of Endothelial Progenitor cells in Peripheral blood in patients with coronary artery disease. J Cardiovasc. Dis. Res. 2016;7(1):27.
- [5]Hastings R, Li N-C, Lacy PS, Patel H, Herbert KE, Stanley AG, Williams B. Rapid telomere attrition in cardiac tissue of the ageing Wistar rat. Exp Gerontol. 2004, 39:855-857.
- [6]Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai KK, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013, 4:1597.
- [7]Serrano AL, Andrés V. Telomeres and Cardiovascular Disease Does Size Matter?. Circ Res. 2004; 94:575-584.
- [8]Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis role of telomere in endothelial dysfunction. Circulation 2002; 105:1541-1544.
- [9]Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. HYPERTENSION-DALLAS-.2003;42(6):1206-52.
- [10]Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995; 92:11190-11194.
- [11]Ryan US. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984; 56:103.
- [12]Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001; 89:e1-e7
- [13]Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004; 24:1442-1447.
- [14]Lambiase PD, Edwards RJ, Anthopoulos P, Rahman S, Meng YG, Bucknall CA, Redwood SR, Pearson JD, Marber MS. Circulating humoral factors and endothelial progenitor cells in patients with differing coronary collateral support. Circulation. 2004; 109:2986-2992
- [15]Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002; 30:e47-e47.
- [16]Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473.
- [17]Aviv H, Khan MY, Skurnick J, Okuda K, Kimura M, Gardner J, Priolo L, Aviv A. Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis. 2001;159:281–287.
- [18]Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398.
- [19]Chauhan A, More RS, Mullins PA, Taylor G, Petch C, Schofield PM. Aging-associated endothelial dysfunction in humans is reversed by L-arginine. J Am Coll Cardiol. 1996;28:1796–1804.
- [20]Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 1996;27:849–853.
- [21]Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D,Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476.
- [22]Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 1998;105:32S–39S.
- [23]Watson JD. Origin of concatemeric T7 DNA. Nature New Biol. 1972; 239:197–201.
- [24]Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460.
- [25]Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA,Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200.
- [26]von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193.
- [27]Lacy F, O’Connor DT, Schmid-Schonbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens. 1998;16:291–303.
- [28]Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196.
- [29]Kasper M, Funk RH. Age-related changes in cells and tissues due to advanced glycation end products (AGEs). Arch Gerontol Geriatr. 2001; 32:233–243.
- [30]Wautier JL, Guillausseau PJ. Diabetes, advanced glycation end products and vascular disease. Vasc Med. 1998;3:131–137.
- [31]Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens 2001;19:891–897.
- [32]Michael Pittilo R. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Exp Pathol 2000;81:219–230.