A brief review on Japanese Encephalitis in Swine with its diagnostic strategies
Abstract
Japanese encephalitis is a viral disease affecting humans, mostly the children and is a major concern to public health. The single stranded flavivirus is mainly transmitted by mosquitoes of the Culex genus and causes neurological disorders with high fatality. The virus is well amplified in animals like pigs (swine) that act as a host. Close proximity of the domesticated pigs to human settlements increases the risk of human infection. The animal to human zoonoses of this antigen is occurred through the mosquitoes bite. Recent developments have led to discovery of various vaccines for the treatment of the disease. This review discusses various aspects of the Japanese encephalitis disease with their transmission, pathogenesis and diagnostic strategies. Moreover it highlights the importance of prevention of transmission of the disease over its cure by proposing a hypothetical kit design model to detect presence of the Japanese encephalitis antigen in the swine populations. This detection process may help to detect infected animals and thus help to keep such animals away from the human settlements so as to prevent transmission as well as outbreak of the disease. This may further help to create a disease free environment, thus saving many lives.
INTRODUCTION
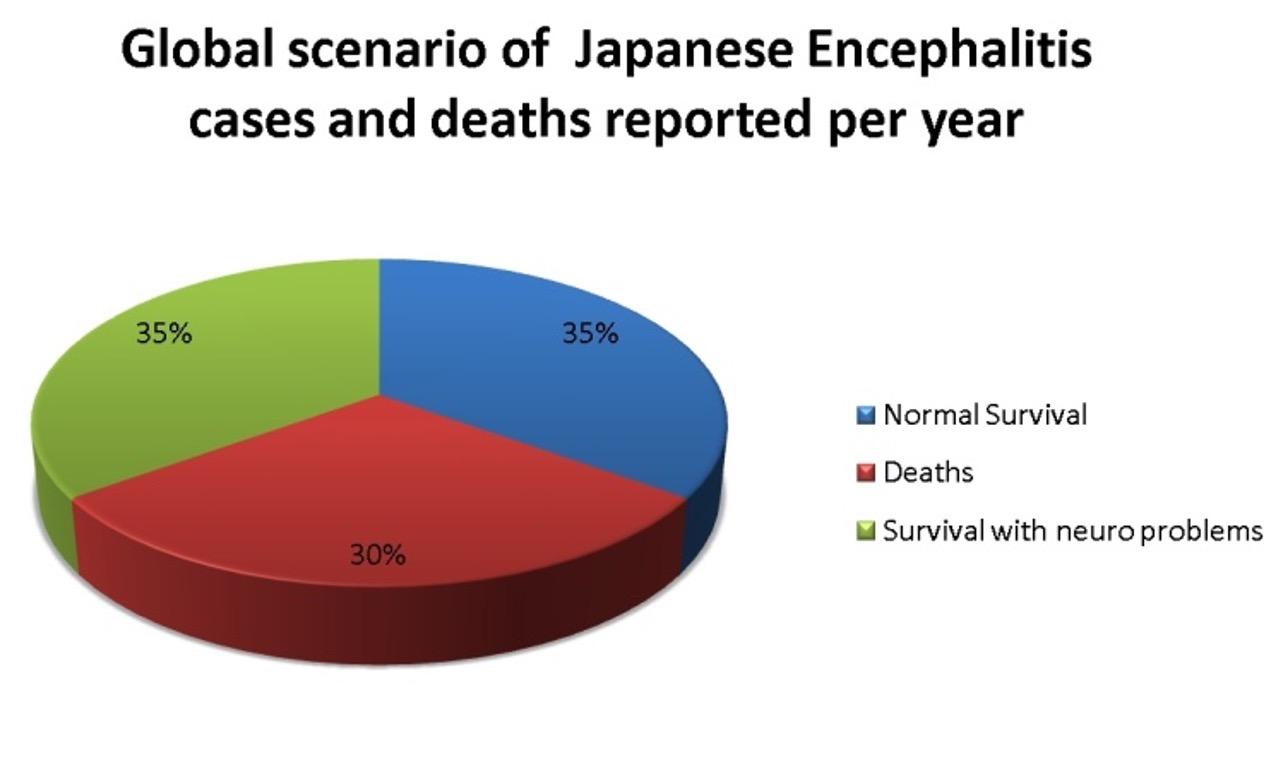
Japanese encephalitis is a viral disease affecting humans and is caused by the Japanese Encephalitis Virus (JEV). The disease is found worldwide and is mostly prominent in the south East Asian regions [1]. Japanese encephalitis is a disease of major public concern and accounts for approximately 20-30 % of fatalities [2]. Japanese encephalitis virus is a flavivirus belonging to the family flaviviridae. The virus is a single stranded positive sense RNA virus. Vertebrates like birds and pigs act as a host, playing an important role in the maintenance and growth of the virus while the invertebrate mosquitoes act as vector for transmission of the virus from the hosts to other living beings, especially to the humans [3]. The disease mostly affects children aged between 0-14 years [4], however children of 3 to 6 years of age are the victims of highest attack rates of the virus in the endemic areas [5-6]. Non-immune adults are also prone to the disease in endemic areas [7]. Japanese encephalitis is a disease of major public concern, thus causing an estimated 50,000 cases and accounting to 15,000 deaths (30%) per annum [8]. Among the survivors, almost half (35%) of them suffer from various neurological diseases [9] (Figure 1).
The disease is spread over three epidemiological regions – Endemic region, Intermediary subtropical region and temperate endemic region (Table 1). The disease transmission is variable in nature and is dependent on environmental temperature and conditions [10]. Five genotypes of the Japanese encephalitis virus can be found – Ia, Ib, II, III and IV [11, 12]. Genotype III was predominant among the early isolates and is now being gradually replaced by the genotype I [13].
The review focuses on various aspects of the Japanese encephalitis disease which includes its mode of transmission, various stages and pathogenesis in humans. Moreover, the review also briefly describes current approaches for detection of the viral antigen in animals and their shortcomings. The authors emphasize the use of citrate stabilized gold nanoparticles for possible and efficient detection of the antigen in serum samples of swine. A hypothetical kit design method has been proposed that makes use of these citrated gold nanoparticles for rapid, efficient and easy detection of the antigen in swine population.
Table 1. Epidemiological regions and spread of Japanese encephalitis
DISEASE TRANSMISSION
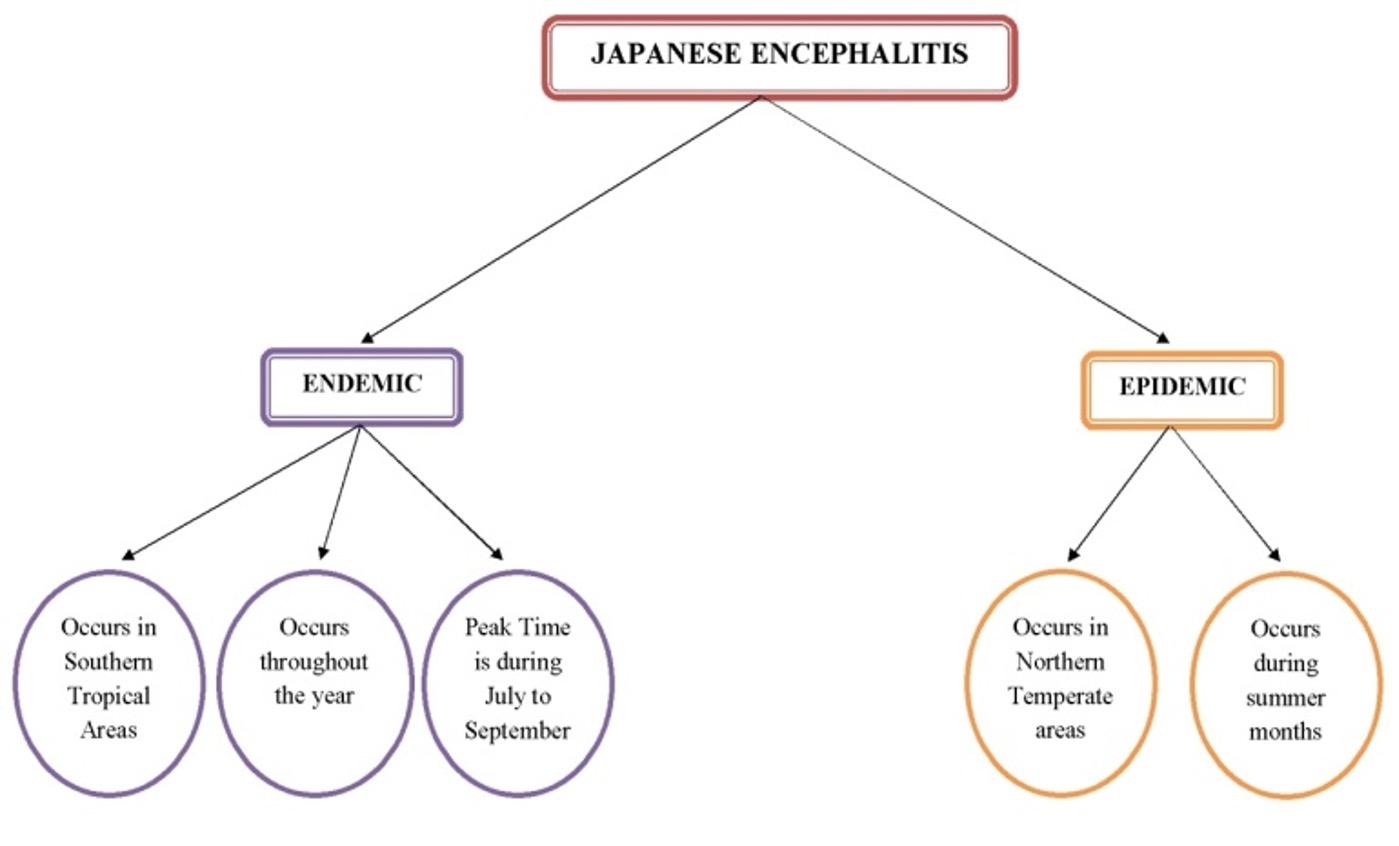
Mosquitoes, especially the Culex species, generally act as a vector for the transmission of Japanese encephalitis to vertebrates [14]. The Japanese encephalitis virus cycle mainly involves water birds and Culex mosquitoes. Besides, pigs also act as an amplifying host, thereby linking to the humans due to their close proximity to dwellings [15]. In general, there are two recognized epidemiological patterns of Japanese encephalitis – the endemic pattern and the epidemic pattern. (Figure 2) describes the details about occurrence of both the patterns.
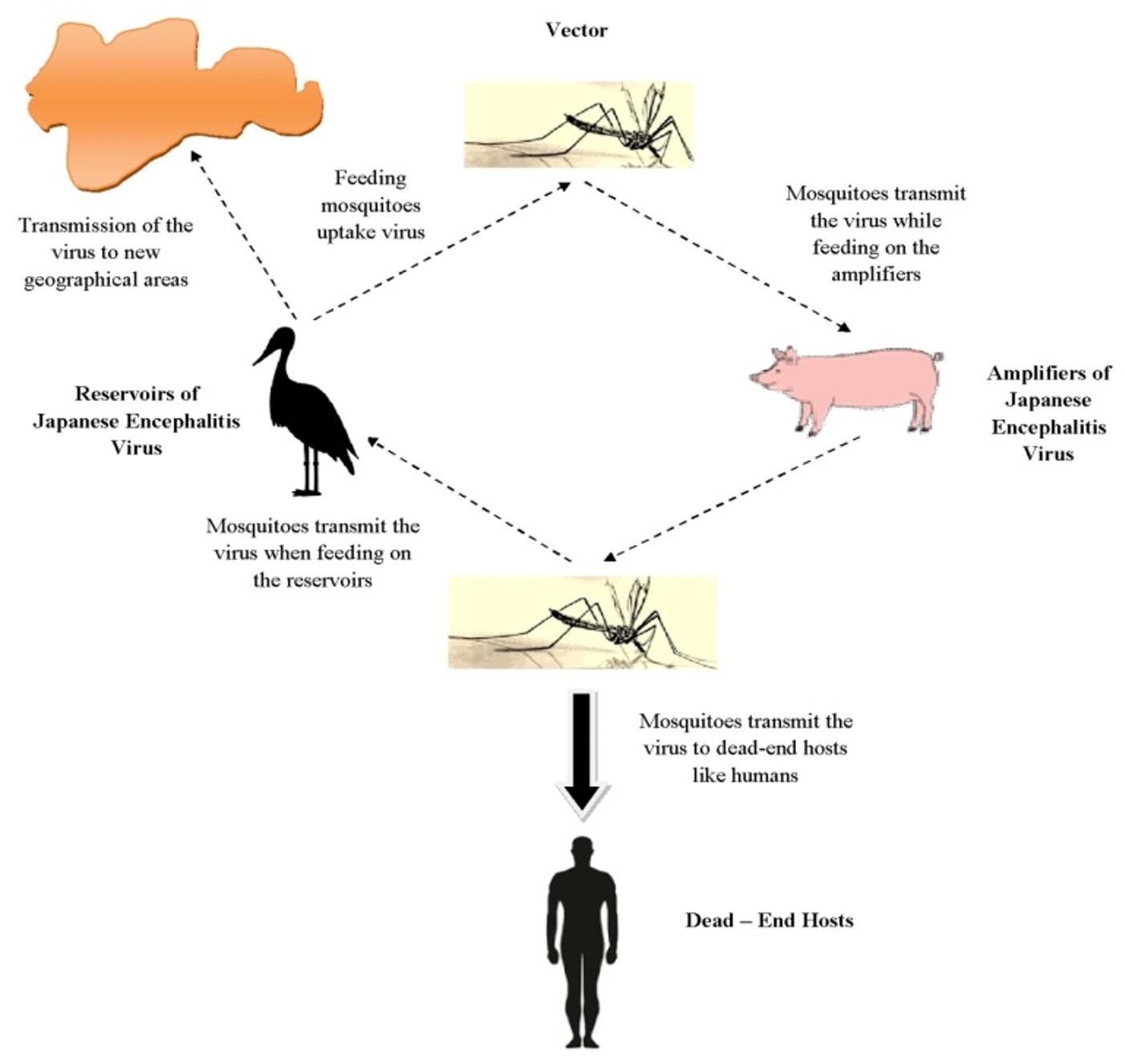
The transmission cycle of Japanese encephalitis (Figure 3) makes it very much difficult to control the spread of the disease. The virus is mostly found in the aquatic birds that act as a reservoir. These viruses are taken up by the mosquitoes when they feed on these aquatic birds. On the other hand the virus is transmitted by the mosquitoes from the birds to animals like pigs which act as an amplifier, thus multiplying the virus inside their body cells. Mosquitoes bite these infected animals and take up the virus again and have more chances of transmitting the same to humans because of the close proximity of these animals to human dwellings. Moreover, these mosquitoes breed in stagnant water bodies or irrigated fields thus posing a greater chance of transmission to humans who act as a dead-end host. This is because the humans do not pose the ability to amplify the virus in sufficient amount to be taken up again by the mosquitoes. Further transmission of this disease to new geographical areas is mediated by the birds migrating from one place to another. Experiments have also confirmed that vector less transmission of the virus between pigs can occur via nasal secretion [16].
STAGES OF JAPANESE ENCEPHALITIS
Patients infected by the Japanese encephalitis virus show signs of ‘acute encephalitic syndrome’ [17]. Stages of Japanese encephalitis can be broadly classified into 4 stages. The first stage also known as the prodromal stage, is marked by a sudden inception of high fever and headache along with certain other non-specific symptoms like malaise, nausea, vomiting and anorexia. The second stage is the ‘acute stage’ which includes decreased consciousness leading to mild clouding or even more adverse conditions like semi-coma or coma. These symptoms are also accompanied by convulsions, neck stiffness and weakness. Patients with more serious conditions generally die at this stage. The third stage or the ‘late stage’ is characterized by improved neurological conditions in patients who do not have any complications. The fourth and final stage also called the ‘sequelae stage’ includes complete recovery in mild cases. However, patients with high severity are found to be left with neurological defects even after improvement of their health conditions.
PATHOGENESIS OF JAPANESE ENCEPHALITIS
Following inoculation of the virus into the skin by the mosquitoes, replication of the viral cells occur within the ‘Langerhans dendritic cells’ similar to that observed in case of dengue virus [18] and or in the ‘Keratinocytes, as observed in case of West Nile virus. This is followed by transfer of the virus to local lymph nodes where further replication occurs. This leads to Viraemia and the virus crosses through the blood-brain barrier to enter the central nervous system (CNS). Once the virus reaches inside the CNS, uncontrolled replication takes place [19]. Inside the CNS, the neurons are the principal target cells for replication of the virus [20-22].
The severity in pathogenesis of Japanese encephalitis has prompted many researchers worldwide to work towards efficient diagnosis of the disease and its possible prevention. Many diagnostic processes have been applied either as single or in combination with each other for the purpose. The section below describes some of the current major diagnostic processes being followed.
CURRENT DIAGNOSTIC SCENARIO
The Japanese encephalitis infection is generally asymptomatic in nature and thereby presents a challenge as far as the diagnostic perspective is concerned. The diagnostic procedures rely highly on a combination of clinical, serological and molecular findings [23], thus making the process highly complicated.
Clinical diagnosis
In vitro isolation of the viral antigen from the central nervous system samples of the animals is possible using tissue culture techniques. The isolated cells can be visualized using a cell dye such as crystal violet. In vivo isolation and detection is also carried out in 2-4 day old mice by administering an intracerebral injection of the homogenized central nervous system tissue sample obtained from the affected animals. If the sample contains JEV antigen then, the mice would show neurological symptoms and death occurs within 14 days. Following the death, the brain of the mice can be removed and the isolation and detection of the antigen can be carried out using cell culture techniques. However, proper identification of the viral antigen requires further serological or molecular steps.
Serological diagnosis
The serological diagnostic procedures mostly rely on the reaction of antibodies with the antigen present in either cerebrospinal fluid or serum samples of the infected animals. Manual ELISA (Enzyme-linked immunosorbent assay) assays and commercially available IgM ELISA kits are mostly used for the diagnosis of Japanese encephalitis [24]. However, these methods require significantly high concentration of antibody in the serum sample for proper detection. The processes may also pose a chance of giving false results due to cross-reactivity between other flaviviruses if present in the serum samples [25], and thus requires separate serological methodologies for accurate interpretation of the results [26].
Molecular diagnostic methods
Several molecular methods have been described and tested for detection of Japanese encephalitis virus by reverse transcription polymerase chain reaction (RT-PCR) [27-28]. Molecular assays compatible for application directly in the field where there is limited availability of equipments have been developed. Parida et al., 2006 described the reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the above purpose [29]. Multiplex assays involving detection and differentiation of a wide range of viruses along with Japanese encephalitis virus has acquired greater interest [30]. Whole genome sequencing techniques have been able to provide a more thorough genetic identification of the virus [31] along with their geographical origin. The molecular techniques are highly specific. However, they simultaneously require highly skilled personnel for their proper execution and also involve high cost in terms of the various equipments, chemicals and primers used.
CITRATE STABILIZED GOLD NANOPARTICLES IN THE DIAGNOSIS OF JAPANESE ENCEPHALITIS
Nanoparticles are defined as ‘very small particles which behave as a complete unit in terms of their transport and properties’ [32] with a diameter ranging below 100 nm [33]. These particles are widely studied and have wide range of applications [34, 35] which include applications in the field of biology and medicine too (Table 2).
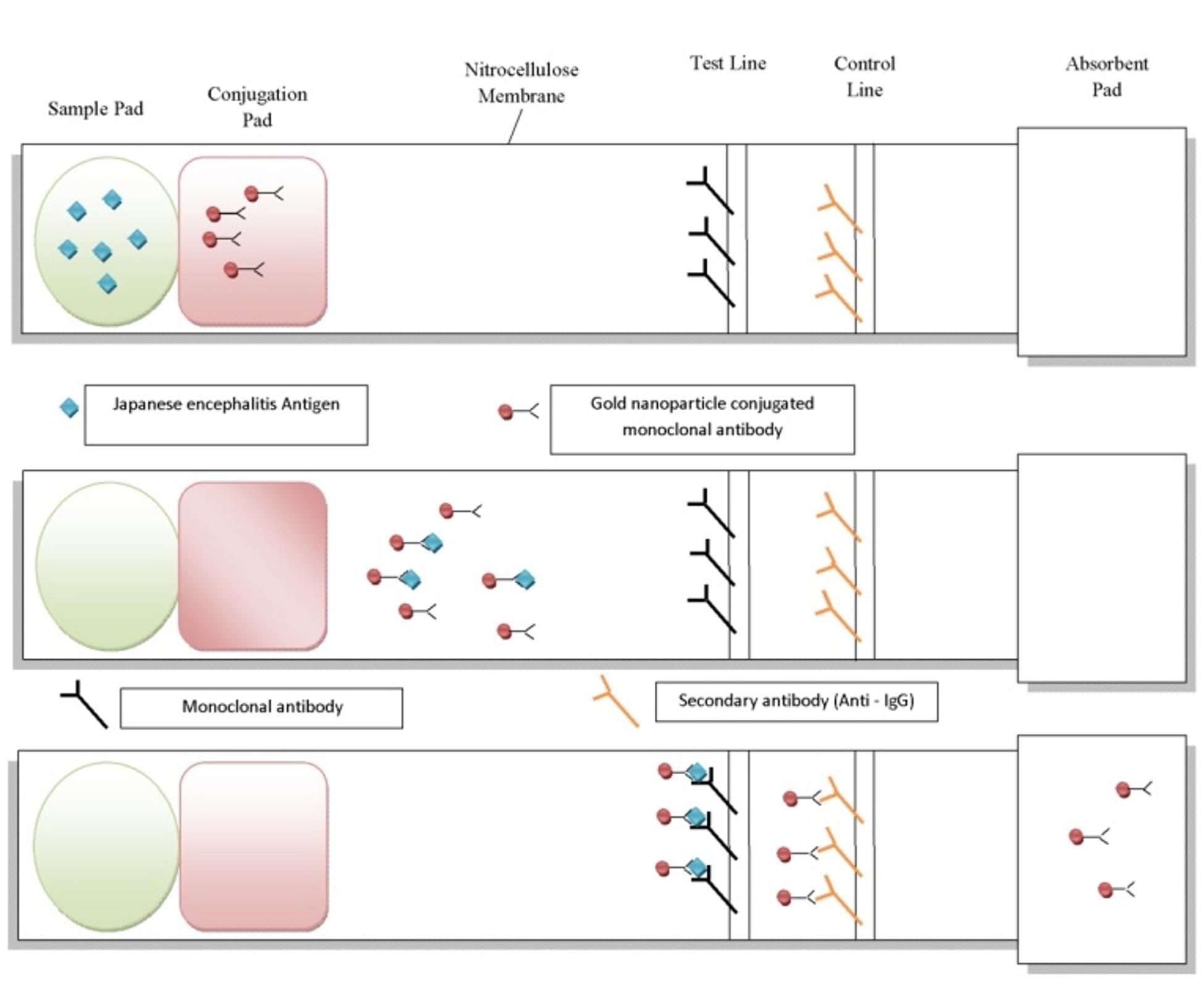
Among the nanoparticles being used, gold nanoparticles (Au NPs) carry high significance which can be mainly attributed to the unique optical properties possessed by them. Au NPs are currently in trend for use in various biomedical applications because of their biocompatibility, functionalization, lesser toxicity and easy detection [45]. These AuNPs undergo colour changes depending on the environment [46] and can thus be used as a sensor for detection of antigens in blood serum. Citrate (in the form of trisodium citrate or citric acid) plays a major role in providing stability to the gold nanoparticles. Apart from stabilizing, it also acts as a reducing agent during formation of various sized AuNPs. Citrate stabilized AuNPs have been a subject of major focus which may be attributed to their wide range of utilities in various fields. Citrated AuNPs have been used to develop a rapid and easy colorimetric method for detection of cyromazine contamination in environmental samples [47]. Citrate stabilized AuNPs have also been found to hinder fibrillogenesis of globular proteins that are responsible for many severe disorders like the Parkinson’s and Alzheimer’s disease [48]. Colorimetric assay for detection of creatinine in urine has been developed that makes use of citrate stabilized AuNPs resulting in a cross-linking reaction leading to aggregation of the nanoparticles and thus a characteristic color change from red wine to blue [49]. This method could be possibly used to diagnose any disease or malfunctioning related to the kidneys. Quantitative comparison between citrated AuNPs and transferrin-coated nanoparticles showed greater uptake of the citrated AuNPs into the mammalian cells. This indicates that these nanoparticles can be used efficiently as vehicle for drug delivery inside mammalian cells [50]. Citrated stabilized AuNPs coated with anti epidermal growth factor receptors have been used successfully to target squamous cells in human oral carcinoma [51]. Citrate capped AuNPs are also used for general detection of bacterial pathogens. Gold nanoparticles synthesized by citrate mediated reduction of gold salts have been used in a simple spectroscopic assay for detection of Cronobacter sakazakii [52], a bacterium that is known to cause fatal infection of the bloodstream and central nervous system. Keeping in mind the wide utilities of citrate stabilized AuNPs, we provide a hypothetical method for designing a kit that utilizes these nanoparticles and may prove to be useful in detecting the Japanese encephalitis virus in pigs. Gold nanoparticles can be synthesized by Turkevich method [53] and then utilized to develop a sensor with the ability as well as accuracy to detect the Japanese encephalitis antigen in animals especially pigs (Figure 4). Citrate stabilized gold nanoparticles can be used for the purpose as they give a red colour in solution and are also detectable at low concentrations [54]. A lateral flow paper that is made up of nitrocellulose membrane can be generally used for the purpose. The strip of paper should consist of a sample pad at one end followed by a conjugation pad, a test zone, a control zone and finally an absorbent pad at the other end. The conjugation pad will have gold nanoparticles conjugated on to absorbed primary antibodies specific for the Japanese encephalitis antigen. The test zone or the test line will contain adsorbed monoclonal antibodies specific for the antigen and the control line will contain secondary antibodies (Anti-IgG) that can easily bind to the IgG present in the blood serum.
Blood samples obtained from animals (pigs) can be directly put on the sample pad. The sample would start to flow laterally to the opposite direction. Once the blood sample reaches the conjugation pad, gold nanoparticles conjugated primary monoclonal antibodies specific for the Japanese encephalitis antigen will bind to the viral antigen (if present) in the sample and move forward towards the test zone. Upon reaching the test zone the monoclonal antibodies present will bind to the viral antigen-gold nanoparticle complex. Deposition of gold nanoparticle on the test line will give a red coloured line [55] indicating a positive result i.e. presence of Japanese encephalitis antigen in the sample. The control line that consists of anti – IgG secondary antibodies will then bind to the IgG present in the blood serum to give another red line. The control line is just to confirm that the kit is working properly and there is unidirectional flow of sample from the sample pad to the absorbent.
The proposed model may prove to be beneficial and may possibly overcome the limitations faced by the current diagnostic methods in practice. The proposed kit may help in detecting the Japanese encephalitis antigen from swine serum samples with high specificity and low cost. The kit can also be easily marketed and can be easily afforded by marginal people living in villages. Further, it does not require a well established lab nor any high-end specific instruments and skilled workforce for its operation. It will also provide results in quick time and will thus facilitate to control the spread of the disease to humans in a timely manner.
Table 2. Applications of nanoparticles in the field of biology and medicine
CONCLUSIONS
Many vaccines have been developed for the treatment of Japanese encephalitis. However, most of the vaccines are associated with certain side-effects. Keeping in mind the severity of Japanese encephalitis and the death rates involved therein, there arises an immediate need to prevent the disease transmission from swine to humans. As such, the hypothetical model may help to design a kit that may successfully detect the antigen in the serum of swine. The current diagnostic methods in practice involve a large cost of analysis, skilled work force, consume more time and sometimes provide results with very low specificity. The current model may prove as an easy, rapid, cost-effective and user friendly approach for detection of Japanese encephalitis in animals like swine. Timely detection of the virus in the swines could possibly help contain future outbreaks and thus save many lives.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Madhusmita Ghana was involved in collection and sorting of data from various databases like Scopus, Science Direct and Google Scholar. Madhusmita Ghana and Pratyush Kumar Das were involved in framing the outline of the review and drafting of the article. Both the authors were equally involved in designing the hypothetical model. Pratyush Kumar Das contributed to revising it critically for important intellectual content and made the final approval of the version to be published.
References
- [1]Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009; 15: 1-7.
- [2]Malhotra S, Sharma S, Kumar P, Hans C. Japanese Encephalitis and its Epidemiology. J Infect Dis Ther. 2015; 3(5): 243. doi:10.4172/2332-0877.1000243.
- [3]Halstead SB, Jacobson J. Japanese encephalitis vaccines. In: PlotkinSA, Orenstein WA, Offit (eds.) Vaccines (5thedn.) Elsevier, Philadelphia, PA, 2008; pp. 311-352.
- [4]Tiwari S, Singh RK, Tiwari R, Dhole TN. Japanese Encephalitis: a review of the Indian perspective. Braz j infect dis. 2012; 16 (6):564–573.
- [5]Grossman RA, Edelman R, Chiewanich P, Voodhikul P, SiriwanC. Study of Japanese encephalitis virus in Chiangmai valley,Thailand. II. Human clinical infections. Am J Epidemiol. 1973; 98:121–32.
- [6]Hoke Jr CH, Vaughn DW, Nisalak A,Intralawan P, Poolsuppasit S, Jongsawas V et al. Effect of high-dosedexamethasone on the outcome of acute encephalitis due toJapanese encephalitis virus. J Infect Dis. 1992; 165:631–7.
- [7]Hills SL, Griggs AC, Fischer M. Japanese encephalitis in travelers from non-endemic countries, 1973–2008. Am. J. Trop. Med. Hyg.2010; 82: 930–936.
- [8]Tsai TF. Factors in the changing epidemiology of Japaneseencephalitis and West Nile fever. In: Saluzzo JF, editor. Factorsin the Emergence of Arboviral Diseases. Amsterdam: Elsevier; 1997. p. 179–89.
- [9]Solomon T, Dung NM, Kneen R,Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. JNeurolNeurosurg Psychiatry.2000;68:405–15.
- [10]Tiwari PM, VigK , Dennis VA , Singh SR . Functionalized goldnanoparticles and their biomedical applications .Nanomaterials.2011; 1 : 31 – 63 .
- [11]Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia.J. Virol.2003;77: 3091–3098.
- [12]Schuh AJ, Ward MJ, Brown AJ, Barrett AD. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoSNegl. Trop. Dis. 2013; 7: e2411. doi: 10.1371/journal.pntd.0002411
- [13]Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol.2011; 85: 9847–9853.
- [14]Kanojia PC, Shetty PS, Geevarghese G. A long-term study onvector abundance & seasonal prevalence in relation to theoccurrence of Japanese encephalitis in Gorakhpur district,Uttar Pradesh. Indian J Med Res. 2003; 117:104–10.
- [15]Kabilan L, Rajendran R, Arunachalam N,Ramesh S, Srinivasan S, Samuel PP et al. Japaneseencephalitis in India: an overview. Indian J Pediatr.2004; 71:609–15.
- [16]Ricklin ME, Nicolas OG, Brechbuhl D, Python S, Zumkehr B, Nougairede A et al. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016; 7: 10832. doi: 10.1038/ncomms10832.
- [17]Ghosh D, Basu A. Japanese encephalitis-a pathological andclinical perspective. PLoSNegl Trop Dis. 2009; 3:e437. doi:10.1371/journal.pntd.0000437
- [18]Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R et al. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med.2000;6: 816–820.
- [19]Huang CH, Wong C. Relation of the peripheral multiplication of Japanese B encephalitis virus to the pathogenesis of the infection in mice. ActaVirol.1963;7: 322–330.doi:10.1038/77553
- [20]German AC, Myint KS, Mai NT, Pomeroy I, Phu NH, Tzartos J et al. A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model.Trans. R. Soc. Trop. Med. Hyg. 2006; 100: 1135–1145.doi:10.1016/j.trstmh.2006.02.008
- [21]Johnson RT, Burke DS, Elwell M, Leake CJ, Nisalak A, Hoke CH et al. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann. Neurol. 1985; 18: 567–573.doi:10.1002/ana.410180510
- [22]Desai A, Shankar SK, Ravi V, Chandramuki A,Gourie-Devi M.Japanese encephalitis virus antigen in the human brain and its topographic distribution.ActaNeuropathol. 1995; 89: 368–373.
- [23]OIE, 2012. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th edition World Health Organisation for Animal Health (OIE), Paris, France.
- [24]Litzba N, Klade CS, Lederer S, Niedrig M. Evaluation of serological diagnostic test systems assessing the immune response to Japanese encephalitis vaccination. PLoS Trop. Negl. Dis. 2010; 4: e883.
- [25]Mansfield KL, Horton DL, Johnson N, Li L, Barrett AD, Smith DJ, Galbraith SE, Solomon T, Fooks AR. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011; 92: 2821–2829.
- [26]Yeh JY, Lee JH, Park JY, Seo HJ, Moon JS, Cho IS, et al. A diagnostic algorithm to serologically differentiate West Nile virus from Japanese encephalitis virus infections and its validation in field surveillance of poultry and horses. Vector Borne Zoonotic Dis. 2012; 12: 372–379.
- [27]Gao X, Lui H, Wang H, Fu S, Guo Z, Liang G. Southernmost Asia is the source of Japanese encephalitis virus (genotype 1) diversity from which the viruses disperse and evolve throughout Asia. PLoS Negl. Trop. Dis. 2013; 19: e2459.
- [28]Do LP, Bui TM, Hasebe F, Morita K, Phan NT. Molecular epidemiology of Japanese encephalitis in northern Vietnam, 1964–2011: genotype replacement. Virol. J. 2015; 12: 51.
- [29]Parida MM, Santhosh SR, Dash PK, Tripathi NK, Saxena P, Ambuj S, Sahni AK, Lakshmana Rao PV, Morita K. Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J. Clin. Microbiol. 2006; 44: 4172–4178.
- [30]Zeng Z, Liu Z, Wang W, Tang D, Liang H, Liu Z. Establishment and application of a multiplex PCR for rapid and simultaneous detection of six viruses in swine. J. Virol. Methods. 2014; 208: 102–106.
- [31]Li MH, Fu SH, Chen WX, Wang HY, Cao YX, Liang GD. Molecular characterization of full-length genome of Japanese encephalitis virus genotype V isolated from Tibet, China. Biomed. Environ. Sci. 2014; 27: 231–239.
- [32]Buzea C, Pacheco I, Robbie K. “Nanomaterials and Nanoparticles: Sources and Toxicity”. Biointerphases2.2007; 2: MR17–MR71.doi:10.1116/1.2815690.
- [33]Das PK. Phytoremediation and Nanoremediation : Emerging Techniques for Treatment of Acid Mine Drainage Water. Def. Lif. Sci. J. 2018; 3(2) : 190 – 196. doi: 10.14429/dlsj.3.11346.
- [34]Geethalakshmi R, Sarada DVL. Gold and silver nanoparticlesfrom Trianthemadecandra: synthesis, characterization, and antimicrobialproperties .Int J Nanomed.2012;7 : 5375 – 5384 .
- [35]MateiA ,Cernica I, Cadar O , Roman C , Schiopu V . Synthesisand characterization of ZnO-polymer nanocomposites .Int J MaterForm.2008;1 : 767 – 770.
- [36]Mah C, Zolotukhin I, Fraites TJ, Dobson J, Batich C, Byrne BJ: Microsphere- mediated delivery of recombinant AAV vectors invitro and in vivo. Mol Therapy.2000;1:S239.doi:10.1006/mthe.2001.0636
- [37]Panatarotto D, Prtidos CD, Hoebeke J, Brown F, Kramer E, Briand JP, Muller S, Prato M, Bianco A: Immunization with peptide-functionalizedcarbon nanotubes enhances virus-specific neutralizingantibody responses. Chemistry&Biology.2003;10:961-966.
- [38]Nam JM, Thaxton CC, Mirkin CA.Nanoparticles-based bio-barcodes for the ultrasensitive detection of proteins. Science.2003;301:1884-1886.
- [39]Mahtab R, Rogers JP, Murphy CJ.Protein-sized quantum dot luminescence can distinguish between “straight”, “bent”, and “kinked” oligonucleotides. J Am Chem Soc.1995;117:9099-9100.
- [40]Yoshida J, Kobayashi T.Intracellular hyperthermia for cancerusing magnetite cationic liposomes. J MagnMagnMaterI.1999;194:176-184.
- [41]Ma J, Wong H, Kong LB, Peng KW.Biomimetic processing ofnanocrystallite bioactive apatite coating on titanium.Nanotechnology.2003;14:619-623.
- [42]de la Isla A, Brostow W, Bujard B, Estevez M, Rodriguez JR, Vargas S,CastanoVM:Nanohybrid scratch resistant coating for teethand bone viscoelasticity manifested in tribology. Mat ResrInnovat.2003;7:110-114.
- [43]Molday RS, MacKenzie D.Immunospecific ferromagnetic irondextran reagents for the labeling and magnetic separation ofcells.J Immunol Methods.1982;52:353-367.
- [44]Edelstein RL, Tamanaha CR, Sheehan PE, Miller MM, Baselt DR, WhitmanLJ, Colton RJ.The BARC biosensor applied to the detectionof biological warfare agents. Biosensors Bioelectron.2000;14:805-813.
- [45]Tiwari PM, Vig K , Dennis VA , Singh SR. Functionalized goldnanoparticles and their biomedical applications . Nanomaterials.2011; 1 : 31 – 63.
- [46]Ahmed S, Bui MPN, Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosensors and Bioelectronics.2016;77: 249–263. doi:10.1016/j.bios.2015.09.038.
- [47]Bai W, Zhu C, Zhang G, Huang Y, Yan J, Yan M, Chen A. Visual colorimetric detection of cyromazine in river water using citrate-stabilized gold nanoparticles. Anal. Methods. 2016; 8: 5869-587. doi: 10.1039/C6AY01845A.
- [48]Cantarutti C, Raj G, Fogolari F, Giorgetti S, Corazza A, Bellotti V et al. Interference of citrate – stabilized gold nanoparticles with β2 – microglobulin oligomeric association. Chem. Commun. 2018; 54: 5422-5425. doi: 10.1039/C8CC01053F.
- [49]He Y, Zhang X, Yu H. Gold nanoparticles – based colorimetric and visual creatinine assay. Microchimica Acta. 2015; 182 (11-12): 2037 – 2043. doi: 10.1007/s00604-015-1546-0.
- [50]Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006; 6(4): 662–668. doi: 10.1021/nl052396o.
- [51]Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Advanced Drug Delivery Reviews. 2008; 60: 1307–1315. doi:10.1016/j.addr.2008.03.016.
- [52]Aly MA, Domig KJ, Kneifel W, Reimhult E. Immunogold nanoparticles for rapid plasmonic detection of C.sakazakii. Sensors. 2018; 18: 2028. doi: 10.3390/s18072028.
- [53]Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. The Journal of Physical Chemistry B. 2006;110(32): 15700–15707. doi:10.1021/jp061667w
- [54]Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing.Chemical Reviews. 2012;112(5): 2739–79. doi:10.1021/cr2001178.
- [55]Su J, Zhou Z, Li H, Liu S. Quantitative detection of human chorionic gonadotropin antigen via immunogold chromatographic test strips. Anal.Methods.2014; 6(2): 450–455. doi:10.1039/C3AY41708E.