Evaluation of density metric grading of agarwood, antioxidant potentiality in agar oil, and prevalence of unknown bacteria in agarwood soaking water
Abstract
The experiments were conducted for grading unknown agarwood, to analyze the total phenolics, total flavonoids, antioxidant status of agarwood and its crude oil and also to identify the existing bacteria that assist in the fermentation of agarwood during soaking period. Grades of unknown agarwoods were determined based on density metric method and cross checked for ether extract. Total phenolics of agar wood and oils were analyzed through Folin-Ciocalteu method and total flavonoid contents of agar oils were estimated using Aluminium chloride colorimetric method. For the determination of antioxidant status of agarwood oil, the inhibitory effects of oil extracts on DPPH radical was measured. To identify the bacterial genus, several biochemical tests were conducted. Results showed that insect infested agarwood was classified as Grade-1 agarwood (Calculated density was 0.641 g/cm3) and contained the highest amount ether extract of 18.90 ± 0.60%, total phenolic content of 3.5 ± 0.06 (mg GAE/g) and flavonoid content of 7.82 ± 0.23 (µg QE/ml). IC50 values obtained by DPPH activity for Aquillaria malaccensis oil extract was found to be 0.904 µg/ml. Bacillus spp. was recognized as the mostly prevalent bacteria responsible for fermentation of agarwood which exhibit special role in increasing the yield of agar oil. If agarwood and agar oil can be graded properly high market value will be obtained by exporting them.
INTRODUCTION
Agarwood is a highly valuable commodity that has been traded in many parts of the world for hundreds of years [1]. The heartwood is light and pale in color, when the wood becomes matured, the tree start to produce a dark aromatic resin in response to infection and results in a very dense, dark and resin embedded heartwood that is called “agarwood” [2]. Formation of agarwood is characterized as a result of pathological and physiological process and only under specific condition as a tree becomes infected and wounded, the scented agarwood forms [3]. There are 21 recognized Aquillarias species and 13 of them are fragrant resin producers. In Bangladesh, Aquillaria malaccensis and Aquillaria sinensis are mainly cultivated in huge amount in Sylhet and Chittagong divisions [4]. Production of agarwood started about 400 years ago in the Suzanagar union under Barlekha Upazila of Moulvibazar district in Bangladesh. Naturally, it is a time consuming process to find best quality agarwood. Therefore, those people in Suzanagar have started production of agarwood and agar oil from ten to fifteen years old trees. At present, there are more than 200 processing industries involved in the production of agarwood and agar oil in Bangladesh [1]. Currently, people involved in agarwood and oil processing business are exporting TK. 5 million to 100 million per year. Presently, it has become Tk. 1000 crore business with agar wood and oil [5]. The oil extracted from agarwood contains distinct aroma producing complex volatile compounds. In high quality agarwood oil three compounds of sesquiterpenes were detected namely,(-)-guaia-1(10), 11-dien-15-al, (-)-selina-3, 11-dien-9-one and (+)-selina-3, 11-dien, 9-ol [6].
The unique aromatic scent of agarwood makes it an important trading commodity in cosmetics, perfumery and incense industries as well as in medical field. Medicinally it is used as stimulant tonic and diuretic. Agarwood is used to treat small pox, rheumatism, illness during and after birth, abdominal pain, nausea, treatment of regurgitation. In northeast part of Bangladesh, agarwood is described as a stimulant, cardiac tonic and carminative [7]. It is considered one of the costliest perfumery raw materials used in high-class perfumery and fixative [8].
A number of scientific studies have revealed that essential oil extracts of Aquilaria possess antioxidant activity [9]. The essential oil of agarwood had a protective effect against oxidative damage induced by hydrogen peroxide in PC12 cells [10]. The most important bioactive constituents of agarwood plants are flavonoids, tannins and phenolic compounds [11]. It has been proved through several research that group of compounds acting as antioxidants was phenolic and flavonoids while the group of compounds that contribute as antibacterial compounds was alkaloids and terpenoids [12]. In some countries, chips of agarwood are usually polished and then colored for the purpose of attracting buyers. Also, many agarwood collectors are engaged in burying immature agarwood in soil with a view to accelerate decomposition. As a result of this kind of act, the product is changed into black and can be sold as higher agarwood. So, it has become increasingly difficult to access agarwood and to identify fake agarwood based on color [13].
With view above discussion, the present experiments were conducted to categorize the grading of agarwood, to determine the chemical compositions of agar wood and oil and to identify the bacteria responsible for fermentation of agarwood.
MATERIALS AND METHODS
Materials collection
Plant materials and agarwood soaking water were collected from an agarwood industry in Barlekha, Moulvibazar. Among those wood some were naturally insect infested, some were artificially screw injected and some were white agar wood free from any infections.
Determination of density of agarwood for their grading
Agarwood of three different categories were collected from Barlekha, Moulvibazar and were taken to the laboratory of Bangladesh Forest Research Institute, Chittagong. Collected agarwoods were dried for two hours at 50ºC to remove residual moisture from those woods. In this process of density metric method, a 200 ml cylinder was taken and filled with water. After that, each of individual samples was soaked in water and the final volume was measured (Figure 1). According to the above-mentioned process, the density of different agar wood was determined for the purpose of grading them into desired class and then those graded woods were cross checked by evaluation of ether extract with three replications.

Evaluation of ether extract from graded agarwood
Ether extract was evaluated with the help of soxhlet extraction apparatus. At first, boiling flask was dried in oven for 1.5 hour at 105ºC and cooled on desiccator, weighed and labeled. The condenser was fixed with water inlet and outlet pipes.
In a thimble, a 2 g sample of grinded agarwood was taken and inside the thimble, a piece of absorbent tissue was placed. The thimble was then transferred into the soxhlet extractor and about 180 ml diethyl ether was poured inside the extractor. The boiling flask was then loaded for heating. The heater regulator was adjusted so that 2-3 drops of diethyl ether per second dribbled from the condenser to the boiling flask with a usual temperature of 40ºC [14].
Determination of total phenolics
For the determination of total phenolics from agarwood oil, the Folin-Ciocalteu method was used and Gallic acid was used as standard [15]. To prepare extract 0.1 g samples were suspended in 10 ml of 75% acetone in 50 ml falcon tubes followed by vortexing for 30 minutes using a vortex mixture. Each extract from individual aliquots were mixed with 0.4 ml of water, 0.25 ml of Folin-Ciocalteu reagent and 1.25 ml sodium carbonate solutions (20%). After waiting for 40 minutes at room temperature, the absorbance was taken at 660 nm wavelength using colorimeter [14].
Estimation of total flavonoid content
To estimate total flavonoid content, Aluminium chloride colorimetric method was followed [16]. Absorbance was taken at 415 nm against the suitable blank [16].
Determination of antioxidant status
For the determination of antioxidant status of agarwood oil, the method described by Susanti and Sirat was followed [17]. To a 3 ml of freshly prepared DPPH solution (0.004%) in methanol, 0.1 ml of each extract and control at various concentrations were added. The reaction was allowed to stand for 30 minutes and absorbance was measured at 515 nm. All experiments were repeated three times independently. The intensity of decolorization of DPPH from purple to yellow resembles the scavenging efficiency of the extract.
The percentage inhibition of DPPH free radical scavenging activity was calculated using the following equation:
Percent inhibition = [(ADPPH–A sample)/ADPPH]*100
Where: ADPPH = Absorbance of DPPH
A sample= Absorbance of sample (extract/ascorbic acid)
Biochemical tests for identification of unknown bacteria
Agarwood soaking water was collected from Barlekha, Moulvibazar in order to identify the genus of unknown bacteria present in that water. After collection, serial dilutions from 1 x 10-1 to 10-10 of the agarwood soaking water was made by using the dilution fluid. The nutrient media and the petri dishes were sterilised by autoclaving at 121ºC for 30 minutes. After the autoclaving the petri dishes were dried and about 20 ml of the sterile media was poured into the petri dishes. About 1 ml inoculum from each of the 10-4 to 10-10 serial dilutions was inoculated into the petri dishes inside the Laminar Air Flow. After inoculation the petri dishes were kept inside the incubator at 37ºC for 24 hrs.
Following biochemical tests were performed for identifying genus of existing bacteria from the agarwood soaking water sample.
Gram-staining
A clean, grease free slide was taken. The smear of suspension was prepared on the clean slide with a loopful of sample. The slide was air dried and heat fixed. Then Crystal Violet was poured and kept for about 30 seconds to 1 minute and rinsed with water. Gram’s iodine was flooded for 1 minute and washed with water. Next, 95% alcohol was used for about 10-20 seconds for washing and rinsed with water. After that, Safranin was added for about 1 minute and washed with water. Finally the slide was air dried, blot dried and observed under microscope [18].
Catalase test
Catalase is an enzyme produced by microorganisms that live in oxygenated environments to neutralize toxic forms of oxygen metabolites; H2O2 and protects them.
A loop or sterile wooden stick was used to transfer a small amount of colony growth in the surface of a clean, dry glass slide. A drop of 3% H2O2 was placed in the glass slide. Finally the slide was observed for the evolution of oxygen bubbles [19].
Oxidase test
An oxidase is an enzyme that catalyzes an oxidation-reduction reaction, specially one involving oxygen (O2) as the electron acceptor.
Strip of Whatman’s No. 1 filter paper were soaked in a freshly prepared 1% solution of tertramethyl-p-phenylene-diamine-dihydrochloride. After draining for about 30 seconds, the strips were freeze dried and stored in a dark bottle tightly sealed with a screw cap. For use, a strip was removed, laid in a petri dish and moistened with distilled water. The colony to be tested was picked up with a platinum loop and smeared over the moist area [19].
Indole test
The indole test is a biochemical test performed on bacterial species to determine the ability of the organism to convert tryptophan into indole.
A sterilized test tube was taken containing 4 ml of broth media. The tube was inoculated aseptically by taking the growth from 18 to 24 hours culture. The tube was then incubated at 37°C for 24-28 hours. A 0.5 ml of Kovac’s reagent was added to the broth culture. The tube was observed for the presence or absence of ring [19].
Carbohydrate fermentation test
Glucose fermentation is a biological technique utilized in microbiology to determine the way a microorganisms metabolizes a carbohydrate.
At first, Trypticase, Sodium chloride, and Phenol red were weighed and dissolved in 100 ml distilled water and transferred into conical flasks. Then, 0.5% of dextrose was added into the flasks. The flask was autoclaved at 115oC for 15 minutes. The mixture was then transferred into fermentation tubes and label properly. Aseptically each labeled carbohydrate broth with bacterial culture was inoculated. The tubes were incubated at 18-24 hours at 37oC [20].
MR (Methyl Red)
Methyl Red (MR) test determines whether the microbe performs mixed acids fermentation when glucose is supplied.
By using sterile inoculating loop, the unknown bacterial culture was inoculated into the fresh, sterile medium. The other broth was left without inoculating (considered as a control).The inoculated tube was incubated at 35-37ºC for two to five days. After incubation, the broths were obtained from the incubator and 5 drops of Methyl Red reagent was added to the broth. The color was observed [20].
VP (Voges Proskuer) test
Voges Proskauer or VP is a test used to detect acetoin in a bacterial broth culture. The test is performed by adding alpha-naphthol and potassium hydroxide to the Voges-Proskauer broth which has been inoculated with bacteria.
A tube was inoculated with MR/VP broth with a pure culture of the test organism and was incubated for 24 hours at 35ºC.At the end of this time, aliquot 1 ml of broth to clean test tube. A 0.6 ml of 5% alpha naphthol was added, followed by the addition of 0.2 ml of 40% KOH. The tube was gently shaken to expose the medium to atmospheric oxygen and allowed the tube to remain undisturbed for 10 to 15 minutes. A pink-red color at the surface within 30 min was observed. The tube was shaken vigorously during the 30-min period [19].
Nitrate reduction test
Nitrate Reduction test is a microbiological test roughly named for its ability to test a microorganism’s ability to produce hydrogen sulfide.
Nitrate broth was prepared and inoculated heavy inoculum of the samples of organism individually in the medium aseptically. Test tubes were incubated at 37°C for 4 hours. After 4 hours incubation, few drops of the reagent A (Sulfanilic acid) and B (α- napthylamine) were added in the culture tubes. Color change was observed due to reduction of nitrate to nitrite [21].
The genus present in the fermented water was identified. Data were analyzed and tabulated through the use of simple statistics like mean, standard deviation etc.
Statistical analyses
All the collected data were then analyzed using IBM SPSS statistics (version 21) software and revealed the results.
RESULTS
Determination of density of agarwood for their grading
After determination of the density of supplied agarwood sample, those three categories of agarwood samples were graded with three replications. Density was highest in case of insect infested agarwoods (0.641 ± 0.85g/cm3) and was graded as Grade-1 agarwood, followed by Nailed (0.436 ± 0.052 g/cm3) and White agarwood (0.361 ± 0.46 g/cm3) and those two type agarwoods were classified as Grade-2 and Grade-3agarwood, respectively (Table 1). Determined densities of different agarwoods were completely similar with the result from eye estimation and also with the information obtained from different agarwood businessman.
Table 1. Density of different agarwood sample.
Evaluation of ether extract from graded agarwood
The results of ether extract are shown in Table 2. The results indicate Insect infested agarwood contained highest amount of ether extract (18.90 ± 0.60%) than Nailed (11.03 ± 0.19%) and White agar wood (1.84 ± 0.04%). Through personal communication with agar industry entrepreneur it has been cleared that market price of insect infested agarwood oil is the highest which is supporting the present results of ether extract content.
Table 2. Ether extract content from agarwood samples.
Determination of total phenolics and total flavonoid
Results of total phenolics and flavonoid were expressed as mg GAE/g of agar wood samples [22] which is shown in Table 3 and µg QE/ml for agar oil samples [23] which is shown in Table 4. The phenolic content in insect infested agarwood, nailed agarwood and white wood were 3.5 ± 0.06 mg GAE/g, 2.98 ± 0.07 mg GAE/g and 2.50 ± 0.05 mg GAE/g, respectively. Results indicated that phenolic content was the highest in insect infested agarwood and the lowest in white agarwood.
In case of oil extracts, phenolic content in insect infested agarwood oil was 33.25 ± 0.66 µg GAE/ml and in nailed agarwood oil was 27.54 ± 1.97 µg GAE/ml. Among the two oil sample, insect infested agarwood oil possessed flavonoid content of 7.82 ± 0.23 µg QE/ml and nailed agarwood contained 6.58 ± 0.62 µg QE/ml.
Table 3. Total phenolics estimation from agarwood.
Table 4. Total phenolics and total flavonoid of agar oil extract.
Estimation of antioxidant status
DPPH radical scavenging capacities of nailed agarwood oil extracts were tested at 0.50, 0.75 and 1.00μg/ml concentrations. The inhibitory concentration of oil extract on DPPH is summarized in Table 5. All oil extracts were found to possess concentration-dependent inhibitory activity against DPPH radical. The antioxidant capacity is also expressed as 50% inhibitory concentration (IC50). A lower IC50 value corresponds to a higher antioxidant activity of the oil extract [24]. The scavenging activity on 1, 1diphenyl-2-picryl hydrazyl (DPPH) radical shown by agarwood oil was 0.904 µg/ ml of IC50 value [25]. IC50value of 0.904µg/ml was observed in oil extracts of nailed agar wood.
Table 5. The inhibitory concentration of oil extracts from nailed agarwood on DPPH radical.
Biochemical tests for identification of genus of microorganisms
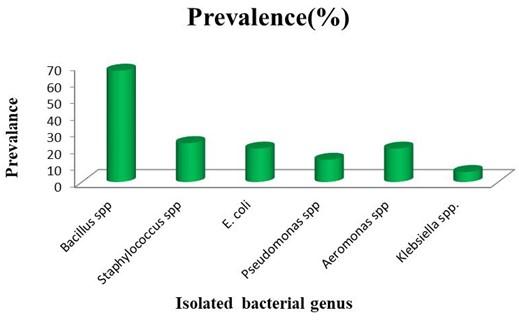
Among, thirty (30) isolates the prevalence percentage of different bacterial genus has been listed in Table 6. Most of the bacteria present in agarwood soaking water belong to Bacillus spp. Also genus of some other microorganism like Staphylococcus, E. coli, Pseudomonas, Aeromonas and Klebsiella were also found but with lower occurrence (Figure 2). It can be concluded that the genus of the most of isolated bacteria was Bacillus spp. with identifying characteristics and may contribute in the fermentation of agarwood to increase agarwood yield [26].
Table 6. No. of bacterial isolates found with their prevalence.
DISCUSSION
The present study results can be concluded that Aquilaria plant contained significant amount of phenols, flavonoids which directly influence the quality of secondary metabolites. Determination of total phenolic and total flavonoid compounds represents that the antioxidant activity is due to the presence of these constituents and suggested that this plant have a potential source of natural antioxidant so it can combat against diseases in where increased free radical produced. Hoq et al. [27] obtained similar result for total phenolics and ether extract in agarwood and oil from Moulvibazar, Bangladesh. Researcher from same laboratory observed 93.92% and 95.37% sesquiterpenes in different agar oil collected from Moulvibazar district [28]. Therefore, same experiments have not been repeated here. There is no easily available and practically established grading system of agarwood. Thus, the present study aimed to develop an easily available grading system for agarwood so that anybody can grade agarwood with little technical knowledge or instruments.
Agarwood is a fragrant resin embedded wood that can sink down in water. Agarwood pieces that sink in water are assumed to have higher resin content and higher density [13]. Therefore, the grading of agarwood was determined based on density metric method. Density was found highest in insect infested wood and classified as Grade-1 agarwood. This grading was also cross checked for ether extract content to increase the acceptance of the grading system. Among several bacterial isolates found in agar wood soaking water, Bacillus prevailed most and assists in agarwood fermentation during soaking period. It is important to identify the species of Bacillus that can help to improve the soaking of agarwood. If the species can be identified, this species can be additionally used during soaking period that would reduce soaking time.
Research should be done to improve the quality of agarwood and agar oil. Extraction method needs to be improved. There is also scope to carry out research on soil quality and quality of agarwood and agarwood oil production. If it is possible to grade the agarwood more accurately, high market value can be obtained by exporting them. Proper regulatory support from the government can play a vital role to make agar sector one of the major foreign currency earning sectors for Bangladesh.
ACKNOWLEDGMENT
The authors extend their appreciation to the Bangladesh Agricultural Research Council (BARC) (Project no.CRG-418, NATP Phase-II) for financing the research work.
AUTHOR CONTRIBUTIONS
JA, MMHK and MAK designed the experiment. JA, SRA and MJH performed the experiments; JA, MMHK and SRA analyzed the data and wrote the draft. MMHK and AK critically revised the manuscript. MMHK contributed to drafting the article. JA and SRA contributed to revising it critically for important intellectual content.
CONFLICTS OF INTEREST
The author declares that no conflict of interest exists.
References
- [1]Abdin J. The agarwood industry: yet to utilize in Bangladesh. Int J Econ Manag Sci. 2014; 3: 163.
- [2]Abdin J. The agarwood industry: yet to utilize in Bangladesh. Int J Econ Manag Sci. 2014; 3: 163.
- [3]Yadav DK, Mudgal V, Agrawal J, Maurya AK, Bawankule DU, Chanotiya CS, Khan F,Thul ST. Molecular docking and ADME studies of natural compounds of Agarwood oil for topical anti-inflammatory activity. Curr Comput Aided Drug Des. 2013; 9(3):360-70.
- [4]Nag LT, Chang YS, Kadir AA. A review on agar producing Aquilaria species. J Trop For Prod. 1997; 2(2):272-285.
- [5]Hansen E. The hidden history of a scented wood.Saudi Aramco World. 2000; 51:1-13.
- [6]Ishihara M, Tsuneya T, Shiga M, Uneyama K. Three sesquiterpenes from agarwood. Phytochem. 1991; 30: 563–566.
- [7]Bhuiyan NI,Jaripa B, Bhuiyan NH. Analysis of essential oil of eaglewood tree by Gas chromatography mass spectroscopy. Bangladesh J Pharmacol. 2009; 4(1):24-28.
- [8]Burt S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods-A Review. Int J Food Microbiol. 2004; 94(3): 223-253.
- [9]Dahham SS, Saghir SM, Ahmed MBK, Al-Suede FS. Bioactive Essential Oil from Aquilaria crassana for cancer prevention and treatment. Glob J Pure Appl Sci. 2014; 4:26-31.
- [10]Wu YC, Lee KS, Song Y, Gehrke S, Lu B. The bantam micro RNA acts through numb to exert cell growth control and feedback regulation of notch in tumor forming stem cells in Drosophila brain. PLoS Genet. 2017; 13(5):e1006785.
- [11]Manasi D, Jayanta KP, Prasanna PP. Phytochemical and antimicrobial screening of extracts of Aquilaria agallochaRoxb. Afr J Biotechnol. 2008; 7(20):3531-3534.
- [12]Hideaki H. Agarwood extracts decrease high protein, high fat diet induced intestinal putrefaction toxins in mice. Pharm Anal Acta. 2012; 3: 152.
- [13]Yang-yang L, Jian-he W, Zhi-hui G, Zheng Z, Jun-chen L. A Review of Quality Assessment and Grading for Agarwood. Chinese Her Med. 2017; 9(1): 22-30.
- [14]Khan MMH. Novel supplements to improve utilization of low quality forages in ruminants. Muktodesh Prokashon, Islami Tower, 11/1 Banglabazar, Dhaka-1100, Bangladesh. 2012.
- [15]Khan MMH, Chaudhry AS. Chemical composition of selected forage and spices and the effect of these spices on in vitro rumen degradability of some forages. Asian Australas J Anim Sci. 2010; 23(8): 889-900.
- [16]Pekal A, Pyrzynska K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal Method. 2014; 7:1776-1782.
- [17]Susanti D, Sirat HM, Ahmad F, Ali RM. Antioxident and cytotoxic flavonoids from the flowers of Melastomamalabathricum. Food Chem. 2007; 103(3): 710-716.
- [18]Beveridge TJ. Use of the Gram stain in microbiology. Biotech Histochem. 2001; 76 (3): 111–118.
- [19]Aneja KR. Experiments in Microbiology, Plant Pathology and Biotechnology. New Age International Publishers, 2003.
- [20]Hemraj V, Sharma D, Gupta A. A review on commonly used biochemical test for bacteria. Innovare J Life Sci. 2013; 1(1): 1-7.
- [21]Campbell WH, Song P, Barbier GG. Nitrate reductase for nitrate analysis in water. Environ Chem. 2006; 4: 69-73.
- [22]Yadav P, Malpathak N. Estimation of Antioxidant Activity and Total Phenol, Flavonoid Content among Natural Populations of Caper (Capparismoonii) from Western Ghats Region, Indian J Pharm Educ. 2016; 50(3): 495-501
- [23]Benslama A, Harrar A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int J Her Med. 2016; 4(6): 158-161.
- [24]Singh K, Deo B. Phytochemical evaluation and in vitro antioxidantactivity of GymnemasylvestreR.Br. J Med Plants Stud. 2014: 2(4): 19-23.
- [25]Nik NANW, Nor AMO, Noorhuda A. In vitro antioxidant activity and phytochemical screening of Aquilariamalaccensis leaf extracts, J Chem Pharm Res. 2014; 6(12): 688-693.
- [26]Bhore SJ, Preveena J, Kandasamy KI. Isolation and identification of bacterial endophytes from pharmaceutical agarwood-producing Aquilaria species. Pharmacogn Res. 2013; 5(2): 131-137.
- [27]Hoque MN,Khan MMH,Mondal MF. Insect infested agarwood: A newly prized product of agarwood market in Bangladesh. Fund Appl Agric. 2019; 4(1): 689-692.
- [28]Hoque MN, Khan MMH, Mondal MF. Chemical composition and Antimicrobial Activity of Essential Oils from Aquilariamalaccensis in Bangladesh. Saudi J Life Sci. 2018; 3(10): 600-608.