In vitro plant regeneration of wild eggplant (Solanum sisymbriifolium) to produce large number of rootstocks for tomato grafting
Abstract
The experiment was conducted to develop a suitable protocol for high frequency plant regeneration of wild eggplant (Solanum sisymbriifolium) in order to produce a large number of rootstocks for tomato grafting for the management of wilt disease. To obtain in vitro seedlings of S. sisymbriifolium, seeds were treated with various concentrations of GA3 (Gibberellic acid) prior to place them in germination media (½ strength Murashige and Skoog) and 750 mg/L GA3 was found as a suitable concentration resulting the highest (76.67%) germination rate. Various factors namely combination of plant growth regulators, explant types and explant age were investigated for development of an efficient plant regeneration system of S. sisymbriifolium. Cotyledon and hypocotyl explants of S. sisymbriifolium were cultured on Murashige and Skoog medium supplemented with various concentrations of BA (6-Benzylaminopurine), NAA (α-Naphthalene acetic acid) and 2,4-D (2,4-Dichlorophenoxy acetic acid), to determine suitable medium for callus and shoot initiation. Fourteen days old cotyledon explants were found more responsive than that of hypocotyl, both in callus and shoot induction. The highest callus initiation (100%) and shoot regeneration (73.33%) were observed in MS media supplemented with 0.5 mg/L NAA + 1.0 mg/L BA and 0.2 mg/L NAA + 3.0 mg/L BA, respectively. MS medium supplemented with 0.1 mg/L NAA showed the highest frequency (86.67%) of rooting. The regenerated plantlets were acclimatized in pot soil and eventually used as rootstock for tomato (Solanum lycopersicum cv. BARI hybrid 4) grafting. The grafted plants showed no wilt disease in field condition until maturity.
INTRODUCTION
Tomato and eggplant belong to the family Solanaceae, are the most important high value, widely consumed, palatable and nutritious vegetables in Bangladesh. They are cultivated commercially throughout the tropical and subtropical region of the world. In respect of, acreage and production eggplant (122,000 acres and 450,000 M. tons) is the second most and tomato (76,000 acres and 414,000 M. tons) is the third most important vegetable crop next to potato (1164,000 acres and 9254,000 M. tons) in Bangladesh [1]. Traditionally, tomato and eggplant are highly consumed in Bangladesh and play a vital role in the national economy as a cash crop. However, the yield potential of these two vegetables is very low in Bangladesh compare to other countries. This lower production rate and higher consumer’s demand lead high price of these two vegetables. The production of these two crops is hampered due to different insects, pests and diseases that exert a deleterious effect on yield, market quality, and storability [2]. Among the 13 different diseases of these crops so far recorded in Bangladesh [3, 4, 5], wilt is one of the major diseases in eggplant and tomato production in the country [6] which causes devastating damage of both crops. Sometime 100% crop failure is noticed in kitchen gardens of Bangladesh due to wilt [7]. Control of wilt is difficult for growers in Bangladesh, particularly for growers with limited capacity to rotate out the solanaceous crops. The wide host range of wilt causing pathogen further restricts rotational options, and effective crop rotation programs in severely infested soils may require multiple years out of tomato production [8]. Even soil fumigants have little success against the causal pathogen of wilt [9, 10]. However, to avoid circumvent of wilt by grafting the tomato on wilt-resistant rootstock is proven an effective technique especially where wilt disease is acute [11]. Moreover, grafting has been utilized to manage wilt in tomato crops worldwide [2, 12, 13, 14].
There are some non-tuberous wild Solanum species and their amphidiploids are being considered to have high resistance against wilt disease and used as rootstocks of tomato and eggplant grafting. S. sisymbriifolium is known as Kata begun is resistant to biotic and abiotic stresses [15-17] and this species is found effective as rootstock to control wilt disease [17, 18]. But the availability of seedlings of S. sisymbriifolium for using as rootstocks is limited due to its poor and not uniform seed germination rate and strong dormancy [11]. To overcome this situation, plant tissue culture offers an efficient method to produce a large number and year round availability of seedlings. Regeneration of valuable economic plants through tissue culture based on the principle of totipotency, individual plant cell is capable of regenerating new plantlets [19]. However, till to date no report was found on tissue culture of S. sisymbriifolium.
To minimize the wilt diseases grafting could be a valuable tool for eggplant and tomato growers in Bangladesh, which is of critical importance of the availability of wilt resistant rootstocks to growers. Therefore, the objective of this study was to establish a suitable protocol for high frequency plant regeneration of S. sisymbriifolium which is a pre-requisite to produce a large number of seedlings for the use of resistant rootstocks for tomato and eggplant cultivation around the year.
MATERIALS AND METHODS
Plant materials
Healthy and disease free seeds of S. sisymbriifolium were collected from Kamalgonj and Rajnagar Upazila of Moulvibazar district of Bangladesh. The seeds were treated with various concentrations of GA3 (90% TC, JiangXiXin Ruifeng Biochemical Company Ltd. China) for 24 h to determine optimal concentration for breaking seed dormancy. After that the seeds were sterilized in the solution of 70% ethyl alcohol (MERCK, Germany) for 5 min and 30% Clorox (Sodium hypochlorite, The Clorox Company, Oakland, USA) for 15 min followed by three rinses in sterilized distilled water. The seeds were then placed on germination medium comprising half strength MS [20] salts and vitamins, 3% sucrose and 1% agar with a density of 10 seeds per culture vessels and incubated in 25±2°C temperature under 16 hours photoperiod provided by 144W white fluorescent lamps (culture condition).
Explant preparation and culture of explant
Cotyledon and hypocotyl explants were prepared from 14-d-old in vitro seedlings of S. sisymbriifolium (Figure 1a) and they were cultured on MS (Murashige and Skoog, 1962) media supplemented with different concentrations of BA (99%, Duchefa Biochemie, the Netherlands) (0.5, 1.0, and 2.0 mg/L), NAA (98%, Duchefa Biochemie, the Netherlands) (0.1, 0.5 and 1.0 mg/L) and 2,4-D (96%, Duchefa Biochemie, the Netherlands) (0.1, 0.5 and 1.0 mg/L) to determine optimal medium for callus initiation. Cotyledons along with 1-2 mm petioles were very carefully excised from the hypocotyl and apical shoot meristems of seedlings. The hypocotyls were then discarded from the root tip and cut into 5-7 mm length segments. The whole procedure was carried out in laminar airflow cabinet. Ten explants were placed on each culture vessels containing 50 ml callus induction media. Cotyledons along with petioles were placed in upward direction with the petiole in contact with the media whereas hypocotyl segments were placed horizontally on the surface of the media (Figure 1b & c).
After 14 days of incubation of explants, when the calli attained a convenient size, were transferred in culture vessels containing shoot induction media (Figure 1d & e). Shoot induction media comprised MS salts and vitamins, 3% sucrose, 1% agar and various concentrations of BA (1.0, 2.0 and 3.0 mg/L), NAA (0.1, 0.2 and 0.5 mg/L) and 2,4-D (0.1, 0.2 and 0.5 mg/L). When the shoots were attained about 2-3 cm in length, these were excised from the callus and transferred into the new culture vessels containing freshly prepared root induction medium. Root induction media contained various concentrations of NAA (0, 0.1, 0.2 and 0.5 mg/L) to develop root. Each of the time, the cultured vessels were sealed with parafilm and marked with a permanent marker to indicate each treatment and were incubated in culture room. Three to four cm in length of plantlets with sufficient root system were taken out carefully from the culture vessels and washed gently in tap water to remove agar medium and sucrose trace elements to discourage infection by fungal contamination. The plantlets were then transplanted to moistened soil in pots and covered with glassware (beaker) for preventing desiccation. After proper hardening, the plantlets were transferred to natural environment. Thirty five days old plantlets of S. sisymbriifolium were used as rootstocks for tomato grafting (S. lycopersicum cv. BARI hybrid 4) and allowed to grown in natural environment.

Statistical analysis
The experiment was arranged in Completely Randomized Design (CRD) with 3 replications. The recorded data for different parameters were statistically analyzed to ascertain the significance of the experimental results. The mean and standard deviation for all treatments were calculated by using MS Excel 2010. The significance and difference between means were evaluated by Dunkan’s Multiple Range Test (DMRT) using R analysis software (version Rx64 3.4.3).
RESULTS
The optimal concentration of GA3 for seed germination
For the optimum germination, seeds were treated with different concentrations of GA3 for 24 h before placing them in germination media. Seeds treated with 750 or 1000 mg/L GA3 showed the highest (76.67%) germination rate while seeds without treated with GA3 showed the lowest germination rate (33.33%) as shown in Table 1.
Table 1. Influence of GA3 pretreatment for germination of S. sisymbriifolium.
The optimal medium for callus initiation
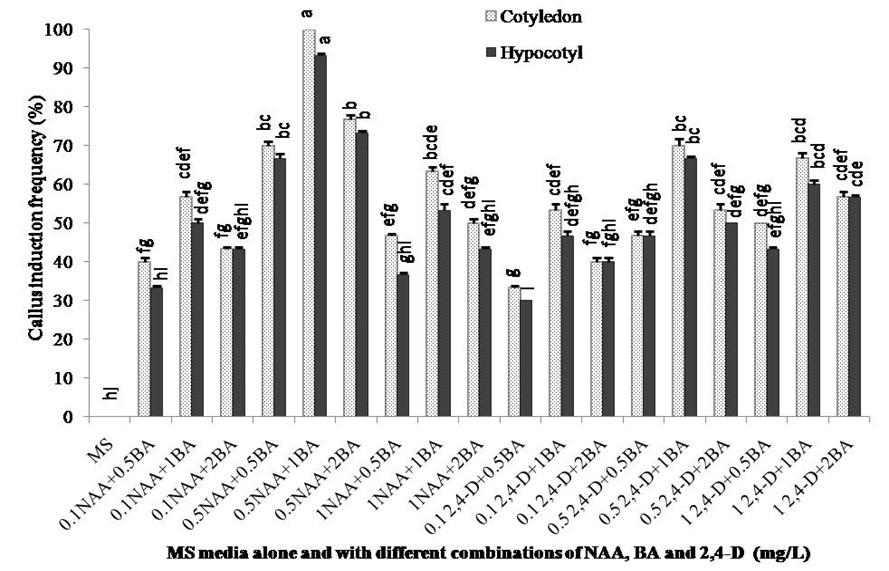
MS media supplemented with various concentrations of BA (0.5, 1.0 and 2.0 mg/L), NAA (0.1, 0.5 and 1.0 mg/L) and 2,4-D (0.1, 0.5 and 1.0 mg/L) were used to determine the suitable media combination for callus induction. Fourteen days old cotyledon and hypocotyl explants of S. sisymbriifolium were used for callus induction. Explants cultured in hormone free MS basal medium (control) did not produce any callus and died after a few days. From a total of 18 different combinations tested, cotyledon explants showed the highest (100%) callus initiation frequency in MS + 0.5 mg/L NAA + 1 mg/L BA combination and the lowest (33.33%) in MS + 0.1 mg/L 2,4-D + 0.5 mg/L BA combination whereas hypocotyl explants showed the highest (93.33%) callus initiation frequency in MS + 0.5 mg/L NAA + 1 mg/L BA combination and the lowest (30%) in MS + 0.1 mg/L 2,4-D + 0.5 mg/L BA combination (Figure 2). A significant difference was found in callus initiation frequency between cotyledon and hypocotyl explants and it is clear that cotyledon explants showed better performance than that of hypocotyl.
The optimal medium for shoot regeneration
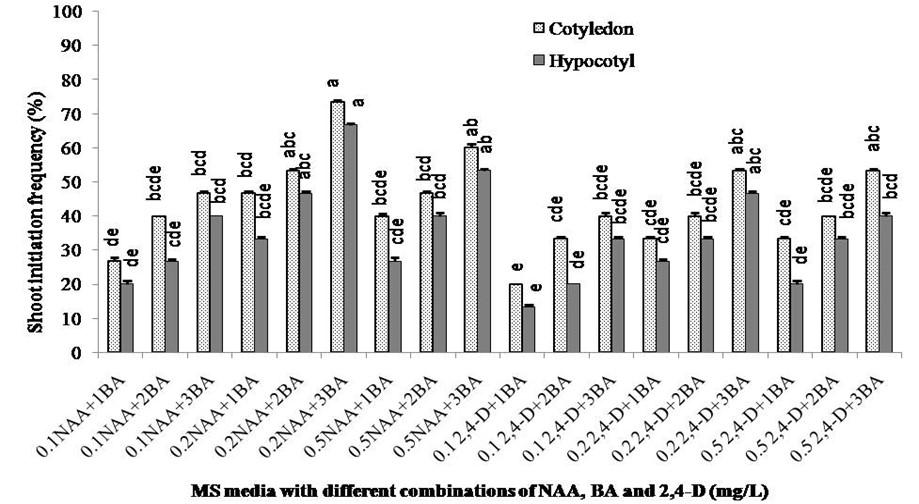
To observe the best shoot regeneration response, various combination of BA (1.0, 2.0, and 3.0 mg/L), NAA (0.1, 0.2 and 0.5 mg/L) and 2,4-D (0.1, 0.2 and 0.5 mg/L) were tested (Figure 3). Two weeks old calli obtained from cotyledon and hypocotyl explants of S. sisymbriifolium were transferred in shoot regeneration media. A total of 18 combinations of supplements showed clear variation in shoot regeneration ability for both the explants. Among the combinations, MS + 0.2 mg/L NAA + 3.0 mg/L BA showed the highest 73.33% and 66.67% shoot regeneration from cotyledon and hypocotyl explants, respectively. The lowest shoot regeneration frequencies (20% in cotyledon explants and 13.13% in hypocotyl explants) were observed in MS + 0.1 mg/L 2,4-D + 1.0 mg/L BA for both cotyledon and hypocotyl explants.
Effect of explant age
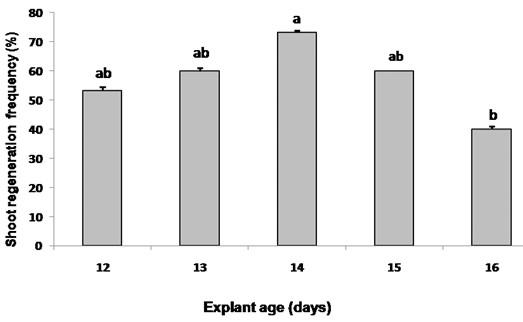
Cotyledon explants of different ages (12 to 16 days) were cultured on the best callus initiation media (MS+ 0.5 mg/L NAA + 1.0 mg/L BA) followed by the best shoot regeneration media (MS + 0.2 mg/L NAA + 3.0 mg/L BA) to investigate the effect of age of explant on shoot regeneration frequency. Explants below 12 days old seedlings were too small and was not used in this experiment. Cotyledon explants of 14 days old seedlings showed the highest (73.33%) shoot regeneration frequency whereas 16 days old seedlings showed the lowest (40 %) shoot regeneration frequency after two weeks of explant incubation (Figure 4).
Root initiation, acclimatization and grafting compatibility
In the final stage of in vitro development, elongated shoots (2-3 cm) were excised out and transferred to rooting media, MS medium supplemented with different concentration of NAA (0, 0.1, 0.2 and 0.5 mg/L). Among the four tested media, the maximum rooting (86.67%) was observed in MS medium supplemented with 0.1 mg/L NAA whereas the lowest root formation frequency (26.67%) was observed in NAA free MS medium (Table 2). Within 6 days of culture, root formation was started and plantlets produced well developed root system within 15 days (Figure 1f). Plantlets produced well developed roots were transferred to pot soil and acclimatized accordingly (Figure 1g). Then thirty five days old S. sisymbriifolium plants were used as rootstocks for tomato (S. lycopersicum cv BARI hybrid 4) grafting. The regenerated S. sisymbiifolium plants showed well compatibility as rootstocks in tomato grafting (Figure 1h). Until fruit maturity the grafted plants showed no wilt disease (Figure 1i).
Table 2. Frequency of root initiation of S. sisymbriifolium on MS media supplemented with various concentrations of NAA.
DISCUSSION
Since wild Solanum species required long time to germinate and showed poor seed germination and strong dormancy, the seeds need pretreatment with GA3 or any other chemicals for breaking seed dormancy and uniform seed germination. Although GA3 treatment for breaking seed dormancy of some wild Solanum species was not so effective [11], however, in our study GA3 treatment was found to be the most influential factor for facilitating seed germination of S. sisymbriifolium. The germination percentage of seed was increased with the increasing concentrations of GA3 up to 750 mg/L (76.67%) and it is constant in 1000 mg/L GA3. That means the treatment neutralized, further increased concentrations of GA3 may give the same result.
Researchers had used wide range of explants for in vitro regeneration of Solanum species such as hypcotyl, cotyledon and young leaf explants for brinjal [21, 22, 23, 24], and pedicel explants for tomato [25]. Although a study was found on in vitro regeneration of S. torvum [26], till to date there is no report found on in vitro plant regeneration of S. sisymbriifolium. In our investigation, cotyledon and hypocotyl segments excised from in vitro grown seedlings were used as explants to find out their callusing ability. Cotyledon explant was found to be more responsive than that of hypocotyl explant in both callus and shoot initiation. This result compares favorably with recent studies of S. torvum [26] and S. melongena [23]. The use of cotyledon explants for in vitro plant regeneration has several advantages. A large number of cotyledon explants can be obtained by germinating seeds under sterile conditions over a short period of time all year round [27]. Moreover, cotyledon explants possess high morphogenic potential. The maximum 100% callus initiation frequency obtained from cotyledon explant in MS media supplemented with 0.5 mg/L NAA and 1.0 mg/L BA media combination. Plant growth regulator, NAA was more responsive for callus and shoot induction compared to 2,4-D (Figure 2 & 3). Among 9 combinations of 2,4-D and BA, cotyledon explant produced the highest 70% callus in MS + 0.5 mg/L 2,4-D + 1.0 mg/L BA combination. Hypocotyl explant showed maximum 93.33% callus initiation frequency in MS + 0.5 mg/L NAA + 1.0 mg/L BA media combination. Among the media combinations of 2,4-D and BA hypocotyl explant produced the highest 66.67% callus in MS + 0.5 mg/L 2,4-D + 1 mg/L BA media combination. Explants cultured on hormone free MS medium did not produce any callus. The callus initiation frequency of both cotyledon and hypocotyl explants increased with the increase of BA concentrations up to 1.0 mg/L then it declines when the concentration of NAA and 2,4-D increased. Combinations of BA with NAA gave the better result than BA and 2,4-D in case of callus initiation of S. sisymbriifolium (Figure 2).
Cytokinins are mainly responsible for cell division and differentiation of adventitious shoots from callus [28]. These compounds overcome apical dominance and release lateral buds from dormancy, while added to shoot regeneration media [29]. The proportion of growth regulators required for shoot induction varies numerously with the tissue and seems directly correlated to the amount of hormones synthesized at endogenous levels within the cells of the explant [28]. Shoot production induces by higher cytokinin to auxin ratio and lower cytokinin to auxin ratio induces roots with few shoots [30]. Similar as callus initiation, cotyledon explant showed better shoot regeneration frequency than hypocotyl explant and this result is compliant with previous work of S. melongena [23]. In this study, maximum 73.33% shoot regeneration from cotyledon explant obtained in MS + 0.2 mg/L NAA + 3.0 mg/L BA media combination. MS medium supplemented with 3.0 mg/L BA was found to be suitable concentration for high frequency shoot regeneration of S. torvum [26] which is compliant with the present study. The shoot regeneration frequency increased with the higher concentration of BA and lower concentration of NAA or 2,4-D which support the findings of previous work [30].
Cotyledon explants derived from 14-d-old seedlings showed the highest frequency of shoot regeneration of S. sisymbriifolium (Figure 4). The cotyledons derived from 16-d-old and older seedlings exhibited yellowing of the lamina after 10-12 days of culture indicated that younger explants exhibit greater morphogenic potential than older explants, as they might have more metabolically active cells with hormonal and nutritional conditions that are responsible for increased organogenesis [27, 31].
Since, lower cytokinin to auxin ratio induces roots with few shoots [30], we only used auxin (NAA) for root initiation. Shoots regenerated from the cotyledon and hypocotyl explants cultured in MS media and MS media supplemented with various concentrations of NAA (0, 0.1, 0.2 and 0.5 mg/L) for root initiation. The highest (86.67%) root formation was found in MS medium supplemented with 0.1 mg/L NAA whereas the lowest (26.67%) root formation was occurred in MS media without NAA. Increasing concentration of NAA dramatically decreased the frequency of rooting. Most importantly, seedlings obtained from tissue culture technology showed grafting compatibility with tomato plants as a rootstock.
CONCLUSIONS
The highest rate of seed germination was found in case of seeds treated with 750 mg/L GA3 for 24 h before placing them in germination media. Fourteen days old cotyledon explants of S. sisymbriifolium showed higher callus and shoot formation frequency than hypocotyl explants. MS medium supplemented with 0.5 mg/L NAA and 1.0 mg/L BA was suitable medium for high frequency callus induction and MS medium supplemented with 0.2 mg/L NAA and 3.0 mg/L BA was the appropriate medium for high frequency shoot regeneration of S sisymbriifolium. MS medium supplemented with 0.1 mg/L NAA is the best rooting medium of S. sisymbriifolium. Finally, the plants established by tissue culture technology in this study showed well compatibility as a rootstock in tomato grafting and the grafted plants exhibited no wilt disease until fruit maturity.
ACKNOWLEDGEMENT
The research work was supported by a grant (4829.1 for FY 2017-2018) from Sylhet Agricultural University Research System (SAURES), financed by the University Grants Commission (UGC) of Bangladesh.
AUTHOR CONTRIBUTIONS
This work is carried out in collaboration of all the authors. Authors SS designed the experiment. Authors GD carried out laboratory work and performed the statistical analysis. Authors MSUB, KKS and ASP helped in conducting the lab work, collecting the data and interpreting the results. Author GD wrote the first draft of the manuscript and which was edited by the author SS. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare that no conflict of interest exists.
References
- [1]BBS: Year Book of Agricultural Statistics of Bangladesh. Ministry of Planning, Government of the People Republic of Bangladesh, 2015.
- [2]Ray BP, Hassan L, Nasiruddin KM. In vitro regeneration of brinjal (Solanum melongena L.). Bangladesh J Agril Res. 2011; 36: 397-406.
- [3]Khan AA, Badshah K, Khan MW. Resistance of aubergine cultivars to root-knot nematodes. Tests of Agrochemicals and Cultivars. 1998; 19: 40-41.
- [4]Das GP, Ramaswamy S, Bari MA. Integrated crop management practices for the control of the brinjal shoot and fruit borer in Bangladesh. DAE-DANIDA, 2000.
- [5]Rashid MM. A Guidebook of Plant Pathology. Department of Plant Pathology. HSTU, Dinajpur, Bangladesh, 2000; pp 58.
- [6]Ali M. Workshop on Research and Development of vegetable crops. Institute of Post graduate Studies in Agriculture (IPSA), Gazipur, Bangladesh. 1993; pp 68-75.
- [7]Ali M, Alam MZ, Akanda MAM. Grafting, A technique of control soil borne diseases of tomato and eggplant. Institute of Post graduate Studies in Agriculture (IPSA), Gazipur, Bangladesh.1994; pp 10.
- [8]Lemaga B, Kanzikwera R, Kakuhenzire R, Hakiza J, Maniz G. The effect of crop rotation on bacterial wilt incidence and potato tuber yield. Afr Crop Sci. 2001; 9: 257-266.
- [9]Driver J, Louws FJ. Fumigants and varieties to manage southern bacterial wilt of tomato. Annu. Int. Res. Conf. Methyl Bromide Alternatives Emissions Reductions. Orlando, FL, 2002, pp228-232.
- [10]Enfinger JM, McCarter SM, Jaworski CA. Evaluation of chemicals and application methods for control of bacterial wilt of tomato transplants. J Phytopathol. 1979; 69: 637-640.
- [11]Ibhrahim M, Munira MK, Kabir MS, Islam AKMS, Miah MMU. Seed germination and graft compatibility of wild Solanum as rootstock of tomato. Online J Biol Sci. 2001; 1: 701-703.
- [12]Grimault V, Prior P. Grafting tomato cultivars resistant or susceptible to bacterial wilt-analysis of resistance mechanisms. J Phytopathol. 1994; 141: 330-334.
- [13]Lin C, Hsu S, Tzeng K, Wang J. Application of a preliminary screen to select locally adapted resistant rootstock and soil amendment for integrated management of tomato bacterial wilt in Taiwan Plant Dis. 2008; 92: 909-916.
- [14]Dhivya R, Sadasakthi A, Shivakumar M. Response of wild solanum rootstocks to root-knot nematode (Meloidogyne incognita Kofoid and White). Int J Plant Sci. 2014; 9: 117-122.
- [14]Tikoo SK, Mathai PJ, Kishan R. Successful graft culture of tomato in bacterial wilt sick soils. Curr Sci. 1979; 48: 259-260.
- [15]Hebert Y. Comparative resistance of 9 Solanum species to bacterial wilt (Pseudomonas solanacearum) and to the nematode Meloidogyne incognita: Importance for breeding aubergine (Solanum melongena L.) in a humid tropical zone. Agronomie. 1985; 5: 27-32.
- [16]Shetty KD, Reddy DDR. Resistance in Solanum species to root-knot nematode Meloidogyne incognita. Indian J Nematol. 1985; 15: 230.
- [17]Petran. interspecific grafting of tomato (Solanum lycopersicum) onto wild eggplant (Solanum torvum) for increased environmental tolerances. A thesis submitted for MS degree to the University of Minnesota, USA, 2013, pp 25-30.
- [18]Krikorian AD, DL Berquam. Plant cell and Tissue culture: The role of Haberlandt. Bot. Rev. 1969; 35: 59-88.
- [19]Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant, 1962. 15:473-497.
- [20]Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant, 1962. 15:473-497.
- [21]Solanki JJ, Pawar PK, Maheshwari VL. Efficient plant regeneration in Solanum melongena L. Physiol Mol Biol Plants. 2006; 12: 307-311.
- [22]Sharma P, Rajam MV. Genotype explant and position effects on organogenesis and embryogenesis in eggplant (Solanum melongena L.). J Exp Bot. 1995; 46: 135-141.
- [23]Dobariya KL, Kachhadiya JR. Role of genotype, explants and culture medium on in vitro morphogenesis in brinjal (Solanum melongena L.). Orissa J Hort. 2004; 32: 52-54.
- [24]Hendrix CR, Litz ER, Kirchoff KB. In vitro organogenesis and plant regeneration from leaves of Solanum candidum Lindl. S. quitoens Lam. (naranjilla) and S. sessiliflorum Dunal. Plant Cell Tissue Organ Cult. 1987; 11: 67-73.
- [25]Compton ME, Veillux RE. Shoot, root and flower morphogenesis on tomato inflorescence explants. Plant Cell Tissue Organ Cult. 1991; 24: 223-231.
- [26]Thirunahari U. In vitro plantlet regeneration from cotyledonary explants in Solanum torvum (Swartz) a medicinally important plant. European J Biomed Pharma Sci. 2016; 3: 207-213.
- [27]Bhuiyan MSU, Min SR, Choi KS, Lim, YP, Liu JR. Factors for high frequency plant regeneration in tissue cultures of Indian mustard (Brassica juncea). K J Plant Biotechnol. 2009; 36: 137-143.
- [28]Pampanna Y. Studies on in vitro regeneration and transformation of tomato (Lycopersicon esculentum L.) Cv. Vybhav with Chitinase gene. Department of horticulture University of Agricultural Sciences G.K.V.K. Campus, Bengaluru. 2002.
- [29]George EF. Plant Propagation by Tissue Culture. Exegetics Ltd: UK, 2008, pp 205-476.
- [30]Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissue cultures in vitro. Symp Soc Exp Biol .1957; 11: 118-131.
- [31]Wayase UR, Shitole MG. Effect of Plant Growth Regulators on Organogenesis in Tomato (Lycopersicon esculentum Mill.) cv. Dhanashri. Int. J. Pure Appl. Sci. Technol. 2014; 20: 65-71.