Phytochemical profiling and antioxidant potentiality of medicinal plants along with their antibacterial efficacy
Abstract
The aim of this study was to explore phytochemical profiling, antioxidant and antibacterial activity of four medicinal plants including Catharanthus roseus, Aegle marmelos, Moringa oleifera, and Ageratum conyzoids grown in Sylhet district, Bangladesh. In this study, total 11 phytochemicals were screened from methanol extract of four medicinal plants, wherein flavonoid, tannin, sterol, phenol were present in all four medicinal plants. In vitro, antioxidant activity of these medicinal plants extract was investigated by DPPH-radical scavenging assay. The Aegle marmelos exhibited the highest antioxidant activity followed by Moringa oleifera, Ageratum conyzoids, and Catharanthus roseus extract. Methanolic extracts of same medicinal plants were subjected to a test of their antibacterial activities against Staphylococcus aureus, Escherichia coli, Klebsiella sp., Pseudomonas sp .and Salmonella sp. by agar disc diffusion method. The highest antibacterial potential was observed in the extract of Aegle marmelos against Salmonella sp. followed by Catharanthus roseus against Pseudomonas sp .with zone of inhibition of 18.67 mm, 15.0 mm, respectively. This study confirmed the efficacy of some native medicinal plants extract as potential source of phytochemicals, along with natural antioxidant and antimicrobials, which provide new possibilities to employing them against disease causing test organisms.
INTRODUCTION
Natural products have been a part and parcel of phytomedicines to treat diseases subsequently from time ancient [1]. Medicinal plants are a big source for a wide range of chemical ingredients as drug candidate [2]. The World Health Organization (WHO) estimates that 65-80% of the people relied upon traditional medicine, notable from plant origin, to combat diverse ailments [3].
Medicinal plants possess some organic compounds which influence certain physiological action on the human body and these bioactive substances include alkaloids, tannins, carbohydrates, terpenoids, steroids and flavonoids [4]. These compounds are synthesized in living organisms by means of primary or rather secondary metabolism. Bioactive compounds derived from plants exhibit various biological activities. They are widely used in many areas such as human therapy, agriculture, veterinary, scientific research [5].
Antioxidants play a major role in protecting the biological systems against oxidative stress, which is associated with development of many chronic diseases and disorders [6]. Antioxidants protect our body against free radicals that leads to various pathological conditions such as anaemia, asthma, arthritis, ischemia, inflammation, neurodegeneration etc. It also delays ageing process [7]. Due to the harmful side effect of synthetic antioxidant, the search for safe, nutritional and therapeutic natural antioxidant has increasingly demanded for future prospect [8]. On the other hand, pathogen, which creates major health problem by causing infectious diseases, has acquired resistance to the available antibiotics. In recent years, there has gained a great interest to discover new drugs especially from plants with a view to minimizing remarkable side effects associated with synthetic antimicrobials [9].
Moringa oleifera having multiple utilities is cultivated all over the world. Every part of Moringa is used for certain medicinal propose. Moreover, being an excellent source of protein, vitamins, oils, fatty acids, micro-macro minerals elements and various phenolics [10]. Both Aegle marmelos and Moringa oleifera plant have great potential to treat diseases like antimicrobial, peptic ulcer, inflammation, anticancer, hepatoprotective, antioxidant, cardio protective and many more [10] [11]. Cathranthus roseus, one of the best-studied medicinal plants, has immense medicinal value for its alkaloids. All parts of the plant including leaf, root, shoot and stem are used for therapeutic purposes against several diseases [12] [13]. Ageratum conyzoides, a herb with a long history, has been used as a traditional medicine in many countries, especially in the tropical and subtropical regions. Extracts and metabolites from this plant have been found to exert several pharmacological activities [14].
Therefore, the current study aimed to evaluate the phytochemical constituents, antioxidant, and antibacterial activity of mentioned plants of Sylhet region in Bangladesh.
MATERIALS AND METHODS
Collection of plant materials
Fresh leaves of Aegle Marmelos, Catharanthus roseus, Moringa oleifera and Ageratum conyzoids were collected from different location of Sylhet district and identified with great care by plant specialist in the department of plant and environmental biotechnology, Sylhet Agricultural University.
Preparation of crude extract
The leaves were sun dried for three days following their collection and blended using electric blender. 10 g of each powdered leaves were placed in conical flask and 200 ml of methanol was added and plugged with cotton and soaked with methanol for 72 hours at room temperature with continuous stirring. After 72 hours the supernatant was collected by filtration using clean cloth and Whatmann filter paper. Finally, the solvent was evaporated to make the crude extract which was considered as 100% concentrated extract and used for further analysis.
Phytochemical screening
The crude plant extracts were subjected to various biochemical tests for phytochemical analysis using standard procedures as described previously [15] [16].
Total Phenolic content
Folin-Ciocalteu reagent assay was conducted to determined total phenolic contents where gallic acid was used as standard active compound. In this assay, a volume of 1ml extract was added to 0.5 ml of 10 fold diluted Follin-Ciocalteu reagent followed by adding 1 ml Na2CO3 (7.5%) after 10 minutes and 4.5 ml distilled water to make reaction mixture. After 30 minutes of reaction occur, the absorbance was taken at 680 nm against reagent blank which was prepared by mixing all reagents except plant extract [16] [17].
Total flavonoid content
Total flavonoid content was determined by aluminium chloride containing colorimetric assay [18]. Briefly, 1ml extract or 1ml varying concentration of standard solution (12.5, 25, 50, 75, 100 μg/ml) mixed with equal volume (200 ul) of 10% Aluminium chloride and 1M potassium acetate (1:1), followed by adding distilled water. All the prepared solutions were filtered through Whatmann filter paper before measuring their absorbance. Similar manner was adopted to prepare sample blank by replacing aluminium chloride solution with distilled water. The absorbance was taken at 510 nm. The content was quantified from Standard calibration curve of quercetin and expressed in mg of quercetin equivalent (QE) per gram of dry extract.
Antioxidant activity
Varying concentration (25, 50, 75, 100 µg/ml) of standard or crude extract was made by serial dilution with methanol from stock solution where the concentration was 1 mg/ml. At a concentration of 0.004%, DPPH solution was freshly prepared by mixing with methanol solvent. The reaction mixture comprised with 1 ml crude extract or 1 ml standard, 3 ml DPPH solution and 1ml methanol. The reference standard compound was ascorbic acid, whereas 1 ml methanol added to 3 ml solution of DPPH was used as control. Blank was made with methanol. After incubating the reaction mixture in dark condition for 30 minutes at room temperature, the absorbance of control, crude extract and standard was measured at 517 nm [19] [20].
Free radical scavenging activity was expressed by using following formula:
%scavenging = [(A517CONTROL –A517SAMPLE)/A517CONTROL]×100
Where, A517CONTROL = Absorbance of DPPH solution
A517SAMPLE = Absorbance of sample
[The IC50 value means the concentration of extract to scavenge 50% of DPPH]
Antibacterial activity
Medicinal plants extract were subjected to a test of their antibacterial activities against five pathogenic bacteria based on agar disc diffusion method [21] [22]. The growth of test organism was maintained by sub culturing on nutrient broth and incubating overnight at 350 C. Gentamycin was used as positive control whereas methanol without plant extract was used as negative control. Methanol extract containing discs (5 mm in diameter) were placed on spread culture of bacterial agar plate subsequently plates were kept in an incubator at 350 C for incubation at least 24 hours.
Statistical analysis
All experiments were conducted three times. Linear Regression analysis was used to calculate IC50 values of antioxidant. All data were analysed using Microsoft Excel 2007 software.
RESULTS
Phytochemical screening of four different medicinal plants in methanol extracts
In this study, total 11 phytochemicals were screened from methanol extract of four medicinal plants, wherein flavonoid, tannin, sterol, phenol were present in all four medicinal plants. Saponins and terpenoids were detected in three extracts out of four. Quinone was present only in Aegle marmelos extract and coumarin was present in three extract except Aegle marmelos. Anthraquinones was present in Moringa oleifera whereas cardiac glycosides were absent in same extract out of four extract (Table 1).
The total phenolic contents and total flavonoids contents in four leaves extracts were examined in mg GAE/g respectively. The leaves of A. marmelos and M. oleifera contained highest amount of total phenolics, amounting 41.45 mg GAE/g and 36.51 mg GAE/g and the highest amount of total flavonoid content was exhibited in M. oleifera extract (15.11 mg QE/g) followed by A. marmelos extract having 11.37 mg QE/g (Table 2).
Table 1. Phytochemical profiling of four medicinal plants in methanol extract.
Table 2. Estimation of total phenolic and total flavonoid content in methanol extract.
Determination of antioxidant activity
Higher DPPH radical scavenging percentage and lower IC50 value indicates higher antioxidant activity, In Aegle marmelos, Moringa oleifera, Ageratum conyzoids and Catharanthus roseus leaves extract, the DPPH radical scavenging (%) were 67.14%, 57.14%, 42.86%, 35.71% and IC50 value were determined to be 70.83 μg/ml, 81.01 μg/ml, 117.03 μg/ml and 142.85 μg/ml respectively (Table 3). IC50 values of these four medicinal plants indicated that Aegle marmelos and Moringa oleifera had higher antioxidant activity and then a little bit low in Ageratum conyzoids, and lower antioxidant activity was recorded in Catharanthus roseus.
Table 3. Antioxidant activity of medicinal plant in MM extract.
Determination of antibacterial activity:
The highest antibacterial activity against S. aureus and Salmonella sp. was exhibited in Aegle marmelos, having zone of inhibition with 10.66 mm and 18.67 mm respectively, while C. roseus exhibited highest antibacterial efficacy against Pseudomonas sp. with zone of inihibition of 15 mm. As described by figure 1 and table 4, methanolic extract of Moringa oleifera exerted highest antibacterial activity against Klebsiella sp. followed by E. coli, having zone of inhibition of 12.67 mm and 11 mm respectively, whereas, overall, Ageratum conyzoids extract exerted lowest antibacterial efficacy.
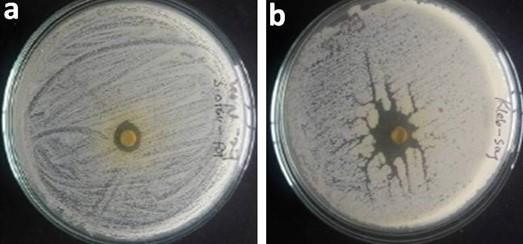
Table 4. Zone of inhibition of plant samples.
DISCUSSION
This research work was carried out on four selected medicinal plants which showed various phytochemical constituent’s i.e., terpenoids, flavonoids, alkaloids, phenols, cardiac glycosides, tannins , saponins and quinones were either present or absent in these plants. In previous studies it was reported that flavonoids and terpenoids were present in leaves extract of the A. marmelos, C. roseus, A. conyzoids that is similar with the present study result [23]. In case of alkaloids and tannins, the present research result and previous research results were slightly different, it might be due to the change in location and genetic variation and that’s why their genetic makeup was changed and showed the different results. It was observed that the antioxidant values were increased with increase in concentration of crude extracts which indicated that antioxidant values may be dependent on the presence of different
phytochemicals such as alkaloids, flavonoids, saponins, tannins etc. It has been determined that the antioxidant effect of plant products is mainly due to radical scavenging activity of phenolic compound [24]. It is well known that polyphenols are widely distributed in the plant kingdom and that they are sometimes present in high concentrations [25]. Since structural features of phenolic compounds are reportedly responsible for antioxidant activity, measurements of phenols in infusions may be related to their antioxidant properties [26].
In the present study, a colorimetric quantification of flavonoids with aluminum chloride was used, which has previously been described for the quantification of flavonoids in propolis extracts [27]. Medicinal plants are a very important and widely available resource for primary healthcare and complementary healthcare systems large numbers of plants are constantly being screened for their antibacterial effects [28]. All the plant extracts showed antibacterial activity against both Gram-positive and Gram-negative organisms and this was conformity with earlier findings that plant extracts have a significant scope to develop a novel broad spectrum of antibacterial herbal formulations [29]. The present study illustrated that Gram-negative bacteria were more susceptible to plant extract as compared to Gram-positive bacterial species. This is probably due to the differences in chemical composition and structure of cell wall of both types of microorganisms [30].
CONCLUSIONS
In conclusion, the present study revealed the presence of diverse active constituents in medicinal plants, including Catharanthus roseus, Moringa oleifera, Aegle marmelos and Ageratum conyzoids. Aegle marmelos and Moringa oleifera can be considered as promising resources for antioxidants and potent antimicrobials. Further study is needed to isolate chemical compounds responsible for such natural bioactivities and may lead to their use as safe alternatives to synthetic drugs.
ACKNOWLEDGEMENT
The authors would like to acknowledge Dean, Faculty of Biotechnology and Genetic Engineering, Sylhet Agricultural University and Chairman, Department of Biochemistry and Chemistry for allowing using the laboratory for conducting the experiment.
AUTHOR CONTRIBUTIONS
SRA, MMHK and MH designed the experiment. IJR, SRA, JA and RR performed the experiments; MH and SRA analyzed the data and wrote the draft. SRA and JA critically revised the manuscript. SRA contributed to drafting the article. JA and SRA contributed to revising it critically for important intellectual content.
CONFLICTS OF INTEREST
The author declares that no conflict of interest exists.
References
- [1]Bandyopadhyay U, Biswas K, Chattopadhyay I, Banerjee RK. Biological activities and medicinal properties of neem (Azadirachta indica). Currnt Sci. 2002; 82(11):1336-1345.
- [2]Mustafa G, Arif R, Atta A, Sharif S, Jamil A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Mat Sc Pharm. 2017; 1(1): 17-26.
- [3]Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med. 2009; 9: 30.
- [4]Edoga, HO, Okwu DE, Mbaebie BO. Phytochemicals constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005; 4(7): 685-688.
- [5]Vasu K, Goud JV, Suryam A, Singara Chary MA. Biomolecular and phytochemical analyses of three aquatic angiosperms. Afr J Microbiol Res. 2009; 3(8):418-421.
- [6]Abhishek RU, Mohana DC, Thippeswamy S, Manjunath K. Antioxidant properties of some selected Indian medicinal plants: Their correlation with total phenolic contents. Int J Green Pharm. 2013; 7(2): 117-121.
- [7]Polterait O. Antioxidant and free-radical Scavengers of Natural origin. Curr Org Chem. 1997; 1: 415-440.
- [8]Gupta VK, Sharma SK. Plants as Natural antioxidants. Nat Prod Rad. 2006; 5(4): 326-334
- [9]Cunha BA. Antibiotics side effects. Med Clin North Am. 2001; 85: 149 185.
- [10]Farooq F, Rai M , Tiwari A , Khan A A, Farooq S. Medicinal properties of Moringa oleifera: An overview of promising healer. J Med Plants Res. 2012; 6(27): 4368-4374.
- [11]Patel P K, Sahu J , Sahu L , Prajapati N K, Dubey B K. Aegle marmelos: A Review on its Medicinal Properties. Int J Pharm Phytopharmacol Res. 2012; 1(5): 332-341.
- [12]Van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004; 11: 607-628.
- [13]Kaushik S, Singh R, Monika T, Raghvendra G, Mishra K. An overview of Catharanthus roseus and medicinal properties of their metabolites against important diseases. Eur Acad Res, 2017; 2: 1237-1247.
- [14]Okunade A L. Ageratum conyzoides L. Asteraceae. Fitoterapia. 2002; 73: 1-16
- [15]Bhuiyan FR, Hasan M, Imran AS, Ahmed SR, Shanzana P,Marufatuzzahan. Antimicrobial activity screening for three Citrus pulp extract and phytochemical constituency profiling. J PharmacogPhytochem. 2019; 8(4): 157-161.
- [16]Ahmed SR, Roy R, Romi IJ, Hasan M, Bhuiyan MK, Khan MM. Phytochemical Screening, Antioxidant and Antibacterial Activity of Some Medicinal Plants Grown In Sylhet Region. IOSR J Pharm Biol Sci. 2019; 14(1): 26-37.6
- [17]Saeed N, Khan MR, Shabbir M. 2012. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilisleptophylla L. BMC Comp. Altern Med. 2012; 12: 221.
- [18]Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods.J Food Drug Anal.2012; 10: 178-182.
- [19]Susanti D, Sirat HM, Ahmad F, Ali RM, Aimi N. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricumL. Food Chem. 2007; 103: 710–716 .
- [20]Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod.2001; 64: 892–895.
- [21]Bauer AW, Kirby WM, Sheris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method.Am J ClinPathol.1966; 45:149–158.
- [22]Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Menthalongifolia L. ssp. longifolia. Food Chem. 2007; 103(4):1449-56.
- [23]Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000; 63(7): 1035-1042.
- [24]Lacine A, Erdi K, Yasin A, Zeyneb A, Mustafa K. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J Biol 2013; 20(3): 235–239.
- [25]Harbone JB, Mabry TJ. The flavonoids: advances in research. Chapman &Hall, New York, 1982.
- [26]Katalinic VM, Milos T, Kulisic, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006; 94: 550-557.
- [27]Chang C, Yang M, Wen H, Chern, Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002; 10: 178-182.
- [28]Raina R, Parwez S, Verma PK, Pankaj NK. Medicinal plants and their role in wound healing. Onl Vet J. 2008; 3(1): 21.
- [29]Mizanur R, Raquib S, Nigar SM, Tasneema I, Pravas CR. Antimicrobial activity of some medicinal plant extracts against Gram positive and Gram negative bacteria in Bangladesh. Asian J Med Biol Res. 2017; 3(4): 405-411.
- [30]Kumar U, Kumar K, Hindumathy CK. Study of antimicrobial activity of Rosa indica against gram positive and gram negative microorganisms. Int J Microbiol Res. 2012; 4(3): 182-185.