A comparative review on TLQP-21 receptors: rodent versus human
Abstract
Growing functional information regarding bioactive TLQP-21 has led to the ubiquitous demand for identification of receptors associated to the peptide, which resulted in the first wave of murine TLQP-21 receptors, gC1qR and C3AR1. gC1qR was identified as receptor of TLQP-21 using chemical crosslinking and monomeric avidin column purification following by MS analysis. TLQP-21 responsive CHO-K1 cells were used to search for its receptor, C3AR1. Putative GPCRs, which may partake in regulating intracellular biological functions induced by TLQP-21, were indexed after the CHO-K1 cellular transcriptome was sequenced using unbiased Genome Wide Sequencing. TLQP-21 binding in the cells were found to be reduced by the gene knockdown with the siRNAs targeting C3AR1. C3AR1 antagonist, SB290157 was shown to prohibit TLQP-21 activity in CHOK1 cells. But this finding was not demonstrable in human cell line. The differences of human TLQP-21 sequence with that of murine TLQP-21 explains the poor binding of the human orthologue with its corresponding receptor. This may suggest a different set of receptors when considering human and rodent variants of TLQP-21. The identification of HSPA8 as receptor was performed using affinity based chromatography and mass spectrometry from human SHSY-5Y cells. Molecular studies in silico revealed that the peptide binding pocket in HSPA8 is an appropriate fit for TLQP-21 docking. Cross-linking and FACS methods presented the TLQP-21 binding to cells from the SHSY-5Y line. The establishment of HSPA8 as a putative receptor for human TLQP-21 can be exploited to explore new horizon in diagnosis and therapies for VGF related human diseases.
INTRODUCTION
The term VGF (non-acronymic), derived from clonal selection in plate V of the nerve growth factor induced PC12 constructed cDNA library [1-2]. It is recognized a neurosecretory protein identified as a member of the extended granin protein family being ~68 kDa in size. The protein is originally described to be gene product induced by a nerve growth factor. It is mostly synthesised conditionally within the neuroendocrine cells and neurons themselves. The VGF sequence is cleaved by endoproteolytic enzymes about the paired R–Arginine and K–Lysine residues into a number of shorter peptides. They require a stimulation provided by regulations in the secretory pathway for release in vitro or in vivo. Current data suggests peptides derived from VGF participate in intercellular communication through vesiculation and secretion. This helps to explain the large range of VGF amalgamated biological activities. Among the aforementioned short peptides, many are identified as factors in pain modulation processes and sexual behaviour as well as other functions in the body such as mood affective pathways and energy balancing in vivo [3-5].
Further studies discover one of these derived peptides, TLQP-21, to be involved in numerous physiological roles. TLQP-21 plays a key role in energy expenditure [6-8], metabolic pathways [9], lipolysis [6], glucose-stimulated insulin secretion (GSIS) [10], nociception [11-13], blood pressure/hypertension regulation [14], gastric contractility [9, 15], regulating release of gastric acids [16-18], reproduction [19-20], stress [21-22], neuroprotective agent [23], anorexia [7-8] (Figure 1). To sum up: Out of the VGF derived peptides, the chronicle of the TLQP-21 is the most denoted to date. Even with a large augmentation of the significant ontological data, regarding TLQP-21 partaken biochemical processes, regrettably, we still very poorly understand the molecular interactions regarding TLQP-21. Identification of receptor of TLQP-21 could open new possibilities to search the molecular approaches of its physiological actions, and of pharmacological modulation thereof.
HUMAN VS RODENT TLQP-21 ANALOGOUS RECEPTOR(S)
Very little knowledge regarding the molecular mechanism of TLQP-21 in physicochemical systems has been generated even after over twenty years of inundated studies of its effects. Being one of the bioactive peptides of VGF, the elucidation of the functions of the peptide is under investigation but there is lacking of the information on the receptor-dependent or receptor independent signalling pathways [3-5].
TLQP-21 showcases stimulatory prolypolytic activities by associating to membranes in adipose tissues [6] and unique binding sites of TLQP-21 were revealed on living Chinese hamster ovary (CHO) cell lines with the aid of atomic force microcopy [24] but there was little information about the mechanisms of action of the peptide as well as about putative receptor that mediate the TLQP-21 effects. Complement component-3a receptor 1 (C3AR1) [25-26], and the head of C1q receptor (Gc1qR) [12] have been determined as possible murine TLQP-21 receptors.
gC1qR as rodent TLQP-21 receptor
gC1qR or C1QBP is the globular head region of the receptor for C1q, a complement component. Also denoted as p32, p33, HABP1 and TAP, gC1qR expresses as a ubiquitous protein which conducts multiligand binding. It is found in mitochondria [27], nuleaus, endoplasmic reticulum (ER) [28], cytosol [29] and cell surface [30]: Interacting with numerous ligands of cellular, bacterial and viral origin [31-33]. It has been observed to play important role in carcinogenesis [34], bacterial and viral infections [35], immune cell modulation and complement activation [31], adipogenesis [36] and pain modulation [12]. Recently, it has been validated as a receptor of murine TLQP-21 highlighting its role in pain modulation [12].
In order to search for the receptor, Chen et al., 2013 [12] used chemical cross-linking, affinity chromatography followed by mass spectrometry analysis: a hetero-bi-functional cross-linker, Sulfo-EMCS was conjugated with biotinylated TLQP-21 followed by crosslinking reaction between this peptide and membrane proteins obtained from whole brain and spinal cord of 6 weeks old female Wistar rat. A ~30 kDa protein was detected by Western blot, probing with HRP-conjugated Streptavidin, from the sample obtained after cross-linking reaction. Further experiment was performed by attaching the peptide to avidin monomeric column followed by incubation with three types of membrane protein fractions of post-natal day 4 rat forebrain: whole cell lysate, cytosolic and membrane & associated protein fractions. The elutants were analysed by silver-staining showing ~30 kDa protein which was identified as gC1qR after nanoLC-MS/MS analysis [3-12].
Further experiments showed that electroporation of siRNAs against gC1qR remarkably lowered the gC1qR level in macrophages. The siRNAs also reduced the quantity of macrophages responding to the peptide observed in live cell Ca2+ imaging. Macrophages Incubated with neutralizing gC1qR mAbs yielded a sufficient reduction of reaction with the peptide. Additionally, gC1qR antibodies were introduced to the nerve ligation site of model rats with induced partial ligation in sciatic nerves (PSNL). The experiment was conducted to apprehend the functionality of gC1qR in the pain modulation mechanisms which exhibited a drop in the punctuate mechanical threshold after antibody application. Again, the gC1qR antibody application caused delay of the inception of the hypersensitivity linked with PSNL [12]. All these results validate the gC1qR as a receptor that activates macrophages in response to TLQP-21 [3-12].
C3AR1 as a receptor of rodent TLQP-21
C3AR1 is a G protein-coupled receptor protein that binds both C3a (complement component-3a) and C4a (complement component-4a) though its official name implies that it only binds C3a [37]. Before discovery of its role as a receptor of rodent TLQP-21, its activity was thought to be involved in innate immune system showing roles in the complement system. But its roles are not limited only to complement system, its roles also found in neurogenesis [37], cancer [38] and hormone release from the pituitary [39]. Moreover, C3AR1 knockout mice were found to be resistant to obesity induction and high fat diet induced insulin suggesting its role in metabolism [40].
Hannedouche et al. 2013 [26] conducted studies on signaling activity in rodent cell lines, CHO-K1 and O-342, that led to the identification of its coupled receptor, C3AR1. CHO-K1 cells responded to TLQP-21 showing increase in intracellular calcium [24] though ATP priming was necessary to get a vigorous signal suggesting that the expected TLQP-21 receptor is not Gq- coupled GPCR (G-protein coupled receptor). cAMP or Inositol phosphates accumulation was not found in TLQP-21-induced signaling events in CHO-K1 cell line. Moreover, increased intracellular Ca2+ influx induced by TLQP-21 treatment was obstructed by pertussis toxin (PTX). The authors therefore concluded that it was highly likely that the G-protein mediated effects were to be G0 due to all the findings regarding PTX sensitivity and lack of cAMP modulation [26].
Subsequently, RNA sequencing was carried on both TLQP-21 responsive, CHO-K1 and TLQP-21 non-responsive, CCL39 cell line in order to have more informations on the molecular mechanisms of TLQP-21 induced actions. A total of 21 GPCRs were identified after unbiased genome-wide sequencing of the transcriptome that were speculated to bring about TLQP-21 mediated biological activity, considering that GPCRs expression would be more in CHO-K1 than CCL39 cell line. To confirm the identity of GPCR of TLQP-21, a panel of antagonists were used to inhibit the peptide response in CHO-K1 cell line. Out of the well characterized antagonists, SB290157 was proved as the most potential antagonist which was a C3AR1 antagonist [41]. In another TLQP-21 responsive cell line O-342, the antagonist SB290157 was tested to confirm that this cell line would demonstrate a homologous receptor like CHO-K1 that was further confirmed by qRT-PCR showing that O-342 cells express the C3AR1 [25-26].
To further validate C3AR1 to be a TLQP-21 receptor, siRNA screening was performed. A total 63 siRNAs were tested against all the 21 genes that were confirmed by the transcriptomic analysis earlier. The only gene knockdown was found to reduce the TLQP-21 mediated response consistently was siRNAs against C3AR1. Thus, C3AR1 was confirmed as a potential receptor for TLQP-21 in rodents using defined receptor antagonist and siRNA techniques. However, this finding was not renderable to the human receptor. For example, the authors tried to show the TLQP-21 signaling in HEK293 cells expressing the human C3AR1 but failed whereas in hamster and rat C3AR1, it was successful. This might be due to the fact that human TLQP-21 is remarkably different than rodent version [26] (see below, in details, about the difference between human and rodent version of the peptide). All these studies led the authors to conclude that there might be a receptor other than C3AR1 for human TLQP-21 [4-5].
Later on, Cero et al., 2014 [25] confirmed C3AR1 as a receptor of TLQP-21. By photo activated cross linking, the peptide was found to bind to a ~56 kDa (equivalent to molecular weight of C3AR1) protein in 3T3L1 and CHO cell lines. The same binding experiment was performed using SB290157, a C3AR1 antagonist [41] and the binding was found reduced in presence of the antagonist suggesting that SB290157 competes with C3AR1 for binding with TLQP-21. Furthermore, human C3AR1 transfected HTLA cells were assessed using β-arrestin recruitment assay in which added more credence to the interaction of TLQP-21 and C3AR1 as it demonstrated that TLQP-21 acts as an agonist for the human C3AR1 [42]: half maximal effective concentration (EC50) for mouse TLQP-21 was about 3 times lower than that of C3A while the EC50 for human TLQP-21 was about 22 times lower than that of C3A, suggesting that human and mouse version of the peptide show activity toward human C3AR1 with different potency [25-26].
Further experiments were targeted to find out the hot spots for biological function in the sequence of the peptide based on the bioassay for the peptide function of the contraction of the fundus strips in the gastrointestinal tract [15]. The peptide showed ~69% contraction stimulated by acetylcholine treatment at a dose of 3 μM of the peptide. Two truncated TLQP-21 peptide fragments were used, TLQP-11 (the C-terminus sequences with the first 11 amino acids) and HFHH-10 (the N-terminus sequences with the latter 11 amino acids) showing that TLQP-11 is completely inactive whereas HFHH-10 is modestly active demonstrating 2.6 %, 62%, 75% response at a dose of the peptide 3, 6, 10 μM, respectively while TLQP-11 showed zero response even at the dose of 10 μM). This suggests that hot spots in the peptide responsible for biological activity lies in the C termini, not in the N-termini. Accordingly, Ala scanning mutagenesis in the C-terminal of the peptide was performed to observe the biological effect of the mutated peptide. It was found that this mutation had no significant effects excepts P18A mutant, P19A and R21A that showed biological activity 65%, 19%, nil, respectively. Amidation of the capping of the C-terminal resulted in the complete loss of biological activity. Notably, in this experiment human TlQP-21 showed 20% response while rodent version of the peptide showed 100% activity as expected suggesting that human peptide shows five times less biological activity to the rodent receptor compared to the rodent version of the peptide [25].
Moreover, C3AR1 has been confirmed as a receptor of TLQP-21 pointing out the folding-upon-binding mechanism : TLQP-21 is intrinsically disordered but upon binding with its receptor C3AR1, it goes from disordered to ordered shift adopting α- helical conformations [25] in consistent with a molecular dynamics study exploring that the peptide is not absolutely random coil but it also possesses stretches of distinct structural region that adopts a very much compact globular structure stabilized by π-cationic interactions and several hydrogen bonding interactions, finally validating that the peptide exhibits very flexibility in solution and that three C-terminal residues, P19-R21, and the region A7-R9 shows strong helical tendency [43].
In fact, according to the report from Cero et al., 2014 [25] supplemented the finding of Hannedouche et al. 2013 [26] that there is possibly a receptor other than C3AR1 in humans, as human TLQP-21 is different from its rodent version sequence by four amino acids [22], highlighting the dissimilar biological function of human and rodent TLQP- 21[25]. Cero et al. 2014 [25] suggested that this finding might be connected with the human susceptibility to obesity due to less potency of human TLQP-21 toward receptor C3AR1 resulting in less lipolysis of adipocytes [6]. This could be one of the reasons of being more susceptible to obesity by human than other species [25]. All in all, considering all potential functions of TLQP-21 (as mentioned earlier), there should be another receptor in humans to which human version of the peptide will bind and potentiate 100% biological activity.
It is noteworthy that almost all of these works with TLQP-21 were performed with rat/mouse TLQP-21, except, so far it has been known, very few works that were performed by Hannedouche et al., 2013 [26] and Cero, et al., 2014 [25] with human TLQP-21; provided that due to the difference in biological activities between human and rodent TLQP-21 [25] (as discussed earlier) as well as due to the difference in respect of the sequences between human (TLQPPSALRRRHYHHALPPSR) and rodent version (TLQPPASSRRRHFHHALPPAR) of the peptide, it is highly expected that there is different receptor(s) for human as mentioned by Hannedouche et al., 2013 [26]. No doubt left, identification of the receptor of VGF-derived human peptide TLQP-21 will offer a very interesting pharmacological target to develop drugs for the treatment not only of obesity related disorders but also for improving the treatment for hypertension [14], stress [21-22], reproduction [19-20] and diabetes [10]. In addition, it should help understand the molecular mechanisms in human underlying these ailments [4-5].
It was proposed by Chen et al., 2013 [12] that both receptors, gC1qR and C3AR1 functions in response to TLQP-21, either simultaneously or sequentially as it has not yet been disclosed that whether the receptors interact each other or not, in response to the peptide. However, the functional and structural similarity between gC1qR and the peptide TLQP-21 is apparently absent [44]. Moreover, the possibility of the existence of the second receptor, in addition to C3AR1, in CHO or 3T3L1 cells is not supported by the recent and previous works [6, 24-26].
RECOGNITION OF HSPA8 RECEPTOR FOR HUMAN TLQP-21
71 kDa heat shock cognate protein A8, also nomenclated as HSPA8 is included in the heat shock protein (HSP70) family. It is expressed constant rate regardless of physiological demand but can be induced with exposure to stress. HSPA8 is centralized in the cytosolic and nuclear domain of the cell and performs a number of biochemical processes. It is also located in the cytoplasmic surfaces in membrane associated interactions, especially in oncocytes, pluripotent human ESCs and transformed B cells [45-48]. Rat neurocerebral studies have found evidence of its presence in the form of membrane rafts [49].
HSPA8 was recognized as a TLQP-21 receptor in human through affinity based chromatographic approaches followed by mass spectrometry. Cross-linking and FACS experiments established the TLQP-21–HSPA8 binding on SHSY-5Y cells which is in accordance with the previously confirmed profusion of HSPA8 and other HSPs on cellular surfaces observed by Pino et al., 2013; Shin, et al., 2003; Vega, et al., 2008; Altin and Pagler, 1995 [50-53]. Molecular modeling studies predict the docking of TLQP-21 into the HSPA8 peptide binding pocket withal [4-5].
Molecular dynamics study
A docking and simulation study, prompted by the identification of HSPA8 as a receptor of TLQP-21, was carried out concluding that the ligand human TLQP-21 directly fits into the receptor HSPA8.
Molecular dynamics simulation study explored the structural dynamics of TLQP-21 bound to the HSPA8 receptor binding site. The mechanism by which ligand-peptide recognition occurs between TLQP-21 and HSPA8 was investigated by designing a protein-peptide docking model. It was apparent that TLQP-21 shows distinct affinity towards the HSPA8 receptor β-sheet region. This enables strong binding of TLQP-21 to occur inside the substrate binding site while maintaining a distinct helical conformation. Hence, proper spatial fitting of ligand to receptor can be verified via molecular docking study. Furthermore, the formation of a stable ligand-receptor complex between TLQP-21 and HSPA8 can be proposed which is strongly supported by the fact that no dissociation was noticed while performing the simulation [4-5, 43].
Down regulation of HSPA8 expression via OMTR
FACS studies, as described before, confirmed that TLQP-21 binds to HSPA8 on the surface of SHSY-5Y cells. But when OMTR, an inhibitor of the expression of HSPA8 [54], was used; binding of TLQP-21 to the surface of cells decreased [4-5, 55-56], strongly suggesting that TLQP-21 binding was through HSPA8.
HSPA8 interacts with surface signaling molecules including CD14, CD40, CD91, TLR2/TLR4, CCR5, CD36, FEEL, Lox-1, SR-A, and SREC [57], initiating extracellular signal transduction cascades. HSPA8 also interacts with TLR2/TLR4, induce cytokine expression, TNF-α, IL- 1β, and IL-6, and finally activates p38MAPK, NF-κB pathways [58]. HSPA8 has been found to be an important signaling molecule interacting with a wide range of molecules, the molecular mechanisms involved remained largely elusive [4-5].
There are multiple evidences showing the ability of HSPA8 to interact with variety of ligands/binding partners of cellular, bacterial, and viral origin, illustrating their functional consequences downward, though in most cases they are still to be more clarified. Already HSPA8 has been known as a multifunctional and multiligand binding protein with multi-location. It also localizes to the cell surface for cell recognition by immune cells, even more directly performing as a receptor [59-64]. For example, the first ever demonstration of HSPA8’s involvement in cell protection from complement mediated damage showed that sublytic complement activation provoked the translocation of HSPA8 from the cytosol to the exoplasmic side of human erythroleukemia cellular plasma membrane, K562. Treatment with HSPA8 inhibitor, deoxyspergualin (DSG) sensitized K562 cells to complement lysis, on the other hand, treatment with HSPA 8 inducers like ethanol, butanol and hemin, shielded the cells from complement-activated disintegration. Moreover, HSPA8 was identified by monoclonal antibodies on intact, viable cellular periphery when complement stressed. All these data validated the role of HSPA8 in cell defense against complement as well as its translocation to the plasma membrane of the cells [4, 60].
The HSPA8 proteins were also found to interact with rat CD3+, CD4-, CD8-, T-cell receptor (TCR) alphabeta-, natural killer recetor-P1- T cells [62]. Moreover, the HSPA8 was found to perform directly as a receptor: Splicosomal U1-70K small nuclear ribonecleoprotein derived phosphopeptide designated as P140, exhibited participation in defensive mechanisms in lpr lupus-prone/MRL mice models. The lysosomal degradation pathway hosts a mechanism responsible for the plummet in the autoreactive T-cell differentiation, which was detected when analyzing the P140—HSPA8 interplay. This was promoted by the endogenous (auto)antigen presentation of B lymphocytes which affects the MRL/lpr APCs [61- 65].
With respect to the functions of membrane-associated HSPA8, HSPA8 localized on the cell surface was shown to act as a cellular receptor for HTLV-1 induced syncytium assembly [63]. Also, a peptide analogue phosphorylated on Ser140 termed peptide P140 was seen to bind ‘a unique cell surface receptor’, HSPA8 [61-65] through the HSPA8 N-terminal domain [66], and with the successful execution of phase II clinical trials—P140 is now considered a potential therapeutic intervention of systemic lupus erythematosus [67-68]; accordingly phase III clinical trials are ongoing [66].
HSPA8 possesses a peptide binding domain [69], a feature it shares with other members of the HSP70 family although each one exhibits exclusive and diversified peptide binding features [70]. When assessing a phage display peptide library, heptamers seems to have an amplifying capacity of the binding affinity of HSPA8 domain when compared with hexamers containing at least KK, KR or RR [71]. Interestingly, the peptide sequence of TLQP-21 contains RR sequence at the region R9- R10- R11 [4-5].
Further, a circular dichroism study has shown the conformational change of HSPA8 due to binding of a decapeptide [72]. HSPA8 has also been shown to bind proteins, such as the prion protein [73] or listeria specific adhesion protein, for which it acts as a receptor in caco-2 cells [74]. HSPA8 has been proved to react with phosphatidylserine on PC12 cellular surface [75]. Binding site has also been unveiled on HSPA8 for 15-deoxyspergualin, DSG [76].
It is also suggested that chaperones like HSPA8 function through ‘transient exposure’ of their ‘interactive surface’ (regions of intramolecular or intermolecular contact) [77] important in maintaining their interactions with other proteins [72].
RECEPTOR(S) FOR HUMAN VS RODENT TLQP-21
Both human and rodent version of the peptide contains basic sequence RR to which the binding affinity of the HSPA8 was found to increase considerably [71]. A strong correspondence with HSPA8 binding site was found for R-10-R11 of TLQP-21, which was explored through simulating molecular dynamics of receptor bound TLQP-21 [4].
However, to date most of the works focusing the characterization of TLQP-21 effects were based on rodent models, rather on human beings [78]. In fact, very few studies have been carried out with TLQP-21 using human cells. It is important to note here that for the first time Stephens et al., 2012 [10] showed the controlled TLQP-21 secretion from human islets and effect of TLQP-21 on human pancreatic islet cells was established by the same group. Later on, in 2013, Zhang et al [78] ascertained that in human umbilical vascular endothelial cells (HUVECs) TLQP-21 was capable of stopping apoptosis when the cells were treated with high glucose concentration; additionally they validated the signaling pathway in HUVECs: TLQP-21 uplifts the synthesis of NADPH and GSH (glutathione) in response to the boost up expression of G6PD (glucose-6-phosphate dehydrogenase), finally restoring the ROS (reactive oxygen species). All these data suggest that the peptide has effects in human cells but there might be different processing mechanism than rodents leading to a different receptor recognition in humans [26]. Recently, Cero et al., 2014 [25] exhibited how an S20A substitution in human TLQP-21 enable binding with human C3AR1 – but we observe that in comparison, rodent TLQP-21 have a higher binding tendency. Moreover, rodent TLQP-21 also surpasses their human orthologues by nearly five times more exertion, considering biological activity [26], upon interaction with the rodent C3AR1 receptor [25].
All these data support the existence of different receptors for human vs rodent TLQP-21 that will potentiate 100 % biological activity in humans. Molteni et al., 2018 [79] recently provided the evidence of having different receptors for TLQP-21 in rodent versus human cells.
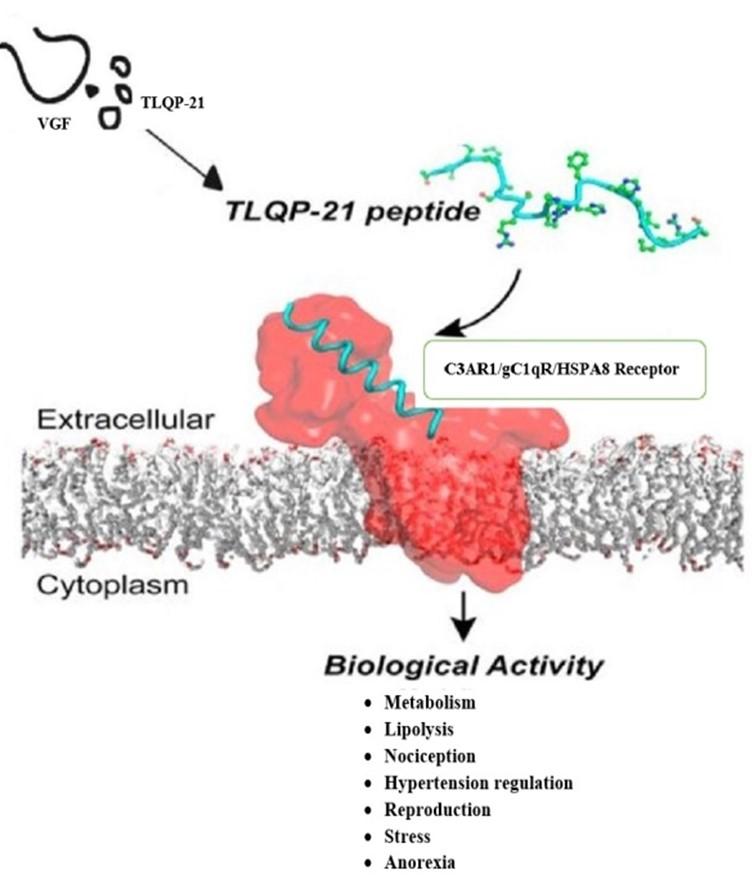
DOWNSTREAM EFFECTS OF HUMAN TLQP-21 BINDING TO ITS RECEPTOR
Until now, what is known about the downstream effects of binding of murine TLQP-21 to its receptors: TLQP-21 was found to increase the calcium levels in macrophages in a gC1qR dependent manner in rats [12]. The bioactivity of TLQP-21 was also reported to be GPCR-mediated in the CHO-K1 cell line. In CHO-K1 cells it was found that there is increase in intracellular calcium in response to TLQP-21, though to get strong signal ATP priming was required [26]. However, these outcomes were not possible to translate in human suggesting that human TLQP-21 might not work very well in those systems.
Since it is unknown that whether similar effects, as cited by Chen et al., 2013 [12] and Hannedouche et al., 2013 [26], would be exerted by human TLQP-21 in the experimental system of this study, a proteomic as well as phosphoproteomic study was carried out. Of note, it is also important to validate that TLQP-21 is indeed having a biological effect in the model used in this study. Further, this is a starting point and pioneer work for future studies regarding the binding of the human version of the peptide to its receptor HSPA8.
Till now, HSPA8 was shown to interact with variety of ligands/binding partners with its functional consequences downward. But in most cases they are still to be more clarified. HSPA8 was reported to interact with TLR2/TLR4, induce cytokine expression, TNF-α, IL- 1β, and IL-6, initiating p38MAPK, NF-κB pathways [58]. It was also found to interact with surface signaling molecules including CD14, CD40, CD91, TLR2/TLR4, CCR5, CD36, FEEL, Lox-1, SR-A, and SREC [57], commencing signal transductions [45]. HSPA8 found to provoke the autogenic (auto) antigen processing affecting antigen-presenting B cells, reducing autoreactive T-cell priming; via a signalling mechanism involving a lysosomal degradation pathway [61].
Both the proteomic and the phosphoproteomic study showed altered protein expressions. Further investigations are required to validate the result, also to confirm whether HSPA8 is involved in this altered protein expression or not. As a whole, further studies are required to clarify the overall downstream signaling pathways involved in the interaction of TLQP-21 and HSPA8. Also to be noted, the exploitation of HSPA8–TLQP-21 binding can only be achieved through extensive research to further enhance our interpretation of this ligand-receptor interplay, which is still in its early phase. In order to unravel the molecular channels associated with TLQP-21 activity in the intracellular domain, it is imperative that we establish clearer cognizance of the downstream consequences for TLQP-21 treatment of cells – apprehending the protein regulation mechanism of TLQP-21 in treated cells for qualitative and quantitative investigation [4-5]. Recently, stromal interaction molecule proteins and Ca+ channels were found to be involved in the intracellular pathways of TLQP-21 [79]. Regarding the pharmacokinetics of TLQP-21, Guo et al., 2018 and very recently, in 2019 Sahu et al. postulated that TLQP-21 acts on adipose tissue to promote lipolysis and, therefore, potentially a future medication for obesity therapies by reducing body fat in vivo via potentiating either the β-AR system [80] or adrenergic-receptor-induced lipolysis system [81], respectively.
FUTURE DIRECTIONS AND CONCLUSION
The major task moving forward is to elucidate the signaling pathways of the ligand TLQP-21 and its receptor HSPA8. Till to date, HSPA8 is the first putative receptor of TLQP-21 in human cells. Further advance exploration into the HSPA8-TLQP-21 interaction emphasizing, specially the downstream consequences is an essential need now. The mechanisms by which HSPA8 is released and acts later on after having bound with TLQP-21 will provide valuable information regarding different physiological processes as well as will provide viable therapeutic strategies for numbers of pathologic conditions treatment.
ACKNOWLEDGEMENT
This research received no external funding. The author is grateful to Jesus Rodriguez Requena, CIMUS Biomedical Research Institute, University of Santiago de Compostela-IDIS, Santiago de Compostela-15782, Spain for his supervision and support during the work.
CONFLICTS OF INTEREST
The author declares no conflict of interest.
References
- [1]Levi A, Eldridge JD, Paterson BM. Molecular cloning of a gene sequence regulated by nerve growth factor. Science 1985; 229(4711): 393-95.
- [2]Possenti R, Eldridge JD, Paterson BM, Grasso A, Levi A A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. EMBO J.; 1989; 8(8): 2217-23.
- [3]Ayub M. Investigating the mechanisms of action of VGF-derived peptides in the nervous system. 2012
- [4]Akhter S. Isolation of VGF derived neuropeptide receptor. 2015.
- [5]Akhter S, Chakraborty S, Moutinho D, AÂ lvarez-Coiradas E, Rosa I, Viñuela J. The human VGF-derived bioactive peptide TLQP-21 binds heat shock 71 kDa protein 8 (HSPA8) on the surface of SHSY-5Y cells. PLoS ONE 12(9):e0185176.
- [6]Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L, Riedl MS, Manieri M, Frontini A, Giordano A, Cinti S, Govoni P, Graiani G, Quaini F, Ghè C, Bresciani E, Bulgarelli I, Torsello A, Locatelli V, Sanghez V, Larsen BD, Petersen JS, Palanza P, Parmigiani S, Moles A, Levi A, Bartolomucci A. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012; 441 (1): 511-22.
- [7]Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JW, Carter WG, Bolton N, Bruggraber M, Morgan PJ, Barrett P.VGFderived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology 2007; 148: 4044-55.
- [8]Bartolomucci A, Corte GL, Possenti R, Locatelli V, Rigamonti AE, Torsello A, Bresciani E, Bulgarelli I, Rizzi R, Pavone F, D’Amato FR, Severini C, Mignogna G, Giorgi A, Schinina ME, Elia G, Brancia C, Ferri GL, Conti R, Ciani B, Pascucci T, Dell’Omo G, Muller EE, Levi A, Moles A. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity, Proc. Natl. Acad. Sci. U. S. A. 2006; 103 : 14584–89.
- [9]Bartolomucci A, Moles A, Levi A, Possenti R. Pathophysiological role of TLQP-21: gastrointestinal and metabolic functions. Eat Weight Disord. 2008; 13(3): e49-54. PMID: 19011364.
- [10]Stephens SB, Schisler JC, Hohmeier HE, An J, Sun AY, Pitt GS, Newgard CB. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 2012; 16 (1): 33-43. doi: 10.1016/j.cmet.2012.05.011.
- [11]Fairbanks CA, Peterson CD, Speltz RH, Riedl MS, Kitto KF, Dykstra JA, Braun PD, Sadahiro M, Salton SR, Vulchanova L. The VGF-derived peptide TLQP-21 contributes to inflammatory and nerve injury induced hypersensitivity. Pain 2014; 155: 1229–37.
- [12]Chen YC, Pristerá A, Ayub M, Swanwick RS, Karu K, Hamada Y, Rice AS, Okuse K. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J Biol Chem. 2013; 288 (48): 34638-46.
- [13]Rizzi R, Bartolomucci A, Moles A, D’Amato F, Sacerdote P, Levi A, La Corte G, Ciotti MT, Possenti R, Pavone F. The VGF-derived peptide TLQP-21: a new modulatory peptide for inflammatory pain. Neurosci Lett 2008; 441: 129–33.
- [14]Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin WJ, Cogliani V, Elste A, Mortillo S, Cero C. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J. 2014; 28: 2120–33.
- [15]Severini C, La Corte G, Improta G, Broccardo M, Agostini S, Petrella C, Sibilia V, Pagani F, Guidobono F, Bulgarelli I. In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. Br. J. Pharmacol. 2009; 157: 984-93.
- [16]Sibilia V, Pagani F, Bulgarelli I, Tulipano G, Possenti R, Guidobono F. Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a VGF-derived peptide, in rats. Amino Acids 2012; 42 (4): 1261-8. doi: 10.1007/s00726-010-0818-6. Epub 2010 Dec 4.
- [17]Sibilia V, Pagani F, Bulgarelli I, Mrak E, Broccardo M, Improta G, Severini C, Possenti R, Guidobono F. TLQP-21, a VGF-derived peptide, prevents ethanol-induced gastric lesions: insights into its mode of action. Neuroendocrinology 2010a; 92 (3): 189-97. doi: 10.1159/000319791.
- [18]Sibilia V, Pagani F, Bulgarelli I, Tulipano G, Possenti R, Guidobono F. Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a VGF-derived peptide, in rats. Amino Acids 2010b; 10.1007/s00726010-0818-6.
- [19]Aguilaera E, Pineda R, Gayta´n F, Sa´nchez-Garrido, MA Romero, M Romero-Ruiz, A Ruiz-Pino, F Tena-Sempere, M and Pinilla L. Characterization of the reproductive effects of the VGF-derived peptide TLQP-21 in female rats: in vivo and in vitro Neuroendocriology 2013; 98: 38–50.
- [20]Pinilla L, Pineda R, Gaytan F, Romero M, Garcia-Galiano D, Sanchez Garrido MA, Ruiz-Pino F, Tena-Sempere M, Aguilar E. Characterization of the reproductive effects of the anorexigenic VGF-derived peptide TLQP-21: in vivo and in vitro studies in male rats. Am. J. Physiol. Endocrinol. Metab. 2011; 300: 837-47.
- [21]Razzoli M, Bo E, Pascucci T, Pavone F, D’Amato FR, Cero C, Sanghez V, Dadomo H, Palanza P, Parmigiani S. Implication of the VGF derived peptide TLQP-21 in mouse acute and chronicstress responses. Behav. Brain Res. 2012; 229: 333–39.
- [22]Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011; 32 (6): 755-97.
- [23]Severini C, Ciotti MT, Biondini L, Quaresima S, Rinaldi AM, Levi A, Frank C, Possenti R.TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem 2008; 104: 534-44.
- [24]Cassina V, Torsello A, Tempestini A, Salerno D, Brogioli D, Tamiazzo L, Bresciani E, Martinez J, Fehrentz JA, Verdié P, Omeljaniuk RJ, Possenti R, Rizzi L, Locatelli V, Mantegazza F. Biophysical characterization of a binding site for TLQP-21, a naturally occurring peptide which induces resistance to obesity. 2013. Biochim Biophys Acta. 2013; (2): 455-60. doi: 10.1016/j.bbamem.2012.10.023. Epub 2012 Oct 30.
- [25]Cero C, Vostrikov VV, Verardi R, Severini C, Gopinath T, Braun PD, Sassano MF, Gurney A, Roth BL, Vulchanova L, Possenti R, Veglia G, Bartolomucci A. The TLQP-21 peptide activates the G-proteincoupled receptor C3aR1 via a folding-upon-binding mechanism. Structure; 2014; 22: 1744–53.
- [26]Hannedouche S, Beck V, Leighton-Davies J, Beibel M, Roma G, Oakeley EJ, Lannoy V, Bernard J, Hamon J, Barbieri S, Preuss I, Lasbennes MC, Sailer AW, Suply T, Seuwen K, Parker CN, Bassilana F. The identification of the C3a Receptor (C3AR1) as the target of the VGF derived peptide TLQP-21 in rodent cells. J Biol Chem. 2013; 20; 288(38): 27434-43. doi: 10.1074/jbc.M113.497214. Epub 2013 Aug 12.
- [27]Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. The multiligand-binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol 1998; 160: 3534-42.
- [28]Petersen-Mahrt SK, Estmer C, Ohrmalm C, Matthews DA, Russell WC, Akusjarvi G. The splicing factor-associated protein, p32, regulates RNA splicing by inhibiting ASF/SF2 RNA binding and phosphorylation. The EMBO journal 1999; 18: 1014-24.
- [29]Peterson KL, Zhang W, Lu PD, Keilbaugh SA, Peerschke EI, Ghebrehiwet B. The C1q-binding cell membrane proteins cC1q-R and gC1q-R are released from activated cells: subcellular distribution and immunochemical characterization. Clin Immunol Immunopathol 1997; 84: 17-26.
- [30]Peerschke EI, Ghebrehiwet B. The contribution of gC1qR/p33 in infection and inflammation. Immunobiology 2007; 212: 333-42.
- [31]Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost 2006; 4: 20352042.
- [32]Ghebrehiwet B, Lu PD, Zhang W, Lim BL, Eggleton P, Leigh LE, Reid KB, Peerschke EI.Identification of functional domains on gC1Q-R, a cell surface protein that binds to the globular “heads” of C1Q, using monoclonal antibodies and synthetic peptides. Hybridoma 1996; 15: 333-342.
- [33]Xu Z, Hirasawa A, Shinoura H, Tsujimoto G. Interaction of the alpha (1B)-adrenergic receptor with gC1q-R, a multifunctional protein. J. Biol. Chem. 1999; 274: 21149- 54.
- [34]Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EI. gC1qR/p33, a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection. Immunol Rev 2001; 180: 6577.
- [35]Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. 2004. Mol Immunol. 2004; 41(2-3):173-83.
- [36]Kim KB, Kim BW, Choo HJ, Kwon YC, Ahn BY, Choi JS, Lee JS, Ko YG. Proteome analysis of adipocyte lipid rafts reveals that gC1qR plays essential roles in adipogenesis and insulin signal transduction. Proteomics 2009; 9: 2373–82.
- [37]Klos A, Wende E, Wareham KJ, Monk PN. International Union of Pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 2013; 65 (1): 500–43.
- [38]Opstal-van Winden AW, Vermeulen RC, Peeters PH, Beijnen JH, van Gils CH. Early diagnostic protein biomarkers for breast cancer: how far have we come? Breast Cancer Res. Treat. 2012; 134: 1–12.
- [39]Francis K, Lewis BM, Akatsu H, Monk PN, Cain SA, Scanlon MF, Morgan BP, Ham J, Gasque P. Complement C3a receptors in the pituitary gland: a novel pathway by which an innate immune molecule releases hormones involved in the control of inflammation. FASEB J. 2003; 17: 2266–68.
- [40]Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, Gordon R, Thomas W, Lamb J, Schadt EE, Kennedy BP, Mancini JA. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes 2009; 58: 2006–17.
- [41]Ames RS, Lee D, Foley J, J Jurewicz, AJ Tornetta, MA Bautsch, W Settmacher, B Klos, A Erhard, KF Cousins, RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001; 15: 166 (10): 6341-8.
- [42]Lin H, Sassano MF, Roth BL, Shoichet BK. A pharmacological organization of G protein-coupled receptors. 2013.Nat Methods. 10(2): 140-6. doi: 10.1038/nmeth.2324.
- [43]Chakraborty S, Akhter S, Requena JR, Basu S. Probing the conformational dynamics of the bioactive peptide TLQP 21 in solution: A molecular dynamics study. Chem Biol Drug Des. 2015 86:938-44. doi: 10.1111/cbdd.12541.
- [44]Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003; 21 278(47): 46974-82
- [45]Liao Y, Tang L. The Critical Roles of HSC70 in Physiological and Pathological Processes. Curr Pharm Design. 2014; 20: 101-7.
- [46]Mambula SS, Calderwood SK. Heat Shock Protein 70 is secretedfrom tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol 2006; 177: 7849-57.
- [47]Kettner S, Kalthoff F, Graf P, Priller E, Kricek F, Lindley I, Schweighoffer T. EWI-2/CD316 is an induciblereceptor of HSPA8 on Human Dendritic Cells. Mol Cell Biol 2007; 27: 7718-26.
- [48]Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and Induces tumor-specific apoptosis. Cancer Cell 2008; 14: 250-62.
- [49]Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J. Neurosci. Res. 2005; 81: 522–29.
- [50]Pino MF, Zhang G, Rosser CJ, Goodison S, Urquidi V. Heat shock cognate protein HSPA8 (Hsc70) regulates migration and xenograft growth of breast tumor cell lines. [Abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6-10; Washington, DC. Philadelphia (PA): AACR; Cancer Res 73(8 Suppl): Abstract nr 3798.
- [51]Shin BK, Wang H, Marie A, Naour FL, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem. 2003; 278: 7607-16. Epub 2002 Dec 18.
- [52]Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 2008; 180: 6, 4299-4307.
- [53]Altin JG and Pagler EB. A one-step procedure for biotinylation and chemical cross-linking of lymphocyte surface and intracellular membrane-associated molecules. Anal Biochem. 1995; 224(1): 382-9.
- [54]Wang YP, Liu F, He HW, Han YX, Peng ZG, Li BW, You XF, Song DQ, Li ZR, Yu LY, Cen S, Hong B, Sun CH, Zhao LX, Kreiswirth B, Perlin D, Shao RG, Jiang JD. Heat stress cognate 70 host protein as a potential drug target against drug resistance in hepatitis B virus. Antimicrob Agents Chemother. 2010; 54(5):2070-7.
- [55]Akhter S, Requena JR Oxymatrine (an inhibitor of HSPA8) reduces binding of VGF derived bioactive peptide TLQP-21 to the surface of live SHSY-5Y cells. Khulna University Studies; 2017; 14 (1 & 2): 71-82.
- [56]Akhter S. A study on the effect of VGF derived bioactive peptide TLQP-21 on ERK/AKT signalling in SHSY-5Y cells. Proceedings of the International Conference on Bioinformatics and Biostatistics for Agriculture, Health and Environment of Department of Statistics, University of Rajshahi, Bangladesh and Bangladesh Bioinformatics and Computational Biology Association (BBCBA), 529-33.
- [57]Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett 2007; 581: 3689-94.
- [58]Zou N, Ao L, Cleveland JC Jr. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol 2008; 294: 2805-13.
- [59]Tsuboi N, Ishikawa M, Tamura Y, Takayama S, Tobioka H, Matsuura A, Hirayoshi K, Nagata K, Sato N, Kikuchi K. Monoclonal antibody specifically reacting against 73-kilodalton heat shock cognate protein: possible expression on mammalian cell surface. Hybridoma 1994; 13: 373-81.
- [60]Fishelson Z, Hochman I, Greene LE, Eisenberg E. Contribution of heat shock proteins to cell protection from complement-mediated lysis. Int Immunol 2001; 13: 983-91.
- [61]Page N, Gros F, Schall N, Décossas M, Bagnard D, Briand JP, Muller S. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann Rheum Dis2011; 70: 837-43.
- [62]Kishi A, Ichinohe T, Hirai I, Kamiguchi K, Tamura Y, Kinebuchi M, Torigoe T, Ichimiya S, Kondo N, Ishitani K. The cell surface expressed HSC70-like molecule preferentially reacts with the rat T-cell receptor Vdelta6 family. Immunogenetics 2001; 53: 401-9.
- [63]Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda. Y.71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J Virol 1998; 72: 535-41.
- [64]Guerrero CA, Moreno LP. Rotavirus receptor proteins Hsc70 and integrin αvβ3 are located in the lipid microdomains of animal intestinal cells. Acta Virol 2012; 56: 63-70.
- [65]Page N, Schall N, Strub JM, Quinternet M, Chaloin O, Décossas M, Cung MT, Dorsselaer AV, Briand JP, Muller S. The spliceosomal phosphopeptide P140 controls the lupus disease by interacting with the HSC70 protein and via a mechanism mediated by γδ T cells. PLoS ONE 4(4): 5273.
- [66]Stricher F, Macri C, Ruff M, Muller S.HSPA8/HSC70 chaperone protein Structure, function, and chemical targeting. Autophagy 2013; 9: (12), 1937–54.
- [67]Muller S, Monneaux F, Schall N, Rashkov RK, Oparanov BA, Wiesel P, Geiger JM, Zimmer R. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early phase II clinical trial.Arthritis Rheum 2008; 58: 3873 – 83.
- [68]Zimmer R, Scherbarth HR., Rillo OL, Gomez-Reino JJ, Muller S, Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis; 2013; 72: 1830 – 5.
- [69]Wang TF, Chmg J-H, Wang C. Identification of the peptide binding domain of hsc70, 18-kilodalton fragment located immediately after atpase domain is sufficient for high affinity binding. J Biol Chem 1993; 268: 35, 26049-51.
- [70]Fourie AM, Sambrook JF, Gething MJ. Common and divergent peptide binding specificities of hsp70 molecular chaperones. J Biol Chem.1994; 2: 269(48): 30470-8.
- [71]Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem. 1995; 270 (34): 19839-44.
- [72]Park K, Flynn GC, Rothman JE, Fasman GD. Conformational change of chaperone Hsc70 upon binding to a decapeptide: A circular dichroism study. Protein Sci 1993; 2: 325-30.
- [73]Wilkins S, Choglay AA, Chapple JP, van der Spuy J, Rhie A, Birkett CR, Cheetham ME. The binding of the molecular chaperone Hsc70 to the prion protein PrP is modulated by pH and copper. Int J Biochem Cell Biol. 2010; 42 (7): 1226-32.
- [74]Wampler JL, Kim K-P, Jaradat Z, Bhunia AK. Heat shock protein 60 acts as a receptor for the listeria adhesion protein in Caco-2 cells. Infect Immun. 2004; 72(2): 931–36.
- [74]Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. HSC70 and HSP70 interact with phosphatidyl serine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004; 18: 14, 1636-45.
- [76]Nadler SG, Dischino DD, Malacko AR, Cleaveland JS, Fujihara SM, Marquardt H. Identification of a binding site on Hsc70 for the immunosuppressant 15-deoxyspergualin. Biochem Biophys Res Commun.1998; 9: 253(1): 176-80.
- [77]Gatenby AA, Ellis RJ. Chaperone function: The assembly of ribulose bisphosphate carboxylase-oxygenase. Annu. Rev. Cell Biol. 1994; 6: 125-49.
- [78]Zhang W, Ni C, Sheng J, Hua Y, Ma J. TLQP-21 protects human umbilical vein endothelial cells against high-glucose-induced apoptosis by increasing G6PD expression. PLoS ONE 2013; 8(11): 79760.
- [79]Molteni L, Rizzi L, Bresciani E, Meanti R, Fehrentz J-A, Verdié P, Omeljaniuk RJ, Biagini G, Locatelli V and Torsello A. STIM Proteins and orai Ca2+ channels are involved in the intracellular pathways activated by TLQP-21 in RAW264.7 macrophages. Front. Pharmacol. 2018; 9: 1386.
- [80]Guo Z, Sahu BS, He R, Finan B, Cero C, Verardi R, Razzoli M, Veglia G, Di Marchi RD, Miles JM, Bartolomucci A. Clearancekinetics of the VGF-derivedneuropeptideTLQP-21. Neuropeptides 2018; 71:97- 103.
- [81]Sahu BS, Rodriguez P, Nguyen ME, Han R, Cero C, Razzoli M, Piaggi P, Laskowski LJ, Pavlicev M, Muglia L, Mahata SK, O’Grady S, McCorvy JD, Baier LJ, Sham YY, Bartolomucci A. Peptide/Receptor Co-evolution Explains the Lipolytic Function of the Neuropeptide TLQP-21. 2019; Cell Rep. 3; 28(10):2567-2580.e6.