Hypoglycemic and antidyslipidemic potential of Pleurotus ostreatus in streptozotocin-induced diabetic rats
Abstract
Oyster Mushroom (Pleurotusostreatus), an edible mushroom, is traditionally being used as a curing. However, hypoglycemic as well as antidyslipidemic effects on methanolic and ethyl acetate extracts of oyster mushroom in streptozotocin-treated diabetic rat model was not investigated yet. Here, we investigated that oral administration of 200 mg/kg oyster mushroom for 30 days were estimated through evaluating the fasting blood glucose level and blood lipid profile of diabetic rat. The results from our studies explained that P. ostreatusoyster mushroom considerably decreased blood glucose level initiated after treatment on second week. However, oyster mushroom was significantly reduced in plasma triglycerides (TG), total cholesterol (TC), as well as low-density lipoprotein-cholesterol (LDL-C). Additionally, high density lipoprotein-cholesterol (HDL-C) was enhanced by the treatment of oyster mushroom. Therefore, the current study suggests that P. ostreatus has antihyperglycemic and anti-dyslipidemic effect on streptozotocin-induce diabetic rat model.
INTRODUCTION
Diabetes mellitus (DM), a metabolic disease, is described as a hyperglycemia, damaged glucose, lipids, and proteins metabolism [1]. Generally, insulin deficiency causes this disorder [2]. Around 220 million people globally affects in this disease and this will be double in the year of 2030 [3]. Oxidative stress which cause lipid peroxidation as well as tissue injury comprising nephropathy, retinopathy, in addition exhibit coronary heart disease as well [4,5]. Additionally, hyperlipidemia or dyslipidemia are intricate in the improvement of cardiovascular problems with a major effect of mortality as well as morbidity [6]. However, traditional food constituent that may decrease glucose absorption or appetite in hepatic gluconeogenesis, intestine, body weight, blood glucose level, in addition to stimulate glucose prompted insulin secretion from pancreatic beta-cells might demonstrated for prevention as well as control of diabetes mellitus. Thus, a diversity of plants could be used in the administration and management of DM, even though their biologically active constituents are unknown yet [7].
To elucidate the effect of oyster mushroom, Pleurotusostreatus, on hyperglycemia as well as dyslipidemia in streptozotocin (STZ)-induced diabetic rat model is investigated in this studies. As we concern that mushrooms are less calories food but highly proteins enriched, carbohydrates, fibers, and vitamins for example, Vit-B complex, Vit-C and minerals [8-12]. From numerous studies it has been well known that the daily mushroom consumption is beneficial effect to health and is usually considered it as a functional foods [13-14]. It has been found that mushroom comprises several bioactive compounds for example proteins, polysaccharides, fats, flavonoids, alkaloids, terpenoids, nucleic acids etc, which show numerous biological activities with antioxidant [15-17], antitumor/anticancer [18], antimicrobial [19], immune-modulatory [20], anti-inflammatory [21-22], anti-atherogenic [23] and hypoglycemic actions [24]. For this, P.ostreatus is used as food and also ingredients in pharmaceuticals purposes.
In the present study, we examine methanolic and ethyl acetate extracts of oyster mushroom on hypoglycemic and antidyslipidemic effects in STZ-induced diabetic rat model.
MATERIALS AND METHODS
Chemicals
STZ as well as all other bio-diagnostic kits were purchased from Human (Germany).
Powder and extracts of oyster mushroom collection and preparation
Fruiting bodies of mushroom were collected from Chapainawabganj Horticulture center, and brought to University of Rajshahi, Dept. of Biochemistry and Molecular Biology to screen their pharmacological efficacy. Fresh mushrooms were washed and disinfected by treating with HgCl2 and washed again. The mushrooms were dried in shade under room temperature for eight to ten days, powdered with grinding machine and sieved. 100 gm of fine powder was separately dissolved in methanol (200 ml) and ethyl acetate (200 ml). The obtained extracts were filtered, concentrated and dried in a rotary flash evaporator maintained at 40°C for proper dehydration. The absolute dried extracts were preserved in separate air tight containers at a constant temperature 4˚C for further investigation.
Maintenance of experimental animal
Long Evan rats were selected as experimental animal to this study. Rat’s weight 185-200 gm was selected from Animal Resource Division, ICDDR’B Mohakhali, Dhaka, Bangladesh. Animals were maintained under standard laboratory conditions according to the guidelines of OECD at ambient temperature of 22 ± 3°C, and relative humidity at 50- 60%, with dark-light cycle of 12 hrs. They were fed with diet; those were made by our research group. Thirty (30) male rats were divided randomly into five groups. Individually rat was grouped with a permanent marker for tentative experimental usage, recording weight; five cages were retained in animal house. The animals were fed on standard laboratory diet with water and kept at room temperature. Rats were acclimatized to the laboratory conditions for one week before experimental works were undertaken. Care should be taken to evade traumatic situations as well as all processes were implemented 8 and 10 am morning. To conduct this study, ethical consent was taken from the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC) at the Institute of Biological Sciences of University of Rajshahi.
Preparation of standard drug solution for oral administration
About 5 mg of glibenclamide was suspended uniformly in 5 ml distilled water and mixed well with a vortex mixture. The drug was not completely dissolved but dispersed in water. This dispersed drug was fed orally once daily with the help of a dropper to the experimental rats at a dose 5 mg/kg. b.wt.
Acute toxicity studies
Numerous doses of mushroom extracts of 100, 200, 400, and 800 mg/kg.b.wt was orally administrated daily for 3 weeks. Rats were continuously monitored from 1 h, 6 h, 12 and 24 h up to three weeks. Toxicity of physical signs such as gasping, gross writhing, behavior, mortality, respiratory rate, and palpitation were followed by OECD guidelines [25]. However, there was no mortality observed during the whole period of experiment.
Induction of diabetes
5% solution of STZ (pH 4.5, 0.1 M sodium citrate buffer) was used to induce diabetes through intraperitoneally (i.p.) single dose injection in overnight fastening rats for 60 mg/kg.b.wt [20]. Diet as well as water consumptions were carefully checked daily after STZ treatment. After 10 days, glucose level was estimated from tail vein blood sample, by a Portable glucometer (Accu-Chek, Roche, Germany). Blood glucose level ≥ 8.0 mmol/L were considered with diabetes mellitus and other symptoms for example epolydipsia, polyphagia, weight loss, and polyuria were considered.
Experimental protocol
Experimental rats were randomly separated into 5 groups with 6 rats in respectively group where group I is normal and group II-V were diabetic groups (Table 1). Group I (Normal) and II (diabetic control) rats received only normal diet. Group III and IV (the extracts groups) diabetic rats received a single daily dose of 200 mg/kg.b.wt of the tested extracts of Pleurotusostreatus initial on the 11th day. This extract of 200 mg/kg.b.wt doses was chosen from an initial small term examination in our laboratory. In Group V (glibenclamide), rats with diabetic expected a distinct daily one dose of glibenclamide of 5 mg/kg.b.wt initial on the 11th day. Glibenclamide as well as experimental extracts were applied orally for 30 days.
Table 1. Grouping of experimental rats.
Collection of blood
Experimental rats blood were collected from cutting the tail of its edge on 0, 7th, 15th and 21th days after treatment. After the completion of treatment schedule, rats of all groups were weighed and sacrificed by using high dose of pentobarbital anesthesia (90 mg/kg.b.wt) injection in peritoneal area. Collected blood was placed into heparinized tubes at 4°C. After clotting, the blood samples were centrifuged at 8000 rpm, 4°C for 15 minutes to separate the plasma. Then the serum was separated into another Eppendorf tubes by micropipette. Finally, the serum was stored at -80°C for biochemical analysis.
Plasma glucose measurements
Plasma glucose levels were estimated via the glucose oxidase-peroxidase enzymatic colorimetric method according to the manufacture protocol (REF-1129005, protocols of LINEAR CHEMICALS, S.L.U, Spain) [26]. The commercially available diagnostic reagents were brought from Human Diagnostics, Germany and the blood glucose level was estimated through the semi-auto biochemistry analyzer (Humalyzer-3000, Germany). Reduces in percentage of glucose level in experimental rat was calculated via using the following formula:
Percent decrease in blood glucose level= (before-after treatment) x100/ (before treatment)
Plasma lipids profile measurements
Total cholesterol (TC), triglycerides (TG) and HDL-cholesterol (HDL-C) were evaluated by colorimetric method according to the protocol (REF-1118005, REF-1155005 and REF-1133010, respectively LINEAR CHEMICALS, S.L.U, Spain) [27]. Reagents were purchased from Human Diagnostics, Germany and all TC, TG, HDL-C of lipid profile were estimated through the semi-auto biochemistry analyzer (Humalyzer-3000, Germany). The decrease in percentage of TC, TG, HDL-C in experimental rat were calculated via the following formula.
Percent decrease in blood TC, TG, HDL-C level= (before-after treatment) x100/ (before treatment).
Blood LDL-Cholesterol level was determined by the following formula:
LDL Cholesterol =Total Cholesterol – (HDL-cholesterol+ Triglyceride/5)
Statistical analysis
Results were presented here as mean values as well as standard deviation (SD) and examined via with Scientific Package of Social Science (SPSS) version 17.0. Two diverse set of numbers were used for analytical and descriptive statistics. One-way analysis of variance (ANOVA) was used to test significance value of these entire experiments (p<0.01).
RESULTS
Pleurotusostreatus extracts reduced plasma glucose level of STZ-induced diabetic rat model
To investigate effects of oyster mushroom-induced plasma glucose level, here we employed to determine blood sugar level by glucose oxidase-peroxidase method [26]. The blood sugar level was compared in STZ induction mediated diabetic rats (Diabetic group) with methanol as well as ethyl acetate extracts of oyster mushroom treated groups shown significant reduction of blood glucose level (Table 2). However, methanol extract treated mouse exhibited 9.8% reduction on the 30th day compared to day 0, and a 48.71% reduction compared to the control values of diabetic group (P<0.01) (Table 2). On the other hand, ethyl acetate extract significantly reduced glucose level 14.56% on 30th day as compared to day 0, ae well as 50.85% (P<0.01) compared to the control of the diabetic group (Table 2). Moreover, glibenclamide reduced glucose level on 30th day 38.56% compared to day 0, and a 69.65% compared to control values of the diabetic group (P<0.01) (Table 2). Day 0, day 7, day 15, Day 21, and day 30 of oyster mushroom supplementation groups-maintained glucose levels 9.8%-50.85% lesser than the diabetic control group, while glibenclamide sustained 38.56%-69.65% lower than diabetic control group. Taken together, these data indicated that Pleurotusostreatus extracts decrease glucose level in diabetic.
Table 2. Effect of Oyster mushroom (Pleurotus ostreatus) extracts on serum glucose level in STZ-induced diabetic rats.
Effect of Pleurotusostreatus extracts on plasma lipid profile
Plasma lipid of total cholesterol (TC), HDL-cholesterol (HDL-C), and triglycerides (TG) were measured via spectrophotometrically by commercial kits. Friedewald formula was used to measure low density lipoprotein (LDL) [27]. Here, we found that diabetic rats displayed a significantly increased in blood the levels of TC, TG, as well as LDL-C. Conversely, reduction of HDL-C was significantly observed when compared to the values of normal control rats (Table 3). Moreover, Oral administration of glibenclamide significantly reduced blood levels of TC, TG and LDL-C. However, the level of HDL-C was significantly increased in glibenclamide treated mice (Table 3). The pretreatment with methanol, ethyl acetate extract and standard glibenclamide was significantly reduced the blood levels of TC (15.38%, 16.92% and 26%); TG (14.66%,17.33% and 29.33%) and LDL-C (16.6%, 17.3% and 20%) respectively. In particulate, the blood level of HDL-C was significantly augmented 15.38, 17.94% and 30.76% with methanol, ethyl acetate extract, as well as glibenclamide, respectively. These results suggested that Pleurotusostreatus extracts reduces TC, TG, and LDL-C level of blood lipid in diabetic.
Table 3. Effect of Pleurotus ostreatus extracts on total Cholesterol (C), TG, HDL-C and LDL-C level in STZ-induced diabetic rats.
DISCUSSION
The following investigation, we induced diabetes mellitus in rats over a lower dose of STZ administration which causes the β-cells destruction in islets of Langerhans [28-29], although the some rats develop permanently diabetic condition [30]. In our study, the results shows that the oral administration of methanol and ethyl acetate extracts oyster mushroom significantly decreases the elevated blood glucose level of experimental diabetic rats’ model as compare to controls groups. Even though, decrease glucose level is less than the glibenclamide standard drug treatment [31]. Moreover, lipids shows a dynamic role in diabetes mellitus pathogenesis. STZ-induced diabetes model enhance glucose level is complemented through enhance in plasma cholesterol, triglycerides, LDL-C and reductions in HDL-C [32], contributing to secondary complications of diabetes [33-34]. Under usual conditions, insulin triggers lipase enzyme which hydrolyzes triglycerides. Insulin enhances acceptance of fatty acids into adipose tissue and rises the synthesis of triglyceride compound. Furthermore, insulin prevents lipolysis. However, concentration of serum free fatty acids is raised consequently free fatty acid in adipose tissues of insulin-deficient diabetes, in where equilibrium of free fatty acids esterification-triglyceride lipolysis cycle is evacuated in lipolysis [35]. Therefore, an additional fatty acid in the plasma formed via STZ-treated diabetes encourages the change of extra fatty acids into phospholipids as well as cholesterol in liver. These two constituents accompanied by an additional triglycerides formed in the liver may be discharged into blood as a form of lipoproteins [36]. As we concern that HDL is an anti-atherogenic lipoprotein that carryings cholesterol from peripheral tissues into liver, also thereby performances as a defensive factor against coronary heart disease. In our results we found that diabetic containing rats shows a significant increasing TC, TG, as well as LDL-C, whereas HDL-C is reduced as well. Additionally, oral administration of Pleurotus ostreatus extracts results in reducing the plasma levels of TC, TG, and LDL-C along with increasing HDL-C level in diabetic rats. These study observed the dropping in plasma lipid in Pleurotus ostreatus treated diabetic rats which proposes that the potential mushroom.
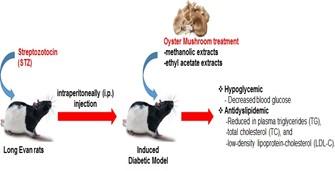
In conclusion, these findings demonstrate that orally administrated methanol as well as ethyl acetate extracts of oyster mushroom produces significant hypoglycemic and antidyslipidemic effects which lowers glucose level and total cholesterol in diabetic rat model. This study exposes that methanol and ethyl acetate extracts of Pleurotus ostreatus have potent antidiabetic and antidyslipidemic effects in diabetic rats (Figure 1). Therefore, additional studies are required to identify the exact chemical constituent of Pleurotus ostreatus responsible for these activities.
ACKNOWLEDGEMENT
The authors acknowledge the cooperation received from all associate concerned with this entire research work specially Department of Biochemistry and Molecular Biology and Department of Zoology of University of Rajshahi, and Department of Biochemistry and Biotechnology, Khwaja Yunus Ali University, Sirajganj, Bangladesh.
FUNDING
This research received no external funding.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
References
- [1]Scheen AJ. Drug treatment of non-insulin-dependent diabetes mellitus in the 1990s. Drugs. 1997; 54(3):355-68.
- [2]Vinik AI, Vinik E. Prevention of the complications of diabetes. American Journal of Managed Care. 2003; 9(3; SUPP): S63-80.
- [3]World Health Organization. Prevalence of diabetes worldwide. 2016
- [4]Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes. Diabetic medicine. 1991; 8(5):411-9.
- [5]Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radical Biology and Medicine. 1991; 10(5):339-52.
- [6]Reasner CA. Reducing cardiovascular complications of type 2 diabetes by targeting multiple risk factors. Journal of cardiovascular pharmacology. 2008; 52(2):136-44.
- [7]Tamil IG, Dineshkumar B, Nandhakumar M, Senthilkumar M, Mitra A. In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthusamarus. Indian journal of pharmacology. 2010; 42(5):280.
- [8]Manzi P, Gambelli L, Marconi S, Vivanti V, Pizzoferrato L. Nutrients in edible mushrooms: an inter-species comparative study. Food chemistry. 1999; 65(4):477-82.
- [9]Kues U, Liu Y. Fruiting body production in basidiomycete. Microbiol. Biotechnol.2000; 54(2):141–152.
- [10]Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finland. Journal of Agricultural and Food Chemistry. 2002; 50(22):6419-22.
- [11]Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricusblazeimurrill: review of literature and pharmaco-toxicological problems. Evidence-Based Complementary and Alternative Medicine. 2008; 5(1):3-15.
- [12]Mishra S, Singh RB. Effect of mushroom on the lipid profile, lipid peroxidation and liver functions of aging Swiss albino rats. Open Nutraceuticals J. 2010; 3:248-53.
- [13]Lakhanpal TN, Rana M. Medicinal and nutraceutical genetic resources of mushrooms. Plant Genetic Resources. 2005; 3(2):288-303.
- [14]Preeti A, Pushpa S, Sakshi S, Jyoti A. Antioxidant mushrooms: a review. Int Res J Pharmacy. 2012; 3(6):65-70.
- [15]Peralta RM, Oliveira AL, Eler GJ, Soares AA, Bracht A. Funcional properties of edible and medicinal mushrooms. Curr. Trends Microbiol. 2008; 4:45-60.
- [16]Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SM, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. Journal of agricultural and food chemistry. 2006; 54(26):9764-72.
- [17]Ferreira IC, Barros L, Abreu R. Antioxidants in wild mushrooms. Current Medicinal Chemistry. 2009; 16(12):1543-60.
- [18]Moradali MF, Mostafavi H, Ghods S, Hedjaroude GA. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). International immunopharmacology. 2007; 7(6):701-24.
- [19]Barros L, Baptista P, Estevinho LM, Ferreira IC. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. Journal of Agricultural and Food Chemistry. 2007; 55(21):8766-71.
- [20]Borchers AT, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity: an update. Experimental Biology and Medicine. 2004; 229(5):393-406.
- [21]Padilha MM, Avila AA, Sousa PJ, Cardoso LG, Perazzo FF, Carvalho JC. Anti-inflammatory activity of aqueous and alkaline extracts from mushrooms (AgaricusblazeiMurill). Journal of medicinal food. 2009; 12(2):359-64.
- [22]Moro C, Palacios I, Lozano M, D’Arrigo M, Guillamón E, Villares A, Martínez JA, García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chemistry. 2012; 130(2):350-5.
- [23]Mori K, Kobayashi C, Tomita T, Inatomi S, Ikeda M. Antiatherosclerotic effect of the edible mushrooms Pleurotuseryngii (Eringi), Grifolafrondosa (Maitake), and Hypsizygusmarmoreus (Bunashimeji) in apolipoprotein E–deficient mice. Nutrition Research. 2008; 28(5):335-42.
- [24]Hu SH, Wang JC, Lien JL, Liaw ET, Lee MY. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotuscitrinopileatus. Applied microbiology and biotechnology. 2006; 70(1):107-13.
- [25]Organisation for Economic Cooperation and Development (OECD). OECD guidelines for the testing of chemicals/section 4: Health Effects Test No. 423; Acute Oral Toxicity Acute Toxic Class Method. Pandit A, Sachdeva T, Bafna P. Drug- induced hepatotoxicity 2012. A review. J. Appl.Pharm. Sci.20042: 233–243.
- [26]Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of clinical Biochemistry. 1969; 6(1):24-7.
- [27]Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972; 18(6):499-502.
- [28]Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of biological chemistry. 1951; 193:265-75.
- [29]Fathallah N, Slim R, Larif S, Hmouda H, Salem CB. Drug-induced hyperglycaemia and diabetes. Drug safety. 2015; 38(12):1153-68.
- [30]Aybar MJ, Riera AN, Grau A, Sanchez SS. Hypoglycemic effect of the water extract of Smallantussonchifolius (yacon) leaves in normal and diabetic rats. Journal of ethnopharmacology. 2001; 74(2):125-32.
- [31]Cameron-Smith D, Habito R, Barnett M, Collier GR. Dietary guar gum improves insulin sensitivity in streptozotocin-induced diabetic rats. The Journal of nutrition. 1997; 127(2):359-64.
- [32]Mitra SK, Gopumadhavan S, Muralidhar TS, Anturlikar SD, Sujatha MB. Effect of D-400, a herbomineral preparation on lipid profile, glycated haemoglobin and glucose tolerance in streptozotocin induced diabetes in rats. Indian J Exp Biol. 1995; 33(10):798-800.
- [33]Palumbo PJ. Metformin. Effects on CardiovascularRisk Factors in Patients withNon–Insulin-Dependent Diabetes Mellitus. Journal of diabetes and its complications. 1998; 12(2):110-9.
- [34]Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes and coronary artery diseases. Indian J Med Res. 2002; 116:163–176.
- [35]Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. Journal of ethnopharmacology. 2004; 91(1):171-5.
- [36]Bopanna KN, Kannan J, Sushma G, Balaraman R, Rathod SP. Antidiabetic and antihyperlipaemic effects of neem seed kernel powder on alloxan diabetic rabbits. Indian journal of Pharmacology. 1997; 29(3):162.