Phytochemical screening and in vitro pharmacological activities of methanolic leaves extract of Caryota mitis
Abstract
The present study design to assess the qualitative phytochemical constituents responsible for anti-inflammatory, thrombolytic and cytotoxic activity of methanol extract of Caryota mitis leaves. The freshly collected Caryota mitis leaves extracted with methanol (MECM) to evaluate the secondary plant metabolites along with anti-inflammatory activity by protein denaturation assay. Thrombolytic and cytotoxic activity investigated using human blood clot lysis and brine shrimp lethality bioassay, respectively. The MECM exhibited several secondary plant metabolites named alkaloid, glycoside, protein, flavonoid, reducing sugar, saponins, and phenolic compound. The MECM extract (62.5-1000 μg/mL) exhibited an extremely significant (P < 0.0001) 6.06-45.45 % inhibition of protein denaturation against positive control diclofenac sodium (43.11-85.48%). In thrombolytic, the MECM exhibited 24.29 % significant (P < 0.0001) protection against blood clotting, whereas the positive control streptokinase (75.35%). In the cytotoxic study, a significant (P < 0.05) percentage of mortality exhibited while 70% mortality of Brine shrimp after 24 h at a concentration of 1000 μg/mL with an LC50 value of 550.57 μg/mL whereas the positive control vincristine sulfate (1.63 μg/mL). The current results suggesting MECM has promising anti-inflammatory, thrombolytic activities with weak cytotoxicity.
INTRODUCTION
Although the role of inflammation in the pathophysiology of arterial thrombosis has revealed in the last decade, the relationship between them is rarely known. Recently, inflammation has acknowledged as a potential system through which distinctive risk factors trigger thrombus formation in veins. A conventional activation pathway followed for inflammation and coagulation, whereas the compositions of thrombus in vein cause inflammation in the vessel wall. Activation of endothelial cells, platelets, and leucocytes leads to the development of thrombus, with the initiation of inflammation and development of microparticles that trigger the coagulation system through the enlistment of a tissue factor. In this manner, the critical start of venous thrombus development is most presumably vein wall inflammation [1]. C-receptive protein (CRP) is an acute stage reactant plasma protein that is available in plasma of healthy humans, whereas a significant plasma concentration increases at acute and chronic inflammation [2]. According to several reports, the CRP concentration independently associated with myocardial infarction [3]. Plasma CRP is inadequately associated with atherosclerosis [4]. According to the report, the CRP plays a vital role in the human body by regulating the blood function, platelets, the fibrinolytic system, etc. CRP dependent on inflammation and thrombosis is functioning in two directions due to activation of the blood clotting system (platelets), which regulates CRP structure and genetic function [5, 6].
The brine shrimp lethality bioassay for cytotoxicity is quick (24 h), basic (e.g., no aseptic strategies are required), easy to understand, reasonable, and requires limited quantities of test material [7]. The bioassay has a correlation with cytotoxic effect in solid tumors of humans and with pesticide activity [7, 8]. This test was recommended by (Michael et al. 1956) and changed by others. Since its recommendation, this in vivo lethality test has progressively utilized for giving a screening for the specific advanced bioassays once the active compounds have been isolated [9].
Traditional medicine has been utilized by most of the population for an extended period. The World Health Organization (WHO) stated that an expected 80 % of the people in a developing country relies upon medicinal plants for their essential therapeutic services [10]. Caryota mitis belongs to the Arecaceae family, which locally known as fishtail palm. These palm fruits and roots reported to use in folk medicine in Bangladesh and India as constipation, to stop vomiting, stomach aches, piles and arthritis [11, 12]. According to the literature review, the C. mitis exhibited antimicrobial and antioxidant activity [13, 14] and there is no report of thrombolytic, anti-inflammatory and cytotoxic activity. So, the present study proves the thrombolytic, anti-inflammatory and cytotoxic activity of methanolic extract of C. mitis leaves.
MATERIALS AND METHODS
Chemicals
The chemicals used in the experiment include methanol, sodium citrate, dextrose, citric acid, hydrochloric acid, sodium chloride, albumin, diclofenac sodium from Merck (Mumbai, India) through Taj Scientific Ltd. Bangladesh. UV visible spectrophotometer (Shimadzu, Japan) used to measure the absorbance. Lyophilized streptokinase vial (1500000 IU) obtained from Square Pharmaceuticals Ltd, Bangladesh, and Vincristine sulfate (1 mg/vial) was procured from Beacon Pharmaceuticals Ltd. Bangladesh.
Identification and preparation of plant extract
Leaves of C. mitis were gathered from Sitakunda, Chattogram and marking off done by Dr. Shaikh Bokhtear Uddin, Professor, Department of Botany, University of Chittagong, Bangladesh. 5 kg of leaves collected and separated all unwanted plant parts. The collected leaves dried for 15 days and dried into a coarse powder. For extraction, 500 gm of the powder submerged in 2.5 ml methanol and vigorously shaken for ten days. The plant extract filtrated by using Whatman filter paper (#1) followed by evaporation in a water bath (40 ºC). A greenish color of semisolid acquired with a percentage of 7.64 yields of methanolic extract of C. mitis leaves.
Phytochemical screening
The methanol extract of C. mitis leaves qualitative phytochemical screening was carried out by standard method to evaluate the alkaloid, carbohydrate, glycoside, protein, oxalate, cholesterol, steroid, reducing sugar, tannin, flavonoid, saponins and phenolic compound [15].
Inhibition of protein denaturation
The anti-inflammatory activity of methanolic extract of C. mitis (MECM) evaluated by albumin denaturation technique followed the previously described method with minor modifications [16, 17]. The test solution (0.5 mL) included test samples (0.05 mL) and 5% w/v aqueous solution of albumin (0.45 mL). The test control solution (0.5 mL) included of 0.45 mL of albumin solution (5% w/v aqueous solution) and 0.05 mL of distilled water. 5% albumin solution prepared by using, 5 gm albumin in 100 ml distilled water (w/v solution). Different concentrations (62.5, 125, 250, 500 and 1000 μg/mL) MECM and diclofenac sodium were taken. All solutions were adjusted to pH 6.3 using 1N HCl. The samples were incubated at 37 °C for 20 min and afterward kept at 57 °C for 30 min. After cooling, 2.5 mL of phosphate buffer was added and the turbidity measured at 416 nm in a UV-visible spectrophotometer. The control represents all the ingredients except the extract or standard solution. The percentage of inhibition of protein denaturation was calculated as follows:
Percentage inhibition of protein= [(Ac– As)/ Ac] ×100
Where Ac=absorbance of control and As=absorbance of sample.
Thrombolytic activity
Thrombolytic activity test was implemented by Prasad et al. [18, 19]. As a stock solution, lyophilized streptokinase vial (1500000 IU) mixed adequately with 5 mL sterile distilled water from which appropriate dilution made. Form healthy volunteers (n=5), 5mL of blood withdrawn by syringe and distributed 0.5 mL per pre-weighted eppendorf tube. At 37 °C for 45 minutes, the eppendorf tubes incubated for the formation of the clot. If any serum formed in the eppendorf tube along with clot removed the serum without disrupting the clot and again reweighed the eppendorf tube for calculating the clot weight where 100 µL plants extract of C. mitis (10 mg/mL) added. 100 µL streptokinase (positive control) and 100 µL distilled water (negative control) were added, respectively and incubated at 37 °C for 90 minutes. After incubation, observed clot lysis and released fluid was removed and reweighed the tube to calculate the difference in weight after clot disruption.
% of clot lysis = (weight of clot after remove of fluid/clot weight) × 100
Brine shrimp lethality bioassay
The cytotoxicity activity evaluated by brine shrimp lethality bioassay by using Artemia salina organisms (shrimp eggs). In the artificial seawater (3.8% NaCl solution), the shrimp eggs hatched for 48 hours for maturing the shrimp called nauplii. The extract serially diluted by using artificial seawater (3.8% NaCl in water) at concentrations of 31.25, 62.5, 125, 250, 500, and 1000 µg/mL. Vincristine sulfate used as a positive control, whereas a serial concentration dilution 0.25, 0.5, 1, 5, and 10 μg/mL used. Ten of the nauplii separately distributed in the premeasured concentration and observed for 24 hours. After 24 hours, count the number of living nauplii in each concentration and recorded [20, 21].
% of mortality = (N0-N1/N0) ×100
Where, N0= the number of nauplii taken; N1= the number of nauplii alive.
Statistical analysis
Results represented as the mean ± SEM (standard error mean). The intergroup comparison was performed using the GraphPad Prism 6 through t-test of one-way ANOVA. P-value (<0.0001) was considered statistically significant.
RESULTS
Phytochemical analysis revealed the presence of secondary metabolites
The qualitative phytochemical analysis revealed several secondary metabolites such as alkaloid, glycoside, protein, flavonoid, reducing sugar, saponins, and phenolic compound. The presence of carbohydrates also exhibited in the Molish test, but the Benedict test not shown carbohydrates (Table 1).
Table 1. Result of phytochemical screening of methanolic extract of C. mitis leaves.
Effect of the extract on protein denaturation in vitro
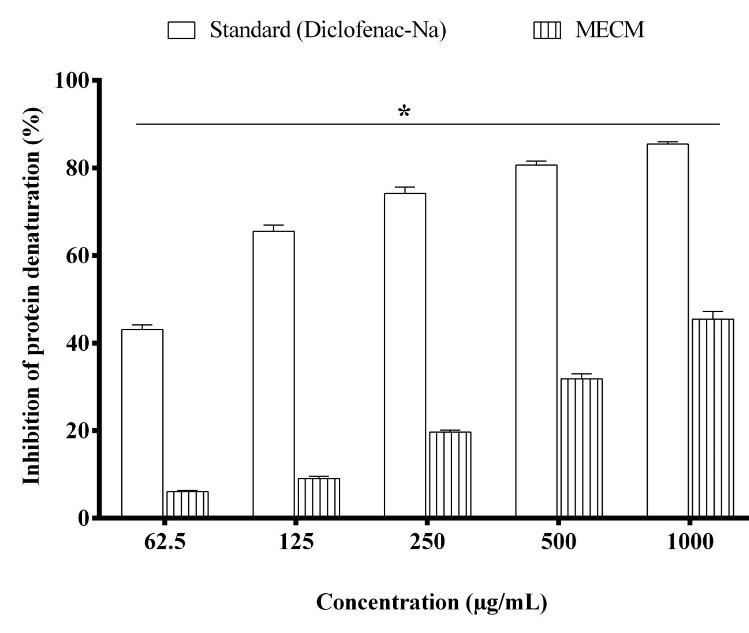
The anti-inflammatory activity evaluated by the protein denaturation method, which summarized in Figure 1. The result exhibited an extremely (P < 0.0001) significant inhibition of protein denaturation of C. mitis vs. positive control Diclofenac-Na. The anti-inflammatory activities showed a dose-dependent manner activity. The MECM showed 6.06 ± 0.29, 9.09 ± 0.47, 19.69 ± 0.39, 31.81 ± 1.16 and 45.45 ± 1.73 % inhibition of protein denaturation at 62.5, 125, 250 500 and 1000 μg/mL, respectively, whereas the positive control diclofenac-Na showed maximum 85.48 ± 0.52 % inhibition at a dose of 1000 μg/mL.
Effect of the extract on clot lysis activity
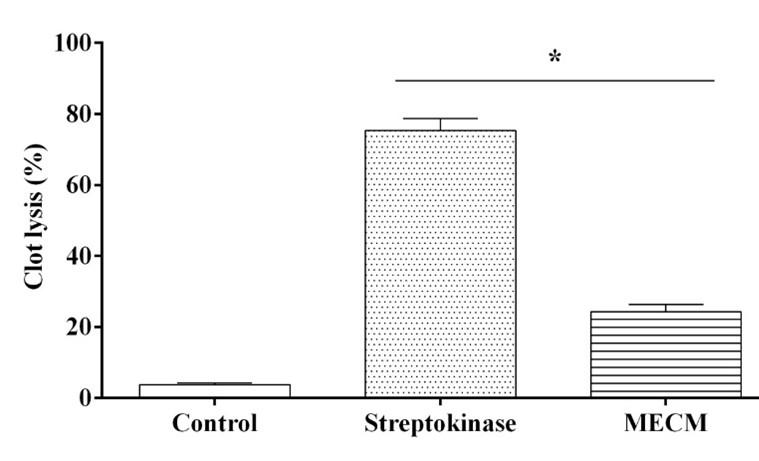
The results of the clot lysis activity of extracts summarized in Figure 2. The positive control Streptokinase showed an extremely significant (P < 0.0001) percentage of clot lysis 75.35 ± 3.38, whereas the negative control water exhibited negligible clot lysis (3.78 ± 0.53 %). After the treatment of clots with 100 µL MECM, a significant (P < 0.0001) 24.29 ± 1.99 % clot lysis observed.
Effect of the extract on the mortality of brine shrimp
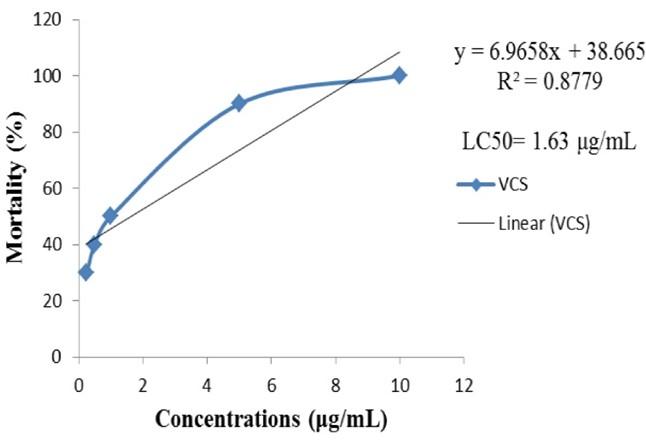
Brine shrimp lethality bioassay followed to evaluate the cytotoxicity, which results is summarized in Figure 3. The percent of mortality is significantly (P <0.05), decreasing with the lowering of concentration in both treatments. The LC50 values of MECM and vincristine sulfate (VCS) were 550.57 µg/mL and 1.63 µg/mL, respectively. MECM is weakly toxic because it exhibited the LC50 between 500-1000 μg/mL.
DISCUSSION
Medicinal plants are a potential source of bioactive compounds and have been utilized as a crude material or as pure compounds for treating different diseases [22]. The methanolic extract revealed the presence of alkaloid, glycoside, protein, flavonoid, reducing sugar, saponins, carbohydrates and phenolic compound. They may help to quantitative estimation and in finding the pharmacologically active substance by qualitative phytochemical tests, which are vital and helpful in discovering chemical constituents in the plant material.
Inflammation is a complex biological reaction of vascular tissue to harmful stimuli, pathogens, pain, warmness and swelling [23]. The prolongation of inflammation leads to atherosclerosis, fever and heart problems [24, 25]. Inflammation is well documented due to the denaturation of protein [26]. According to the literature report, denaturation of protein leads to rheumatoid arthritis because of the production of auto-antigens in arthritic diseases [27]. The Mechanism of denaturation is intricate in the modification of electric force, hydrogen, hydrophobic and disulfide bonds. Anti-inflammatory drugs reported having a dose-dependent ability to inhibit the protein denaturation [28]. From the results, the methanolic extract of C. mitis leaves capable of inhibiting protein denaturation, whereas the maximum 45.45 % inhibition exhibited at 1000 µg/mL while the positive control 85.48 %. The extract revealed a dose-dependent manner effect. This effect may be due to the presence of secondary metabolites such as alkaloid and flavonoid [29] and also validated the traditional use of C. mitis in folk medicine as arthritis [11, 12].
Thrombogenicity of the atherosclerotic plaque is resolved by the immobility of a fibrous cap and substance of tissue factor in its center, which initiates the coagulation when exposed to blood flow. These components connect with one another and with the blood vessel and under biological circumstances, the blood flow to tissues is unimpaired by coagulating or clotting [30]. Tissue plasminogen activator (tPA), streptokinase (SK) and urokinase-type plasminogen activator (uPA) are thrombolytic drugs available in the market which act by dissolving the blood clot formation by converting plasminogen to plasmin and increased clot lysis [31]. Our result suggests that the methanolic extract of C. mitis exhibited an extremely significant (P < 0.0001) clot lysis (24.29 %) compared to negative control water (3.78 %), whereas the positive control streptokinase (75.35 %). This effect may be due to the presence of flavonoids which affect embolus and cardiovascular disease by interfering with platelet activation [32] or due to the presence of phenolic compounds [31].
The brine shrimp test characterizes as fast, economical and basic bioassay for testing the plant extract lethality which connects the cytotoxicity and antitumor properties. Yet, a typical biological reaction isn’t because of one component instead because of several mixtures of bioactive plant components. Hence, the screening of plant extract is necessary to evaluate the biological activity. The brine shrimp lethality bioassay has demonstrated to be a helpful framework for checking the biological reaction of natural plant products [33]. The lethality of plant extracts directly proportional to the concentration with an LC50 of 550.57 μg/mL. This effect may be due to the presence of alkaloids indicates cytotoxic properties [34-48].
CONCLUSIONS
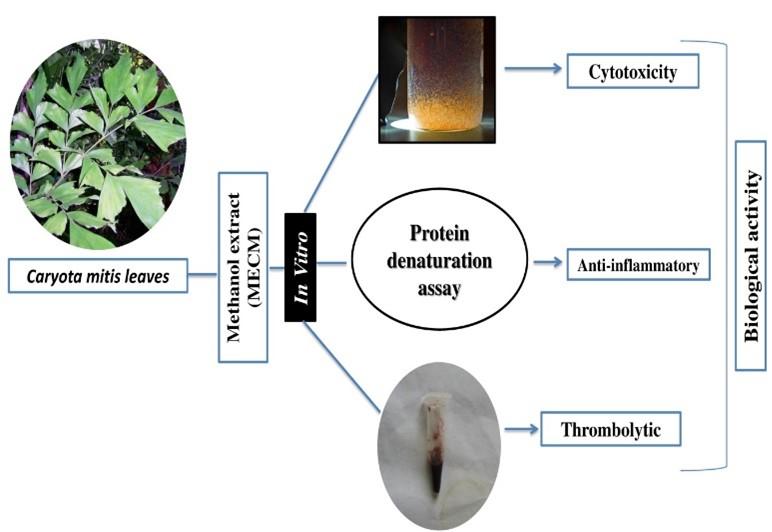
The present study suggests that the methanolic leaves extract of C. mitis exhibited secondary metabolites, which also exert weak toxicity. The methanolic extract of C. mitis leaves have shown an extremely significant concentration-dependent anti-inflammatory effect on bovine serum albumin. Moreover, the extract demonstrated a significant thrombolytic activity on human blood clot lysis. The present study of C. mitis concludes as the potential source for the potent bioactive substances, especially anti-inflammatory and clot lysis activities as summarized (Figure 4). Further advance study required to identify the mechanism for pharmacological properties.
ACKNOWLEDGEMENT
Authors are very much thankful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh for research facilities and other logistic supports.
FUNDING
This work is conducted with the individual funding of all authors.
AUTHOR CONTRIBUTIONS
MRT and AMT together planned and designed the research. MAS arranged the whole facilities for the research and supervised the whole research. IJ, SAS, MHM and MS conducted the entire laboratory works with MRT and AMT. AMT and TBE imparted in study design and interpreted the results putting efforts on statistical analysis and also participated in the manuscript draft and has thoroughly checked and revised the manuscript for necessary changes in format, grammar and English standard. All authors read and agreed on the final version of the manuscript.
CONFLICTS OF INTEREST
Authors declared that they have no conflict of interest.
References
- [1]Poredos P, Jezovnik MK. The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int Angiol. 2007;26:306-11.
- [2]Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-12.
- [3]Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17k-22k.
- [4]Zebrack JS, Muhlestein JB, Horne BD, Anderson JL. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol. 2002;39:632-7.
- [5]Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. Jama. 2001;286:64-70.
- [6]Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217-21.
- [7]Colegate SM, Molyneux RJ. Bioactive natural products: detection, isolation, and structural determination: CRC press; 2007.
- [8]Mclaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Information J. 1998;32:513-24.
- [9]Apu A, Muhit M, Tareq S, Pathan A, Jamaluddin A, Ahmed M. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica J Young Pharm. 2010;2:50-3.
- [10]Palhares RM, Gonçalves Drummond M, Dos Santos Alves Figueiredo Brasil B, Pereira Cosenza G, das Graças Lins Brandão M, Oliveira G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS One. 2015;10:e0127866-e.
- [11]Mollik AH, Hassan AI, Paul TK, Sintaha M, Khaleque HN, Noor FA, Nahar A, Seraj S, Jahan R, Chowdhury MH. A survey of medicinal plant usage by folk medicinal practitioners in two villages by the Rupsha River in Bagerhat district, Bangladesh. Am-Eura J Sustainable Agricult. 2010:349-57.
- [12]Sharief M. Plants folk medicine of Negrito tribes of Bay Islands. 2007.
- [13]Abdelhakim IA, El-Mokhtar MA, El-Baky AMA, Bishay DW. Chemical constituents and antimicrobial activity of the leaves of Caryota mitis (Arecaceae). J Med Plants. 2017;5:250-5.
- [14]Abdelhakim IA, Abdel-baky AM, Bishay DW. In vitro evaluation of antioxidant activity of Caryota mitis Leaves extracts. J Pharmacognosy Phytochem. 2017;6:2559-62.
- [15]Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veterinary Res Forum. 2014;5:95-100.
- [16]Juvekar A, Sakat S, Wankhede S, Juvekar M, N. Gambhire M. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculat a Linn Planta Med. 2009;75(9):12-19.
- [17]Uddin MJ, Ansari P, Rahman MM, Mamun A, Islam M, Hazrat M, Reza ASMA. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. J Basic Clin Physiol Pharmacol. 2016;28.
- [18]Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thrombosis J. 2006;4:14.
- [19]Rahman MA, Sultana R, Emran TB, Islam MS, Rahman MA, Chakma JS, Rashid H-u, Hasan CMM. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement Altern Med. 2013;13:25.
- [20]Hossain S, Kader G, Nikkon F, Yeasmin T. Cytotoxicity of the rhizome of medicinal plants. Asian Pacific J Trop Biomed. 2012;2:125-7.
- [21]Dash R, Emran TB, Paul A, Siddique MKU, Khan MA, Rahman MG, Sarwar MS, Nasir Uddin MM. Effects of five Bangladeshi plant extracts on in vitro thrombolysis and cytotoxicity. Pharmacognosy Res. 2016;8:176-80.
- [22]Dar SA, Yousuf A, Ganai FA, Sharma P, Kumar N, Singh R. Bioassay guided isolation and identification of anti-inflammatory and anti-microbial compounds from Urtica dioica(Urticaceae) leaves. African J Biotechnol. 2012;11:12910-20.
- [23]Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Practice & Res Clin Anaesthesiol. 2004;18:385-405.
- [24]Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. The Am J Med. 2008;121:S21-S31.
- [25]Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosomatic Res. 2002;52:1-23.
- [26]Umapathy E, Ndebia E, Meeme A, Adam B, Menziwa P, Nkeh-Chungag B, Iputo JJ. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plant Res. 2010;4:789-95.
- [27]Dey P, Chatterjee P, Chandra S, Bhattacharya S. Comparative in vitro evaluation of anti-inflammatory effects of aerial parts and roots from Mikania scandens. J Adv Pharm Educ Res. 2011;1:271-7.
- [28]Modi CM, Bhatt PR, Pandya KB, Patel HB, Patel UD. Comparative Evaluation of in vitro Anti-Inflammatory activity of different extracts of selected edicinal plants from Saurashtra Region, Gujarat, India. 2019;8:1686-98.
- [29]Shravan K, Kishore G, Siva K, Sindhu P. In vitro anti-inflammatory and anti-arthritic activity of leaves of Physalis angulata Int J Pharm Ind Res. 2011;1:211-3.
- [30]Ananyeva NM, Kouiavskaia DV, Shima M, Saenko EL. Intrinsic pathway of blood coagulation contributes to thrombogenicity of atherosclerotic plaque. Blood. 2002;99:4475-85.
- [31]Naderi GA, Asgary S, Jafarian A, Askari N, Behagh A, Aghdam RH. Fibrinolytic effects of Ginkgo biloba Exp Clin Cardiol. 2005;10:85-7.
- [32]Dwivedi S. Terminalia arjuna Wight & Arn.—a useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114:114-29.
- [33]Chanda S, Baravalia Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrima aerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat Prod Res. 2011;25:1955-64.
- [34]Wiart C. Medicinal plants of Asia and the Pacific: CRC Press; 2006.
- [35]Rakib A, Ahmed S, Islam MA, Haye A, Uddin SMN, Uddin MMN, Hossain MK, Paul A, Emran, TB. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico Food Sci Nutrition. 2020;8(1):547-556.
- [36]Shifah F, Tareq AM, Sayeed MA, Islam MN, Emran TB, Ullah MA, Mukit MA, Ullah M. Antidiarrheal, cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum J Adv Biotechnol Exp Therapeutics. 2020;3(1):77-83.
- [37]Dutta T, Paul A, Majumder M, Sultan RA and Emran TB. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem Biophy Rep. 2019;21:1-8.
- [38]Ahmed S, Rakib A, Islam MA, Khanam BH, Faiz FB, Paul A, Chy MNU, Bhuiya NMMA, Uddin MMN, Ullah SMA, Rahman MA and Emran TB. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin Phytosci. 2019;5:36.
- [39]Emran TB, Dash R, Uddin MMN, Rahman MA. Sedative, anxiolytic, antinociceptive, anti-inflammatory and antipyretic effects of a chloroform extract from the leaves of Urena sinuata (Borss) L. in rodents. J Appl Life Sci Int. 2018;16(3): 1-19.
- [40]Al Mahmud Z, Qais N, Bachar SC, Hasan CM, Emran TB and Uddin MMN (2017). Phytochemical investigations and antioxidant potential of leaf of Leea macrophylla (Roxb.). BMC Res Notes. 2017;10: 245.
- [41]Dash R, Ahsan MT, Hosen SMZ, Rahman MG, Emran TB and Uddin MMN. Evolution of selective COX-2 inhibitor from Alangium salvifolium: an in silico J Appl Pharm Sci. 2015;5(4):89-93.
- [42]Kabir MSH, Hossain MM, Kabir MI, Rahman MM, Hasanat A, Emran TB and Rahman MA. Phytochemical screening, antioxidant, thrombolytic, α-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia Koch leaves. J Young Pharmacists. 2016;8(4):391-397.
- [43]Uddin MMN, Zahan S, Islam MA, Ahmed S, Tajbiha-E-Mowla, Rahman MS, Sultan RA and Emran TB. Evaluation of the anti-diarrheal activity of methanol extract and its fractions of Urena sinuata (Borss) leaves. J Appl Pharm Sci. 2016;6(12):056-060.
- [44]Emran TB, Rahman MA, Uddin MMN, Rahman MM, Uddin MZ, Dash R and Layzu C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro BMC Complement Altern Med. 2015;15:128.
- [45]Emran TB, Rahman MA, Uddin MMN, Dash R, Hossen MF, Mohiuddin M and Alam MR. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against Staphylococcus aureus. DARU J Pharm Sci. 2015;23:26.
- [46]Momin MAM, Bellah SMF, Rahman SM, Rahman AA, Murshid GMM and Emran TB. Phytopharmacological evaluation of ethanol extract of Sida cordifolia roots. Asian Pac J Trop Biomed. 2014;4(1):18-24.
- [47]Biswas FB, Roy TG, Rahman MA, Emran TB. An in vitro antibacterial and antifungal effects of cadmium(II) complexes of hexamethyltetraazacyclotetradecadiene and isomers of its saturated analogue. Asian Pac J Trop Biomed. 2014;7(Suppl. 2):S534-S539.
- [48]Rahman MA Emran TB and Islam MS. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J Biol Sci. 2013;20:213-225.