Antibacterial and cytotoxic activities of four selected summer season fruits’ seeds of Bangladesh
Abstract
The seeds of fruits which are thrown away as waste can be used in medicinal and therapeutic purposes. The goal of this investigation was to find out antibacterial and cytotoxic potentialities of crude acetone and ethanol extracts of seeds of four common summer fruits, purchased from Khulna region, Bangladesh: Litchi chinensis (Lychee), Phoenix dactylifera (Date Palm), Syzigum cumini (Black Plum) and Artocarpus heterophyllus (Jackfruit). Extracts were screened for their antibacterial activity by agar well diffusion method, followed by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination by colorimetric broth macro dilution method and plating method. The highest antibacterial activity was found from acetone extract of S. cumini against Salmonella typhi where zone of inhibition was 19.67±0.27 mm along with MIC 0.0585 mg/ml and MBC 0.2344 mg/ml. Acetone extract of S. cumini was found to have bactericidal effect on all microorganisms except bacteriostatic on E. coli and S. paratyphi and ethanol extract showed bactericidal effect on all the microorganisms tested. The cytotoxic activity was determined by brine shrimp lethality assay. Among all seed samples, P. dactylifera exerted relatively better cytotoxicity where LC50 value was below 100 µg/ml. The rest of the samples showed relatively less toxicity as LC50 value was more than 100 µg/ml. Among all the seed samples, S. cumini showed most potential antibacterial activity with less cytotoxicity. Overall, the findings of the study endorse antibacterial activity of examined seed extracts with moderate cytotoxic activity, suggesting the isolation of active compounds thorough bioassays.
Keywords
INTRODUCTION
Natural sources are boundless inspiration for developing novel drugs [1]. Natural products do possess enormous diversity both structurally and chemically which are not similar to the library of artificial synthetic small molecules and encourage unique exploration in biochemical and medical sciences [2]. Plants are the simplest sources of natural products and medicines. Numbers of different biologically potent compounds can be produced by plants. Nearly 20% of plants known have been utilized in studies of pharmaceutics and therapeutics impacting the drug industry in progressive ways such as treating cancer and harmful diseases. Presently, according to the estimation of World Health Organization (WHO), eighty percent of the people worldwide use herbal medicine for different aspects of primary health care [3]. Many pharmaceutical companies are showing interest in ‘Green Medicine’, the drugs driven from plants mainly because of the faith of safety and acceptances compare to synthesized drug, which can possess conflicting effects [4].
Antibiotic drugs which have saved and still saving thousands of lives every year, “antibiotic resistance” could be a serious and one in every of the foremost threatening problems worldwide, including Bangladesh [5]. Antibiotics, the frequently recommended medicines in normal medical practicing but drug resistant diseases remain priority concern in public health issues [6]. Resistance to antibiotics and also the rise within the failure of obtainable chemotherapeutics are now resulting in the sorting out therapeutic floras for potential antibacterial, antiviral, antifungal, antiprotozoal and anticancer function, as well. Failure of available synthetic drugs to treat multiple drug resistant bacteria and undesirable side effects of chemotherapeutics suggest to findout alternative drug sources to overcome the problems [7-8]. Traditional medical practitioners in Bangladesh is an integral part of the culture of using plant or its parts as therapeutic agents, without any dependency on synthetic drugs: to them, plants driven compounds are the first choice.
With the advancement of medicinal technology, it’s now easier to spot specific botanical constituents and assess their potential activity. Conducting contemporary research dealing with local plant samples characterize the therapeutic values of plant derived constituents and evaluate their prospective potential activity of the available plants of a specific region. Pharmacological prospecting of plants by established method for the identification of effective compounds can lead to the development of novel medicinal agents. Bio prospecting of plants might show qualitative and quantitative variation of effective pharmacological ingredients due to difference in geographical location. In this favor, Bangladesh is a country full of biodiversity. Lots of plants of different regions are available having medicinal properties for the treatment of assorted diseases. However, scientific proofing are conducted to a limited extent with few medicinal plants [9]. About 5,700 species of angiosperms have been recorded from this country. But only about 500 species have documented scientifically as medicinal plants [10].
Out of all the summer season fruits of Bangladesh, the designated four fruits in this study were selected as they are worth mentioning due to their belongingness to the popular crowd favorites. Interestingly all these four fruits are grown and accordingly found in the market of Bangladesh for comparatively shorter period of time than the rest of the summer season fruits of Bangladesh. On the other hand, normally plant parts: leaf, bark, root, fruits, flowers are extensively tested in vitro and in vivo for their pharmacological profiling. Seeds of plants, more specifically, of ripe fruits that are thrown away as wastes can possess pharmacological activities, too. So, taken together all these into consideration, in this study, seeds of four summer season fruits: S. cumini, P. dactylifera, L. chinensis and A. heterophyllus of Bangladesh have been tested in vitro to evaluate preliminary antibacterial and cytotoxic activities.
MATERIALS AND METHODS
Seed collection
Fresh ripe fruits were purchased from local market of Khulna district of Bangladesh. Fruits were cut into pieces to extract out seeds from ripe fruits. After bringing out seeds of fruits, they were washed, and sun dried with shade to avoid dust.
Sample preparation and extraction
Seeds were grinded into fine powder. One hundred fifty gm of the powder were soaked into 500 ml of absolute acetone and ethanol solvents and kept in dark places for ten days with occasional shaking, followed by, filtration through Whatman filter paper, evaporation using rotary evaporator and air drying at room temperature. Amount and final color of the extracts were as illustrated in Table 1.
Table 1. Amount and color of extract obtained after ten days of soaking.
Antibacterial screening
Agar well diffusion assay
Bacteria were grown on Nutrient Agar Medium (yeast extract 2.0g, beef extract 1.0g, NaCl 5.0g, peptone 5.0g, agar 15g, and distilled water 1 L). Dilution with 105 CFU/ml was used as inoculum for the study. Molted agar medium was poured into petri dishes to obtain a depth of 10 mm. The inoculation of the entire surface area of the agar plate was done with 500 µl inoculum. Then, with the help of a disinfected cork borer four holes of 5 mm span were made by punching in aseptic condition. Fifty and hundred µl of sample was introduced into two holes: one kept blank and in another 5 µl of gentamycin (200 µg/ml) was added as positive control. For agar well diffusion test, sample was prepared at a concentration of 20 mg extract into 1 ml of acetone or ethanol. Agar plates were incubated, and inhibition zone was measured as per Balouiri et al., 2016 [11]. The following bacteria were used: Escherichia coli (American Type Culture Collection, ATCC-8739), Pseudomonas aeruginosa (ATCC-27833), Vibrio cholerae (ATCC-14035), Salmonella typhi (ATCC-14028), Salmonella paratyphi (ATCC-13311), Micrococcus spp. (ATCC-14396), and Staphylococcus aureus (ATCC-25923). The findings of the agar well diffusion assay were expressed in four categories depending on zone (diameter) of inhibition: designated as ‘not active’, ‘partially active’, ‘active’, and ‘very active’ when zone (diameter) of inhibition were <9 mm, 9 – 12 mm, 13 – 18 mm, >18 mm, respectfully [12].
MIC assay
The assay was performed following a modified method [13]: a mannitol phenol red broth was used. A stock solution of samples were prepared: Nine consecutive two fold dilutions of extract with 50% Dimethyl Sulfoxide to obtain stock concentrations of 50 mg/ml to 0.195 mg/ml. One-tenth ml of stock sample from previously prepared was mixed into 0.4 ml broth. For nine different concentrations, nine different tubes were used respectively to obtain final concentrations of 10 mg/ml to 0.039 mg/ml. Now into each test tube, 10µl of freshly prepared bacterial culture was nurtured at 37ºC overnight. As positive control, Gentamycin was used at a concentration from 10 to 0.039 µg/ml. As negative control, test tubes without sample but with bacterial culture were used. MIC was tested for those microorganisms and sample for which zone of inhibition was 13 mm or above.
MBC assay
This method is required to identify the concentration at which 100% microorganisms will be killed. 10 µl of broth from test tubes from where MIC was considered were plated in nutrient agar plate and incubated to see the growth.
MIC (MBC/MIC) indexing
The MIC index was recorded to find out whether an extract is bactericidal (MBC/MIC <4) or not. The range of MIC index value greater than 4 but less than 32 were considered as bacteriostatic [8].
Cytotoxic activity testing
To ascertain the cytotoxic activity of an extract, the brine shrimp lethality test is acknowledged as standard [14]. For the experiment, 5mg of each extract were dissolved in 1ml of seawater and one drop Tween-80; adjusted to a final concentration of 5μg/μl. Then 4ml of seawater was given to each of the test tubes. From the stock solution specific volumes of 5, 10, 20, 40, 80, 160 and 320 μl of samples were poured into the test tubes; adjusted to 10 ml with saline water to get the final concentration of 2.5, 5, 10, 20, 40, 80, 160 μg/ml, respectively. Finally, 10 live brine shrimp nauplii were taken into each test tube [14]. As positive control, vincristine sulfate was used. Following the mortality count, after 24 hours; log concentration versus percent mortality were plotted and median lethal concentration (LC50) were calculated.
Of note, the findings of the agar well diffusion assay were studied in Microsoft Excel 2013. In cytotoxic activity testing, LC50 values were also calculated by using Microsoft Excel 2013. Each test was conducted three times.
RESULTS
Effect on antibacterial activity
The zone of inhibition for 100 µl/well is higher, as expected, than 50 µl/well (Figure 1 and 2). The topmost finding was obtained from S. cumini acetone extract against S. typhi having zone of inhibition for 100µl/well as 19.67±0.27 mm (Figure 1) with MIC and MBC value 0.0585mg/ml and 0.2344 mg/ml respectively (Table 2, 3, 4 and 5; Figure 1, 2 and 3). But from MIC index we found that acetone extract of S. cumini was found to have bactericidal effect on all microorganisms except bacteriostatic on E. coli and S.paratyphi and ethanol extract showed bactericidal effect on all the microorganisms tested (Table 5). Acetone extract of P. dactylifera showed highest zone 16.67±0.27 mm against S. aureus with MIC and MBC value of 1.25 and 5 mg/ml respectively. Zone of inhibition of P. dactylifera acetone extract was in between 13 – 18 mm for all the tests except S. paratyphi. Again, ethanol extract of P. dactylifera showed highest zone 15.67±0.27mm against P. aeruginosa with MIC and MBC value of 1.25 and 7.5 mg/ml, respectively.
Acetone extract of L. chinensis was found active against all the microorganisms tested and found very active against S. aureus (Table 2, 3 and 4). Ethanol extract of L. chinensis was found active against P. aeruginosa, Micrococcus spp., E. coli and S. aureus, and partially active against rest of the tested bacteria. Acetone extract of A. heterophyllus was found partially active against all the tested bacteria except P. aeruginosa (14.67±0.98 mm) and Micrococcus spp. (14.67±0.27 mm). Ethanol extract of A. heterophyllus was found partially active against all the tested bacteria except V. cholera.
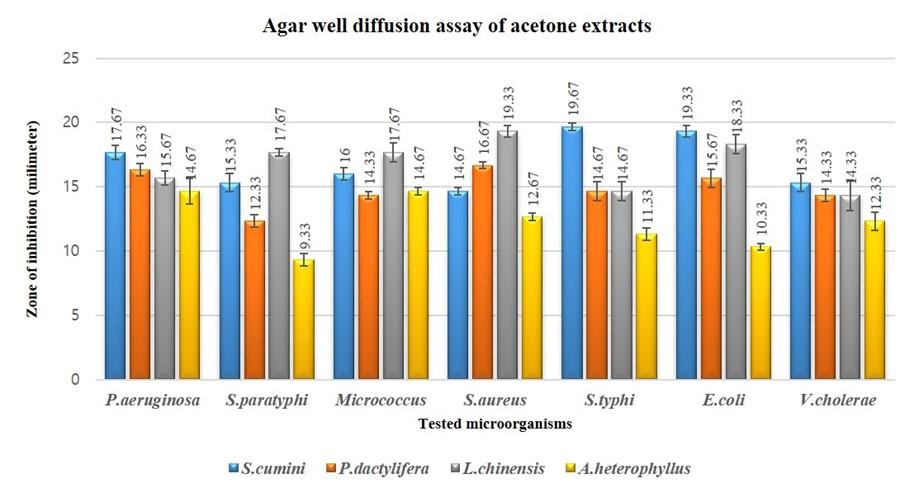
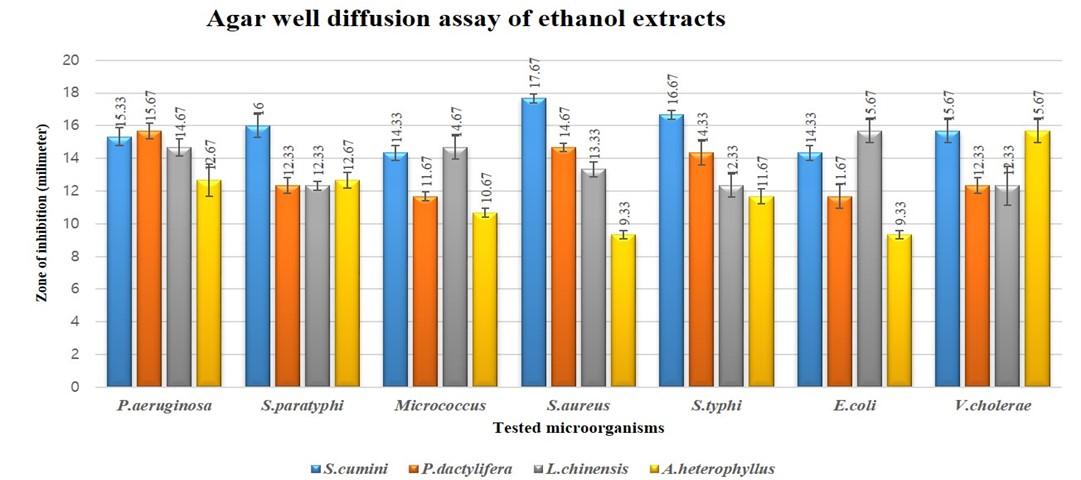
Table 2. Zone of inhibition of the samples against gram positive and gram-negative bacteria.
Table 3. Minimum inhibitory concentration of tested samples against different microorganisms.
Table 4. Minimum bactericidal concentration of tested samples against different microorganisms.
Table 5. MIC Index (MBC/MIC) of tested samples against different microorganisms.
Effect on cytotoxic activity by brine shrimp lethality test
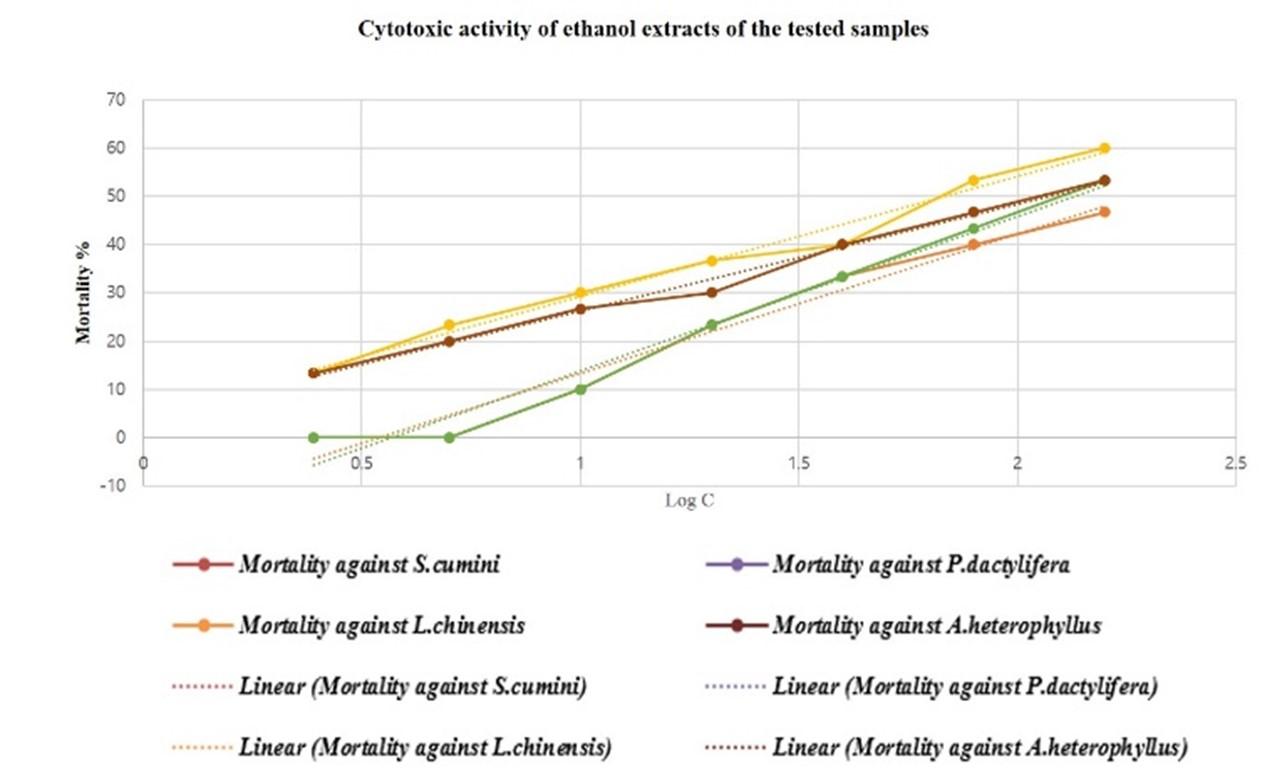
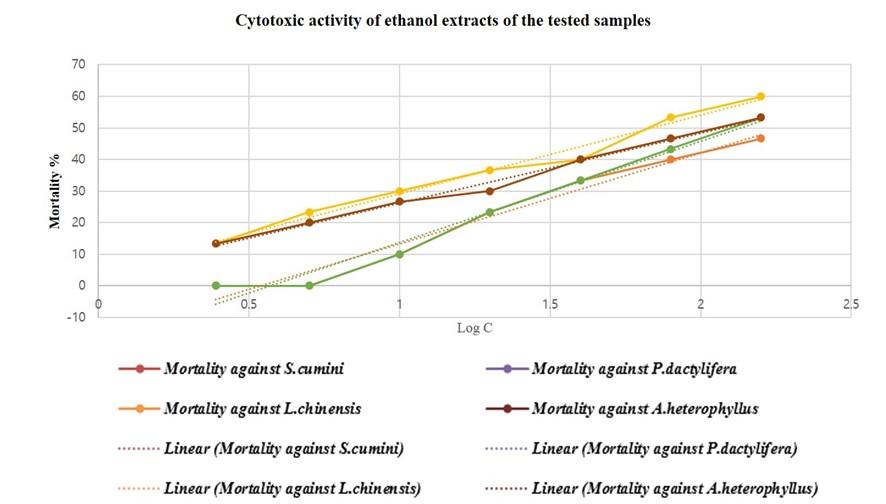
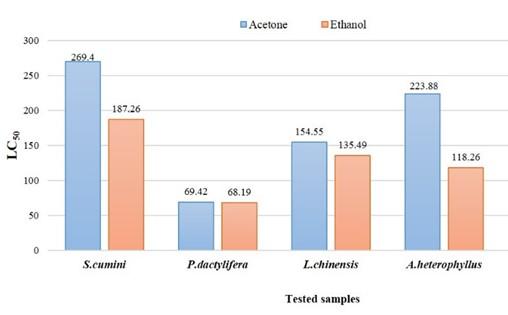
Extracts exhibit LC50 ≤ 30 µg/ml have significant cytotoxic activities, LC50 ˃30 and LC50 ≤100 possess mild toxicity and LC50 ˃ 100 can be considered low toxic [14]. Only seeds of P. dactylifera showed mild cytotoxicity where LC50 values of acetone extracts and ethanol extracts were 69.42 and 68.19 µg/ml respectively. All the other samples were found relatively less toxic as LC50 value more than 100 µg/ml (Figure 4, 5 and 6). The LC50 values were obtained (Figure 6) by plotting log concentration vs percent shrimp mortality (Figure 4 and 5) into MS Excel and generating linear regression equation: Y = a + bX, Y – the dependent variable, a – the y-intercept, b – the slope of the line, and X – the independent/ explanatory variable. The dotted lines in the graph were plotted to produce regression equation for calculating LC50 values (Figure 4 and 5). The values in Figure 6 were obtained from the regression equation as per plot of Figure 4 and 5.
DISCUSSION
Among all the samples, S. cumini was found more active compared to other samples. The best result obtained from acetone extract of S. cumini against S. typhi and E. coli (Figure 1 and 3). Our study, in accordance with another previous study where the MIC value of the seed extract against E. coli, P. aeruginosa was as 6.25 mg/ml whereas against S. aureus MIC value was 3.13 mg/ml [15] suggests antibacterial potentialities of seeds of S. cumini. Seeds of L. chinensis are used in different countries of Asia for the treatment of bacterial infection and the related diseases [16]. Our study supports them and provides scientific prove as both acetone and ethanol extract exhibited good antibacterial function. Our study, in consistent with another previous study of ours [17] confirms antibacterial potentialities of seeds of P. dactylifera. Seeds of A. heterophyllus was found to be active against V. cholerae as the ethanolic extract exhibited bactericidal effects. Thus, it can be used to treat cholera diseases. A study mentioned that extract from the seeds are useful in the treatment of diarrhea [18-19]. Our study also suggests that seeds of A. heterophyllus can be a source of remedy for severe watery diarrhea. The difference of the quality and quantity of plant ingredients, responsible for the bioactivity could be because of the diversified secondary metabolites (either constitutive or inducible: might function as antibacterial and cytotoxic agent) present within the extracts and the solvents used [20]. Different types of polar solvents possess different ranges of soluble capacity for various phytoconstituents. Plant and/or plant parts act as the profound packs or reservoir of secondary metabolites [21-22].
In the study we found two trends of results: in some cases, extracts were found potential against gram positive bacteria and in other cases extracts were found more active against gram negative bacteria. Structure of the membrane of gram-positive bacteria includes low capacity of buffering against protonation generated by plant compounds. Thus, intra-cellular space of bacteria become highly acidified, lessening metabolic energy generation of bacteria. The outer polysaccharide layer consisting of lipid and extra components of minor membranes provide greater capability of buffering to gram negative bacteria, which function as protective wall against hydrophobic compounds. Therefore, gram negative bacteria are lesser sensitive to antibacterial properties. Here, gram negative bacteria showed more susceptibility than gram positive bacteria. The compound that can efficiently fuse the bilayer of lipid with increased fluid passing capability via cellular membrane is considered potential to show the activity as antibacterial agent [23, 24, 25].
Resistance to antibiotic as a huge concern for global public health [26]. In a study conducted in one referral center in 2005 a whole of 57% of S. typhi strains isolated were MDR (multi drug resistant) and NAR (nalidaxic acid resistant) [27]. Methicillin-resistant S. aureus could also be a significant cause of human infections. It is a most wanted explanation for bacteremia, infective endocarditis, osteoarticular, pleuropulmonary, and device-related infections [28]. As antibiotic resistance is a significant problem worldwide, a replacement source of treating bacterial infections could also be a crying need. In line with our study, seeds of the four common summer fruits could be a potential source to isolate bioactive compounds [29-32] that might heal the multi-drug resistant problems worldwide. As a whole, all the four samples can be a candidate for extracting antibacterial and cytotoxic bioactive agents from them.
CONCLUSIONS
Seeds of many plants are utilized by traditional healers as drug and therapeutics to treat different diseases and infections. But most of the times seeds of plants are used only for sowing purposes, more importantly to be noted, sometimes seeds are thrown away as wastes, rather than utilizing or understanding their potentiality as bioactive compounds. More research is needed to evaluate the potentiality of seeds in this regard. In this in vitro study of seeds of four common summer fruits of Bangladesh, provide scientific footing to enhance confidence on the traditional claims of seeds. The antibacterial and cytotoxic activity of tested seed extracts recommends further isolation, purification and characterization of active constituents through bioassays, in-vivo trials and other state-of art technology, leading to sort out the outcome of the effects of bioactive compounds and its role as therapeutics and pharmaceuticals in health care system, as well.
ACKNOWLEDGEMENT
The authors express thanks to Khulna University Research Cell (KURC), Khulna University, Bangladesh for providing the grant to conduct this fundamental research. The authors also express thanks to their respective institute: Khulna University, Bangladesh.
AUTHOR CONTRIBUTIONS
MSA was involved in conception and design of the experiments. SAR contributed to perform the experiments. SAR and MSA analyzed data and contributed to drafting the article. SAR and MSA contributed to revising it critically for important intellectual content. MSA made the final approval of the version to be published.
CONFLICTS OF INTEREST
Authors declared that they have no conflict of interest.
References
- [1]Li F, Wang Y, Li D, Chen Y, Dou QP. Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin Drug Discov. 2019; 14: 417–20. doi:10.1080/17460441.2019.1582639.
- [2]Blockley A, Elliott D, Roberts A, Sweet M. Symbiotic Microbes from Marine Invertebrates: Driving a New Era of Natural Product Drug Discovery. Diversity 2017; 9: 49. doi:10.3390/d9040049.
- [3]Al-Snafi AE. Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger and Hyoscyamus reticulates)-A review. IOSR J. Pharm. 2018; 8:6:18-32.
- [4]Shah Z, Shafi S. Diabetes the Global Economic Burden of Adults and the Role of Herbalism as a safe & altenative cost-effective therapy. An Updated Overview. J. drug deliv. ther. 2019; 9:3: 954-961. doi:10.22270/jddt.v9i3-s.3085.
- [5]Sutradhar KB, Saha A, Huda NH, Uddin, R. Irrational use of antibiotics and antibiotic resistance in southern rural Bangladesh: perspectives from both the physicians and patients. Annu Res Rev Biol. 2014; 1421-1430. doi:10.9734/ARRB/2014/8184.
- [6]Faiz MA, Basher A. Antimicrobial resistance: Bangladesh experience. In Regional Health Forum 2011; 15:1:1-8.
- [7]Datir SS. Plant Metabolites as New Leads to Anticancer Drug Discovery: Approaches and Challenges. In Anticancer Plants: Natural Products and Biotechnological Implements 2018; 141-161. doi:10.1007/978-981-10-8064-7_7.
- [8]Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Potala S, Verma RS. Antibacterial activity of plants used in Indian herbal medicine. Int. J. Green Pharm. 2010; 4:1. doi:10.22377/ijgp.v4i1.114.
- [9]Rahman MS, Begum B, Chowdhury R, Rahman KM, Rashid MA. Preliminary cytotoxicity screening of some medicinal plants of Bangladesh. Dhaka Univ. J. Pharm. Sci. 2008; 7:1: 47-52. doi:10.3329/dujps.v7i1.1217.
- [10]Mukul SA, Biswas SR, Rashid AM. Biodiversity in Bangladesh. In Global Biodiversity, Volume 1: Selected Countries in Asia 2018; 93-107.
- [11]Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Pharm. Anal. 2016; 6:2:71-79. doi:10.1016/j.jpha.2015.11.005.
- [12]Mariselvam R, Ranjitsingh AJA, Selvakumar PM, Krishnamoorthy, R, Alshatwi Eco friendly natural dyes from Syzygium cumini (L) (Jambolan) fruit seed endosperm and to preparation of antimicrobial fabric and their washing properties. Fibers Polym. 2017; 18:3: 460-464. doi:10.1007/s12221-017-1097-6.
- [13]Tippett LO, Zeleznick LD, Robb CA. Modification of the microtiter technique for antimicrobial drug susceptibility testing by incorporation of indicators. Appl. Environ. Microbiol. 1970; 20:3:342-345.
- [14]Meyer BN, Ferrign, RN, Putnam, JE, Jacobson, LB, Nicholas, DE, McLaughlin, JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982; 45:31-34.
- [15]Banerjee J, Narendhirakannan RT. Phytochemical analyses, antibacterial, in vitro antioxidant and cytotoxic activities of ethanolic extract of Syzygium cumini (L.) seed extract. Int. J. Pharm. Sci. 2011; 2:7: 1799.
- [16]Ibrahim SR, Mohamed GA. Litchi chinensis: medicinal uses, phytochemistry, and pharmacology. J Ethnopharmacol. 2015; 174:492-513. doi:10.1016/j.jep.2015.08.054.
- [17]Aamir J, Kumari A, Khan MN, Medam, SK. Evaluation of the combinational antimicrobial effect of Annona Squamosa and Phoenix Dactylifera seeds methanolic extract on standard microbial strains. Int Res. J Biol Sci. 2013; 2:5: 68-73.
- [18]Hari A, Revikumar KG, Divya D. Artocarpus: A review of its phytochemistry and pharmacology. J. Pharm. Res. 2014; 9:1:7-12.
- [19]Debnath S, Rahman SH, Deshmukh G, Duganath N, Pranitha C, Chiranjeevi A. Antimicrobial screening of various fruit seed extracts. Phcog J. 2011; 3:19:83-86. doi:10.5530/pj.2011.19.15.
- [20]Umaarasu T, Padmavathy K, Thirunavukkarasu D, Rajesh SV, Govindaraj J, Shanmugam G. Evaluation of the antimicrobial efficacy and phytochemical constituents of the extracts of Andrographis paniculata against drug-resistant bacterial pathogens. Drug Invent. Today. 2019: 11:10.
- [21]Gopalakrishnan S, Rajameena R, Vadivel E. Antimicrobial activity of the leaves of Myxopyrum serratulum AW Hill. Int. J. Pharm. Sci. Drug. Res 2012; 4:31-34.
- [22]Rahman SA, Akhter, MS. Antibacterial and cytotoxic activity of seeds of white hyacinth bean (Lablab purpureus sweet ‘white’). J Adv Biotechnol Exp Ther. 2018; 1:2: 49-54.
- [23]Rakholiya K, Kaneria M, Desai D, Chanda S. Antimicrobial activity of decoction extracts of residual parts (seed and peels) of Mangifera indica L. var. Kesar against pathogenic and food spoilage microorganism. Microbial pathogens and strategies for combating them: Science, Technology and Education. FORMATEX 2013; 850-6.
- [24]Asif A, Farooq U, Akram K, Hayat Z, Shafi A, Sarfraz F, Aftab S. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci Tech. 2016; 53:102-112. doi:10.1016/j.tifs.2016.05.004.
- [25]Ediriweera MK, Tennekoon KH, Samarakoon SR. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (mango). Evid. Based Complementary Altern. Med. 2017. doi:10.1155/2017/6949835
- [26]Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Salamat MKF. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018; 11:1645. doi: 10.2147/IDR.S173867.
- [27]Hoque R, Mostafa A, Haque M. Intern doctors’ views on the current and future antibiotic resistance situation of Chattagram Maa O Shishu Hospital Medical College, Bangladesh. Ther Clin Risk Manag. 2015; 11:1177. doi: 10.2147/TCRM.S90110
- [28]Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28:3:603-661. doi: 10.1128/CMR.00134-14.
- [29]Martindale WH. Essential oil in relation to their antiseptic powers. Purfum. Essent. Oil Rec. 1910; 1: 266274.
- [30]Hoffmann C, Evans AC. The Use of Spices as Preservatives. Indus & Engin Chem. 1911; 3(11): 835-838.
- [31]Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J of nat. prod. 2012; 75(3): 311-335.
- [32]Shen B. A new golden age of natural products drug discovery. Cell. 2015; 163(6): 1297-1300.