Adaptations of muscular biology in response to potential glucocorticoid treatment in broiler chicken
Abstract
Poultry meat production has been dramatically increased in the last few decades due to increased population rate. Glucocorticoids decrease the growth of poultry and increase fat accumulation in liver and meat. In the coming days, it is important to consider the quality of meat to fulfill the increasing demand of proteins. The morphological and biometric properties of meat are associated with the quality of meat. The present research aimed to study the adaptations of muscular biology in response to potential glucocorticoid treatment in broiler chicken. This experiment was conducted into three groups of broilers (i.e. control group: homemade ration, group A: commercial broiler ration, and group B: a high dose of glucocorticoid at 7 mg/kg) started from day 7 to 28. Meat and blood samples were collected at day 7, 14, 21, and 28. For gross morphology, color and weight of meat were measured. Histomorphology of meat was studied under light microscope by Hematoxylin & Eosin stain. The length and width of meat fibers were measured using calibrated stage micrometer. The blood cholesterol dynamics was measured by spectrophotometer. The color of breast meat was more yellowish and lighter than thigh meat. The weight of meat was negatively affected by glucocorticoid. Glucocorticoid treatment negatively influenced the number of myofibers in breast meat, while positively influenced the thigh meat. Excess dietary glucocorticoid increased the biometry of breast meat and decreased that of thigh meat in broiler. Glucocorticoid non-significantly increased the serum cholesterol level. These findings advance our knowledge about the action of glucocorticoid in the muscular system and provide basis for novel therapies to prevent glucocorticoid-induced muscular atrophy.
INTRODUCTION
Due to the growing human population and increasing demand for proteins throughout the world, the production of poultry meat has dramatically increased in the last few decades. A broiler can attain a body weight of 2 kg by consuming 3 kg of feed within 35 days [1]. The majority of this increase has been rendered possible due to rapid growth by genetic selection of poultry for quantitative traits, which has tremendously augmented the growth rate of meat by inducing hypertrophy in meat fibers [2, 3]. In the coming decades, it is also essential to ensure the quality meat to fulfill the increasing demand of proteins. The synthetic steroid, glucocorticoid is used as a growth promoting agents for increasing the body weight in livestock legally or illegally [4, 5, 6]. Steroid hormones are applied for increasing the growth rate of broiler to meet up the demand of total meat consumption [7].
The color of meat is an important quality attributes both for the consumer’s selection of fresh meat at the retail level and for the consumer’s final evaluation and acceptance of meat product [8]. Morphological and biometric properties of meat fibers play a key role in performance and meat quality in broiler chicken [9]. So, investigation of these characteristics is one of the most practical importance to poultry and meat scientists. Although the current commercial broiler chicken strains are a result of successful selection programs for rapid growth and body conformation, especially favoring the breast muscles. Because, the breast meat is the most valuable portion of the chicken meat in the market and have significant economic impact. For this reason, the broiler industry is constantly interested in evaluating the weight and yield of the breast meat as the most important variables [10].
It has been reported that dietary supplementation of steroid growth promoter; glucocorticoid did not improve overall growth performance (i.e. body weight and dressed weight) of broiler chicken [11]. In chicken, glucocorticoid (dexamethasone) induced-retarded fatty acid utilization in muscle. Dietary dexamethasone promoted the transcriptional activity of genes related to lipid uptake and oxidation in muscles. Unrivalled lipid uptake and utilization are recommended to be involved in the augmented intramyocellular lipid accumulation [12]. A higher dose of glucocorticoid causes greater muscular atrophy in rat in comparison to those receiving lower doses of glucocorticoid [13, 14]. But the mechanism underlying the muscular atrophy in response to glucocorticoid in broiler chicken is not completely understood. It was hypothesized that high dose of glucocorticoid alters the number or the size of myofibers in broiler chicken.
In addition, modern intensive broiler production has been in the efficient and economical production of high-quality chicken meat [15]. The quality of the meat is associated with the total lipid and cholesterol profile in blood. A group of scientists have noted that supplementation of herbal mixture lowers the blood cholesterol level in broiler chicken [15]. There is no research exists which studies the effects of dexamethasone on the blood cholesterol profile in broiler chickens. Therefore, the present study aimed to study the morphology and biometry of breast and thigh meat and on blood cholesterol level in response to high dose of glucocorticoid in broiler chicken.
MATERIALS AND METHODS
Animal ethics
The present experimental procedures were approved and performed in accordance with the guidelines for the care and use of animals as established by Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Bangladesh.
Bird management
The experiment was conducted in the department of Anatomy and Histology, Bangladesh Agricultural University, Mymensingh-2202. In total, 90 (“Cobb 500” strain) one day old broiler chicks were purchased from a commercial hatchery (Provita Feed and Hatchery Ltd., Bangladesh). Upon arrival, broilers chicks were individually weighed, and steel wing tagged before being allotted to one of the 3 pens (thirty birds per pen), equally distributed over three identical climate-controlled rooms, so that each pen had a similar initial total body weight. Each pen had three drinking nipples with a cup underneath connected to a water tank. A feeding tray was placed on the floor of the pen during week 1 and replaced by a feeding trough thereafter. Feed and water were available ad libitum throughout the experiment. Rice husk was used as litter material.
Experimental design and treatments
The experiment was conducted into three groups in three different pens (three pens with thirty birds per pen), and treatments were equally distributed over pens. All birds received the same standard starter diet for the first 7 days of the experiment. After week 1, three dietary treatments were provided which contained homemade ration in control group, commercial broiler ration in group A and homemade ration with a high dose of glucocorticoid (dexamethasone) (7mg/kg) in group B. The homemade ration was formulated according to the commercial broiler type ration to meet the nutrient recommendations for boilers. These dietary treatments were randomly assigned to the broilers in pens. All rations were provided as pellets for the starter and grower diet. The broiler ration did not contain any antibiotic or other growth promoters.
Tissue collection and preservation
The experimental broilers were slaughtered by cervical subluxation method at day 7, 14, 21, and 28 of the experiment. The birds were plucked immediately after slaughtering and meat samples were excised from the breast and thigh of the broilers, rinsed with physiological saline (0.9%). Then the collected meat samples were immediately placed in Bouin’s solution for further histological study.
Blood collection and serum preparation
Blood samples were collected from wing vein of each broiler at day 7, 14, 21 and 28 days of the experiment. About 5 ml of blood was collected from each broiler without anticoagulant, in a sterile glass test tube. The blood containing tubes were placed in a slanting position at room temperature for clotting. The tubes were then placed in a refrigerator at 4ºC over-night. Serum sample was then collected and centrifuged at 1000 rpm for 15 minutes to get rid of unwanted substances. The collected serum was then stored in a screw capped serum vial and preserved at -20º C until use.
Gross morphology
Morphometric (i.e. color and weight) alterations of the breast and thigh meat were examined in the broilers during the experiment. The color of the excised meat samples was compared in between breast and thigh meat through visual inspection. The weight of breast and thigh meat was recorded by electronic digital weighing balance.
Histomorphology and biometric measurements
For histological studies, the collected breast and thigh meat were dehydrated in the series of ascending graded alcohol followed by clearing in three changes of xylene. Then, the tissues were infiltrated with different grades of melted paraffin (49°C, 55°C, and 58°C) at 30 minutes interval. The tissues were then embedded in paraffin (58°C) and finally the sections were cut at 6μ thickness using sliding microtome (MIC 509, Euromex, Japan). After cutting, the sections were floated in a floatation bath at 37°C for stretching. Then the sections were mounted on clean slides using an adhesive (egg albumins) and dried on a slide warmer at 37°C. The sections were then stained using Mayer’s Hematoxylin and Eosin (H & E) stain. The histological characters (i.e. number of meat fibers) of meat tissues were observed under light microscope at 40x magnification. The length and width of breast and thigh meat fibers were considered for biometric measurement which was performed using calibrated stage micrometer in μm (micrometer). Thirty sections (ten sections from each group) were biometrically evaluated from three different groups of experiment.
Photomicrographs
The sections were evaluated at 400-fold magnification by light microscope (Leica DMR; Leica Microsystems, Wetzlar, Germany). The pictures were taken with a digital color camera. For each section, 10 randomly selected fields were captured for the measurement of number, length and width of fibers, as well as the total number of fibers. Results are presented as the mean percentage of the fibers per total fibers in the evaluated fields.
Serum cholesterol analysis
5 ml of blood was collected without anticoagulant in sterile glass test tubes. The blood containing tubes were placed in a slanting position at room temperature for clotting. The tubes were then placed in the refrigerator at 40C over-night. Blood was centrifuged at 1000 rpm for 15 minutes for serum collection and the serum was stored in a freezer at -20°C until analyses. Blood serum cholesterol (TCHOL) was determined by the spectrophotometric method, using Human Humalyzer 2000 (Wiesbaden, Germany). The cholesterol present in the serum sample produced a colored complex. The intensity of the color formed is proportional to the cholesterol concentration in the serum sample.
Statistical analysis
The results were analyzed in the GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). All data were expressed as mean ± standard error and differences among the groups of birds were compared using one-way ANOVA with post-hoc Duncan’s multiple range test. Values of p<0.05 were considered significant.
RESULTS
Gross morphology of breast and thigh meat
The color and lightness of breast and thigh meat were observed by visual inspection. The breast meat exhibited a light-yellow color, while thigh meat appeared pale red in the broilers of group B (Figure 1a). The color of breast meat was more yellowish as compared to thigh meat. The lightness of breast meat was more than the thigh meat.
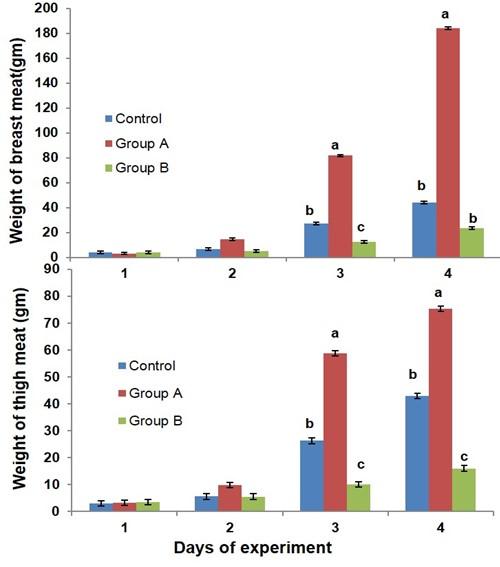
The effects of glucocorticoid on the weight gain of breast and thigh meat in broiler chicken are presented in Figure 2. The breast meat weight was decreased in group B as compared to control and group A in broiler at day 21 of the experiment. The thigh meat weight was decreased in the broilers of group B than the broilers of control and group A at days 21 and 28 of the experiment.
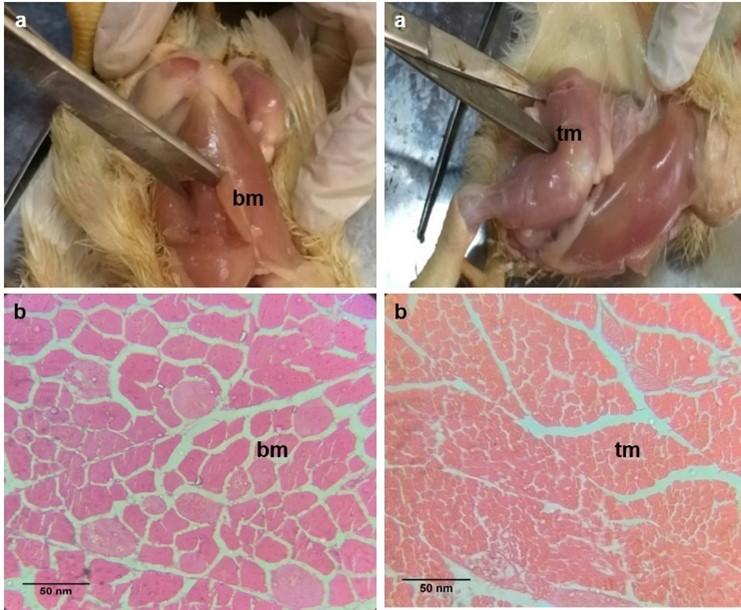
Histology of breast and thigh meat
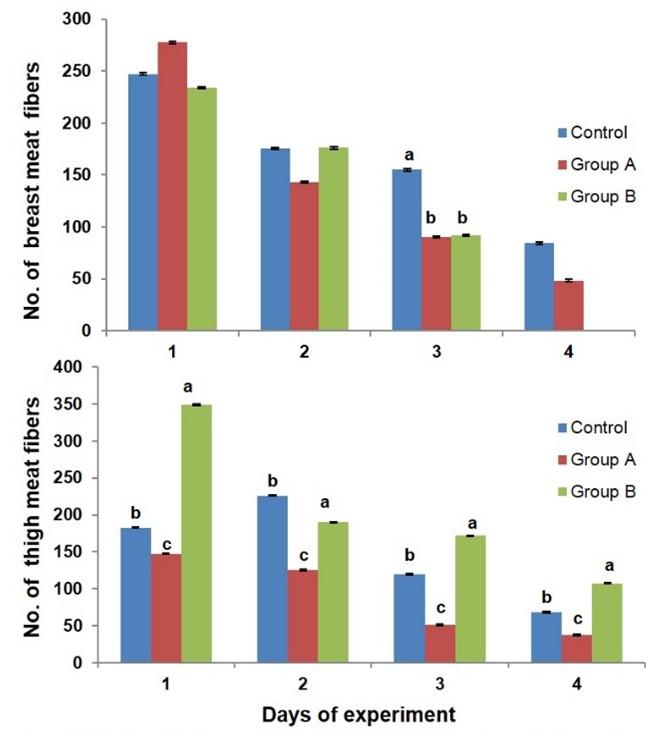
The histological structures of breast and thigh meat fibers are presented in broiler chicken (Figure 1b). Steroid growth promoter, glucocorticoid influenced the numbers of myofibers in breast and thigh meat of broiler chicken (Figure 3). The number of myofibers of breast meat was decreased in the broilers of group A and B as compared to the control birds at day 21 of the experiment. The number of myofibers of thigh meat was increased in the broiler of group B as compared to that of group A and control at day 7, 14, 21, and 28 of the experiment.
Biometry of breast and thigh meat
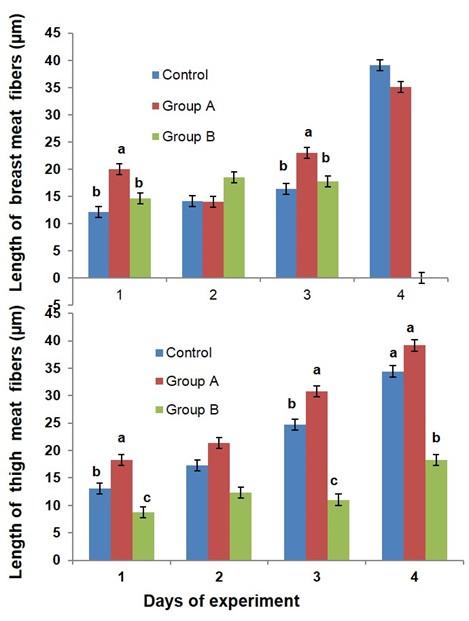
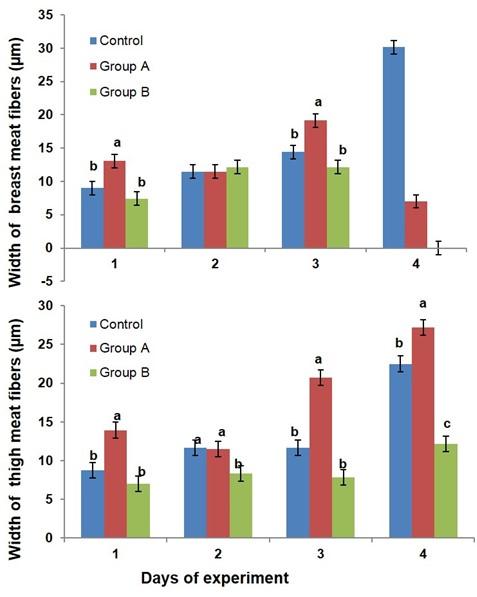
The biometry (i.e. length and width of myofibers) of breast and thigh meat fibers is affected by dietary glucocorticoid in broiler (Figure 4 & 5). The length of breast meat fiber was non significantly increased in group B as compared to the control birds, while the length of thigh meat fibers was significantly decreased in the broilers of group B as compared to control and group A at day 7, 21, and 28 of the experiment. The width of breast meat fiber was non-significantly increased in group B as compared to the control birds, while the width of thigh meat fibers was significantly decreased in the broiler of group B as compared to control and group A at day 14 and 28 of the experiment.
Blood cholesterol profile
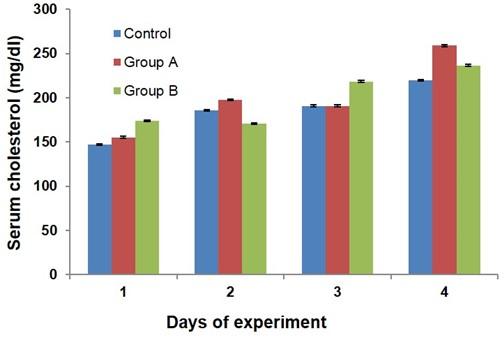
The serum cholesterol data in different groups of broilers are presented in Figure 6. There was a non-significant increase in serum cholesterol level in broilers of group B as compared to control and group A at day 7, 21, and 28 of the experiment.
DISCUSSION
The present study intended to demonstrate the effects of glucocorticoid on gross morphology (i.e. color and weight), histomorphology (i.e. number of myofibers), and biometry (i.e. length and width of myofibers) of breast and thigh meat and blood cholesterol level in broiler chicken. It was hypothesized that high dose of glucocorticoid would result alteration in the morphology of meat, which coincides with reduced body weight. Additionally, it was questioned that the high dose of glucocorticoid could alleviate the blood level of cholesterol in broiler. Therefore, the experiment was conducted with high dose of glucocorticoid in broiler chicken.
The color of meat is an important quality attribute for the consumer’s selection of fresh meat and acceptance of a meat product [8]. Extreme paleness or darkness of meat has reflected badly on poultry industry [16]. The color of poultry meat is highly correlated to the amount of haem containing compounds, myoglobin. However, within most individual experiments the myoglobin content in breast muscle was significantly lower than that of the thigh muscle. The thigh meat has a high proportion of myoglobin as compared to breast meat [17]. The intramuscular connective tissue and tenderness of the meat are varied between the breast and thigh meat in chicken [18]. The color of breast meat is more yellowish as compared to the thigh meat. The lightness of breast meat is more than the thigh. The variations in color and lightness might be related with myoglobin content in meat, the intramuscular connective tissues and tenderness of the meat.
Higher doses of dexamethasone have negative effects on the growth rate and tibia genomic properties of broiler [19]. The immunosuppressive effect of glucocorticoid is related with the muscle fiber loss in muscular dystrophy [20]. In addition, glucocorticoid impairs young myofibers formation in skeletal muscle, increase protein breakdown in skeletal muscle of young adults, decrease protein synthesis in aged rats, and morphologically and functionally damage muscle precursor cells [21].
Glucocorticoid; dexamethasone increases the plasma T3 levels and metabolism of protein in muscle which is responsible for muscular dystrophy [22]. The present study reported that dietary supplementation of glucocorticoid decreased the individual weight of breast and thigh meat in broiler chicken. These results support the study performed by our group and suggested that dietary glucocorticoid did not improve overall growth performance (i.e. body weight and dressed weight) in broiler chicken [11]. This is in agreement with other researches [14, 22] who reported that high doses of dexamethasone cause muscular dystrophy and atrophy in rat.
Histological and biochemical properties of meat fibers are associated with the meat quality. So, investigation of these characteristics is one of the most practical importance to poultry and meat scientists. However, still there is not enough research that definitely demonstrates the deleterious effects of increasing fiber size on meat quality, and some researchers showed that largest breast meat fibers exhibited high meat quality [9]. In the present study, the number of myofibers of breast meat was decreased in the broiler fed the ration supplemented with dexamethasone as compared to the control. And the number of thigh meat fibers were increased in glucocorticoid treated broiler. So, it is assumed that the lower number of breast meat fibers might contribute to the reduced body weight gain in response to dietary glucocorticoid in broiler chicken.
Many researchers reported that excess glucocorticoid either endogenous or exogenous induce muscular atrophy leading to myopathy in skeletal muscle in animals [13, 14]. Thus, high dose of glucocorticoid affects the quality of meat in animals. In addition, A high dose of glucocorticoid causes mild damage in the liver of rat and improves renal failure in multiple myeloma patients [23, 24]. However, there is no study exists about the effects of high dose of glucocorticoid on the biometry of breast and thigh meat in broiler chicken. In this experiment, high dose of glucocorticoid affected the biometry of breast and thigh meat fiber in broiler chicken. The length of breast meat fibers was non significantly increased, while the length of thigh meat fibers was significantly decreased in the broilers. The width of breast meat fiber was non-significantly increased, while the width of thigh meat fibers was significantly decreased in the broiler. High dose of glucocorticoid has positive effects on the breast meat biometry and negative effects on the thigh meat biometric properties in broiler chicken. This result supports that the high dose of glucocorticoid reduces the myotube diameter in thigh meat [25] but opposite to the breast meat profile in broiler.
The cholesterol content in broiler meat is one of the quality indices whose level the consumer wants to have as less as possible. The ingredients of feed are one of the most contributing factors to reduce the cholesterol level in meat in poultry [26, 27]. In the present study, dietary glucocorticoid was used in broiler and their influence on blood cholesterol dynamics was evaluated in the broiler chicken. In this study, the blood cholesterol level was non significantly increased in response to dietary glucocorticoid in broiler chicken. On the contrary, feed supplementation of herbal mixture lowers the blood cholesterol level in broiler chicken [15]. This increased blood cholesterol might release into the body tissues i.e. meat and liver which is necessary to investigate as further research.
In conclusion, the outcomes of the present study reveal that a high dose of glucocorticoid alters the breast and thigh meat morphology in broiler chickens. The reduced number of breast meat fibers might contribute to the decreased body weight gain in broiler treated with glucocorticoid. A high dose of glucocorticoid increased the breast meat biometry and decreased the thigh meat biometric properties in broiler chicken. The effects of glucocorticoid on the biometry of breast and thigh meat fibers might be associated with the excess of glucocorticoid in breast and thigh meat. Morphological and biometric properties of breast and thigh meat fibers can provide beneficial information regarding the nature of mechanically deboned meat and its use in food products. The up regulated blood cholesterol by glucocorticoid might release into body tissues i.e. meat and liver which is necessary to investigate as further research. It would be interesting to assess the effects of glucocorticoid on intramuscular connective tissue, lipid profile and cholesterol level of meat in broiler.
ACKNOWLEDGEMENT
Technicians of the department of Anatomy and Histology, Bangladesh Agricultural University, Bangladesh are thanked for their assistance in performing the experiment. The author, Dr. Nasrin Sultana is gratefully acknowledged to the funding of the University Grants Commission (UGC) of Bangladesh (Project No.: Life 11/2017).
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
AUTHOR CONTRIBUTIONS
FA and MA were involved in conception and design of the experiments. FA and RI contributed to perform the experiments. FA and AK analyzed data. NS contributed to drafting the article. MHS and NS contributed to revising it critically for important intellectual content.
References
- [1]Choct M. Managing Gut Health through Nutrition. Bri Poult Sci 2009; 50: 9–15. 10.1080/00071660802538632.
- [2]Picard B, Lefaucheur L, Berri C and Duclos MJ. Muscle fibre ontogenesis in farm animal species. Reprod Nutri Dev 2002; 42:415–431.
- [3]Scheuermann GN, Bilgili SF, Tuzun S and Mulvaney DR. Comparison of chicken genotypes: myofiber number in pectoralis muscle and myostatin ontogeny. Poult Sci 2004; 83:1404–1412.
- [4]Jeong SH, Kang D, Lim MW, Kang CS, and Sung HJ. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol Res 2010; 26: 301.
- [5]Yuan Y, Xu C, Peng C, Jin Z, Chen W, and Liu L . Analytical methods for the detection of corticosteroids-residues in animal-derived foodstuffs. Crit Rev Anal Chem 2008; 38: 227-241.
- [6]Serratosa J, Blass A, Rigau B, Mongrell B, Rigau T, Tortades M, et al. Residues from veterinary medicinal products, growth promoters and performance enhancers in food-producing animals: a European Union perspective. Rev Sci Tech 2006; 25: 637-653.
- [7]Schumacher HI, Barrantes SQ, Alpízar MZ and Corella MR. Serum sexual steroid hormones and lipids in commercial broilers (Gallus domesticus) in Costa Rica. J Appl Poultry Res 2010; 19: 279–287.
- [8]Fletcher DL, QIAO M, and Smith DP. The Relationship of Raw Broiler Breast Meat Color and pH to Cooked Meat Color and pH. Poult Sci 2000; 79:784–788.
- [9]Tůmová E and Teimouri A. Chicken muscle fibres characteristics and meat quality: a review. Sci Agric bohem 2009; 40 : 253–258.
- [10]Scheuermann GN, Bilgili SF, Hess JB and Mulvaney DR. Breast muscle development in commercial broiler chickens. Poult Sci 2003; 82:1648-1658.
- [11]Afrose M, Sultana N, and Islam MR. Physiological Responses of Corticosteroid, Dexamethasone in Broiler Chicken. IJSR 2018; 7:1459-63.
- [12]Wang XJ, Song ZG, Jiao HC and Lin H. Dexamethasone facilitates lipid accumulation in chicken skeletal muscle. Stress 2012; 15: 443-456.
- [13]Sato AY., Richardson D, Cregor M, Davis HM, ED, Mcandrews K, Zimmers TA, Organ JM, Peacock M, Plotkin LI and Bellido T. Glucocorticoids Induce Bone and Muscle Atrophy by Tissue-Specific Mechanisms Upstream of E3 Ubiquitin Ligases. Endocrinol. 2017; 158: 664–677.
- [14]Fappi A, Neves JC, Sanches LN, Silva PVM, Sikusawa GY, Brandão TPC, Chadi G, and Zanoteli E. Skeletal Muscle Response to Deflazacort, Dexamethasone and Methylprednisolone. Cells 2019; 8: 406. 10.3390/cells8050406.
- [15]Yano AA, Iriyanti N, and Rosidi. Effect of supplemental fermeherbafit on total blood lipid and cholesterol in broiler chickens. J Phys 2018; 1025: 012057.
- [16]Mir NA, Rafiq A, Kumar F, Singh V, and Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. Int. J Food Sci and Tech 2017; 54: 2997–3009.
- [17]Wideman N, O’bryan CA and Crandall PG. Factors affecting poultry meat colour and consumer preferences – A review. World Poultry Sci J 2016, 72: 354-366.
- [18]Mobini B. Histological properties of intramuscular connective tissues in native chickens and their relationship with meat tenderness. Anim Sci J 2015; 2: 105-109.
- [19]Ademu LA, Erakpatobor-Iyeghe GT, Barje PP, Daudu OM and Wafar RJ. Response of Broiler Chickens under Dexamethasone Induced Stress Conditions. AJAVA 2018; 1: 1-9.
- [20]Hussein MR, Hamed SA , Mostafa MG, Abu-Dief_ Nageh EE Kamel F and Kandil MR . The effects of glucocorticoid therapy on the inflammatory and Dendritic cells in muscular dystrophies. Int. J. Exp. Path. 2006, 87, 451–461.
- [21]Otrocka-Domagała I, Paździor-Czapula K and Gesek M. Dexamethasone-induced impairment of post-injury skeletal muscle regeneration. BMC Veterinary Research 2019.15:56.
- [22]Aengwanich W. Effects of dexamethasone on physiological changes and productive performance in broilers. AJAVA 2007; 32: 157-161.
- [23]Darda U, Warsch BS and Pereira D. High‐dose glucocorticoids improve renal failure reversibility in patients with newly diagnosed multiple myeloma. American Journal of Hematology, 2011 86: 224-227.
- [24]Eken H, Ozturk H, Ozturk H and Buyukbayram H. Dose-related effects of dexamethasone on liver damage due to bile duct ligation in rats. World J Gastroenterol 2006, 33: 5379-5383.Schakman O, Kalista S, Barbé C, Loumaye A and Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell B. 2013; 45: 2163–2172.
- [25]Ramasamy K, Norhani A, Syed J, Michael W and Yin WHO. Effects of Lactobacillus feed supplementation on cholesterol, fat content and fatty acid composition of the liver, muscle and carcass of broiler chickens. Anim Res 2006; 55: 77–82.
- [26]Al-Ruwaili M, Herzallah S, Al-Dmoor H and Al-Atiyat R. Effect of Broiler Commercial Strains on Total and Free Cholesterol Levels of Chicken Muscle Tissues. Glob Vet 2014; 12: 381-383.