Hematological leukocytes ratio indices: predictors of acute purulent fecal peritonitis in nonlinear laboratory rats
Abstract
The use of hematological leukocytes ratio indices (HLRI), which show the interrelations between different blood cells, for evaluation the organism state of laboratory rats is questionable. The aim is to investigate the informativity of HLRI in laboratory rats on the model of acute infectious process. The study performed on white nonlinear (7–8 months) male laboratory rats: control (n = 10) and experimental groups (n = 10), which simulated acute purulent fecal peritonitis. Analyzed the number of leukocytes, blood leukocyte formula, phagocytic activity of neutrophils, HLRI. Acute infectious process is accompanied by leukocytosis, neutrophilia with leukocyte blood formula shift to the left and inhibition of neutrophils absorption capacity. A set of changes of HLRI in experimental group animals reflects the predominance of cellular immunity over the humoral, prevalence of granulocytes and their immature forms, increased activity of the inflammatory process, predominance of the microphage system, intense non-specific immunity, disturbance of immune system functional state with a shift of balance towards monokines, formation of mainly delayed-type hypersensitivity reactions, decreased function of affector cells and predominance of effector part of immunological process, the infectious nature of intoxication. Analysis with receiver operating characteristic (ROC) curves displayed that 8 from 16 applied HLRI, such as blood leukocyte shift index, neutrophil to lymphocyte, segmented neutrophil to lymphocyte, banded neutrophil to lymphocyte, eosinophil to lymphocyte, monocyte to lymphocyte, neutrophil to monocyte and segmented neutrophil to monocyte count ratio indices have very high levels of sensitivity and specificity and can be used as diagnostic markers for acute purulent fecal peritonitis.
INTRODUCTION
Acute purulent fecal peritonitis is one of the common and severe diseases of abdominal surgery, because most acute surgical diseases and injuries of the abdominal organs are complicated by peritonitis and require an immediate surgical intervention [1, 2, 3]. The cause of acute peritonitis is predominantly autoinfection, less often – an exogenous infection [4]. Although modern medicine is highly developed, there is still high mortality rate for peritonitis [1, 2, 3, 5], including in elderly patients [6]. The main cause of thanatogenesis is the septicemia and infectious-toxiс shock, which contributes to the development of multiple organ failure [2, 4]. Peritonitis is also a frequent pathology of veterinary medicine [7, 8], which indicates research relevance.
Despite the growing number of laboratory capabilities, the search for available and simple blood tests, associated with systemic inflammation that allow monitoring the state of immune system at the time of examination and predicting the prognosis of disease remains relevant [9]. Practically all changes in mammalian organism are largely related with quantitative and qualitative changes in blood leukocyte formula that depends on nature, strength of external influences and organism reactivity. Different types of leukocytes perform certain functions, so the study of their ratio, content of young, immature and pathological forms is a valuable diagnostic feature [10].
Consequently, usage of some leukocyte counts and their ratio as a marker of patients’ outcome is an easy, cheap and fast method of laboratory diagnostics, so it can be used in daily clinical practice, especially in developing countries [11]. However, it remains not clear enough whether it is advisable to use hematological leukocytes ratio indices (HLRI) for evaluation of organism state of sexually mature laboratory rats, which, unlike humans, have a lymphoid blood profile. So, the aim of the study is to investigate the basic parameters of blood leukocytes and informativity of HLRI in nonlinear sexually mature laboratory rats on the model of acute infectious process – diffused purulent fecal peritonitis.
MATERIALS AND METHODS
Animals
The study performed in accordance with the ethical standards on 20 white nonlinear, sexually mature (7–8 months) male laboratory rats weighing 180–220 g, which have passed the quarantine and had no external manifestations of the disease. The rats were housed in cages under standard laboratory conditions: fixed temperature and humidity and with natural and artificial lighting (from 8:00 to 5:00 h). All the animals had access to fresh water, and they were fed with balanced food. Animals were randomly divided into control/intact (n = 10) and experimental groups (n = 10). Manipulations with animals were performed in compliance with regulated norms and rules for the treatment of laboratory animals: principles of bioethics, legislation and requirements in accordance with the provisions of the European Convention for the Protection of Vertebrate Animals Used for Research and Scientific Purposes (Strasbourg, France, 1986), The Law of Ukraine “On protection of animals from cruel treatment”. All procedures performed were approved by the Bioethics committee of Zaporizhzhia National University Biology faculty.
Experimental conditions
Simulated an acute diffuse purulent fecal peritonitis with 10% fresh filtered autogenic fecal mixture in a dose of 0.5 ml per 100 g of body weight which injected into the abdominal cavity and taken out animals from experiment on day 3 after injection [12]. Fecal mixture was prepared from 2 g of feces received for each rat individually, which was dissolved in 20 ml of 0.9% saline solution, homogenized, filtered through gauze and used it no later than 20 minutes after preparation.
Collection of blood samples and laboratory analysis
The animals decapitated under ethereal anesthesia (Sorbpolimer-Analitic Ltd., Kyiv, Ukraine), the arteriovenous blood was collected, stabilized with heparin (20 mg/ml, Spofa, Prague, Czech Republic) and immediately analyzed. The research carried out at the same time of day. The number of leukocytes analyzed in Goryaev’s chamber (MICROmed TM, Poltava, Ukraine) [13]. In order to perform this, 0.38 ml of 3% acetic acid solution (System Optimum Ltd., Lviv, Ukraine) was added to the well of the serological plate and 0.02 ml of test blood was added. The mixture was mixed thoroughly with a pipette and left for 1-2 minutes till complete lysis of erythrocytes, after which it was again thoroughly mixed and injected at Goryaevʼs chamber. It was left in a horizontal position for 1 minute with subsequent counting of leukocytes in 100 large squares and further multiplication of the obtained number by 50. Blood smears stained by Pappenheim using a solution of eosin methylene blue dye according to May-Grünwald (Biomed Ltd., Shostka, Ukraine) and 10% solution of Romanowsky-Giemza dye (Biomed Ltd., Shostka, Ukraine). Blood leukocyte formula was evaluated according to corresponding morphological signs of leukocytes by Pappenheim staining (not only typical signs were taken into account, but the features of cytoplasmic membrane, signs of cell nucleus activation, presence of atypical nucleus, signs of apoptosis such as pyknosis, karyorrhexis and karyolysis, the presence of cytoplasmic vacuolation, immature and/or toxic granules, Döhle bodies and others) [10, 14] and further calculation of relative and absolute ratio of different leukocytes’ types: eosinophils, basophils, neutrophils, monocytes and lymphocytes (the percentage of pathological forms in each cell type was not calculated separately). Cytological preparations were analyzed using a Primo Star iLED microscope with an AxioCam ERc5s camera (Carl Zeiss, Goettingen, Germany).
Phagocytic activity of neutrophils (PAN) evaluated in test with Saccharomyces cerevisiae Meyen ex EC Hansen, 1883 by standard method: 0.05 ml of whole blood stabilized with heparin and 0.05 ml of 1% yeast suspension were introduced into the well of the serological plate for serological tests, the suspension was prepared on RPMI-1640 culture medium (PanEco, Moscow, Russian Federation); thoroughly mixed by pipetting. Incubated in a thermostat for 30 minutes at a temperature of 37˚C, with periodic shaking every 10 minutes, after which smears were prepared; fixed and stained by Pappenheim. The following indicators of PAN were studied: phagocytic index (PI) as percentage of neutrophils that are involved in phagocytosis; phagocytic number (PN) as average number of microorganisms absorbed by one neutrophil; number of active phagocytes (NAP, ×109/L) as absolute number of phagocytotic neutrophils in 1 L of blood, which was determined based on the absolute number of neutrophils (N) in the blood smear and the percentage of phagocytosis (PI):
| NAP = (PI / 100 | (1) |
phagocytic blood volume (PBV, ×109/L) as count of microbes that can absorb phagocytes of 1 L of blood [15]:
| PBV = PN × N. | (2) |
The following HLRI were determined based on blood leukocyte formula:
- Leukocyte index (LI), or adaptation index, or stress index, or total index of nonspecific reactivity Garkavi et al., or coefficient of resistance:
| LI = LYM / sN. | (3) |
- Lymphocytic index (LYMI):
| LYMI = LYM / (sN + bN + Ml + metaMl). | (4) |
- Blood leukocyte shift index (BLSI), or blood cell index, or granulocyte-agranulocytic index:
| BLSI = (E + B + sN + bN + Ml + metaMl) / (LYM + Mono). | (5) |
- Lymphocyte to granulocyte index (LGI):
| LGI = 10 × LYM / (Ml + metaMl + bN + sN + E + B). | (6) |
- Neutrophil to lymphocyte count ratio (NLCR) or Krebs index:
| NLCR = (bN + sN) / LYM. | (7) |
- Lymphocyte to eosinophil count ratio (LECR):
| LECR = LYM / Е. | (8) |
- Neutrophil to monocyte count ratio (NMCR):
| NMCR = (bN + sN) / Mono. | (9) |
- Lymphocyte to monocyte count ratio (LMCR):
| LMCR = LYM / Mono. | (10) |
- Segmented neutrophil to lymphocyte count ratio (sNLCR):
| sNLCR = sN / LYM. | (11) |
- Immunoreactivity index (ІRІ):
| ІRІ = (LYM + Е) / Моno. | (12) |
- Eosinophil to lymphocyte count ratio (ЕLCR):
| ЕLCR = Е / LYM. | (13) |
- Monocyte to lymphocyte count ratio (MLCR):
| MLCR = Моno / LYM. | (14) |
- Segmented neutrophil to monocyte count ratio (sNMCR):
| sNMCR = sN / Моno. | (15) |
- Banded neutrophil to lymphocyte count ratio (bNLCR):
| bNLCR = bN / LYM. | (16) |
- Segmented neutrophil to banded neutrophil count ratio (sNbNCR):
| sNbNCR = sN / bN. | (17) |
- Modified leukocyte index of intoxication (МLІІ):
| МLІІ = WBC count / (WBC count – LYM count), | (18) |
Where all indicators are measured in ×109/L.
The abbreviation in the presented formulas: WBC – white blood cell; Ml – myelocytes; metaMl – metamyelocytes; bN – banded neutrophils; sN – segmented neutrophils; LYM – lymphocytes; Mono – monocytes; E – eosinophils; B – basophils in the leukocyte formula of peripheral blood [16, 17, 18].
Statistical analysis
Statistical analysis and presentation of experimental results were performed using IBM SPSS Statistics version 20 (IBM corp., Armonk, NY, USA). Normality of quantitative indicators’ distribution was checked by Kolmogorov-Smirnov single-sample test. Two-sample Student t-tests for independent samples with normal distribution was used. The values in the tables are presented in form ± m, where is the arithmetic mean, m is the standard error of the mean. P-values less than 0.05 (*P < 0.05) were considered statistically significant [19].
Receiver operating characteristic (ROC) curves were constructed to evaluate the sensitivity and specificity of HLRI in predicting acute purulent fecal peritonitis. ROC curves displayed sensitivity versus specificity such that the area under the curve (AUC) varied from 0.5 to 1.0, with higher values indicating increased discriminatory ability. Confidence intervals on the AUC were calculated using nonparametric assumptions [20].
RESULTS
Disease symptoms
Fecal peritonitis modeled in laboratory rats for shifting leukocyte indices due to activation of immunogenesis during acute infection. Observation of animals’ behavior, which simulated fecal peritonitis, showed that in rats already on day 3 after injection of 10% filtered fecal suspension, a severe clinical picture of acute purulent peritonitis observed (without death of animals). The rats were adynamic, lethargic with lacklustre eyes, the animals refused water and food. Also ruffled fur, rapid breathing, shortness of breath, bloating, lack of stool were observed.
Laboratory findings of blood cells
Acute purulent peritonitis is one of the manifestations of the acute inflammatory reaction of whole organism, therefore, corresponding changes are also observed in peripheral blood (Table 1, Table 2). The volume of arteriovenous blood obtained after decapitation of animals in the group of healthy (control) laboratory rats was 26.95 ± 1.361 ml/kg, and in the experimental group with fecal peritonitis – 15.83 ± 1.016 ml/kg, which is lower than the control one by 41.26%.
Acute diffuse purulent fecal peritonitis was accompanied by leukocytosis (an increase in leukocytes count by 1.85 times compared with the control group at P < 0.05 level, Table 1). Neutrophilia was observed with a shift to the left: an increase in relative and absolute neutrophils count by 2.5 and 4.67 times (P < 0.05); an increase in absolute banded neutrophils count by 9.25 times and an increase in absolute segmented neutrophils count by 4.26 times (P < 0.05); a twofold decrease in relative lymphocytes (P < 0.05) count was also found too in leukocyte blood formula.
Morphological features of laboratory rats’ blood cells stained by Pappenheim are presented in Figures 1 and 2. There are rouleaux of erythrocytes, echinocytes, polychromatophiles and rarely orthochromatic erythroblasts in blood smears during fecal peritonitis (Figure 1 G, J, P, S; Figure 2 N, S).
In blood smears of rats with fecal peritonitis there are large lymphocytes with signs of activation, which have rounded or bean-shaped nuclei, loose chromatin, where nucleoli can be distinguished, and basophilic cytoplasm (Figure 1 C, D, E, G) in addition to typical small and medium lymphocytes with a dense compact nucleus, narrow rim of basophilic cytoplasm, fine azurophilic granules (Figure 1 A, B, D, F). There are reactive and atypical lymphocytes with different morphological forms of the nucleus, in particular, nuclei with notches, bi-nucleated lymphocytes and ones with signs of apoptosis, in particular with cytoplasmic blebbing (Figure 1 H-K).
Monocytes in most common morphology are with bean-shaped, horseshoe-shaped or irregularly shaped nucleus and gray-blue cytoplasm (Figure 1 L-O), there is vacuolation of the cytoplasm in blood smears during fecal peritonitis (Figure 1 M, N). In laboratory rats, both banded and segmented eosinophils are normally found, but more often band-shaped eosinophils (Figure 1 Q-T). In fecal peritonitis, eosinophils with cytoplasmic degranulation are found (Figure 1 T).
Laboratory rats are normally characterized by band-shaped, mostly a ring-shaped nucleus (Figure 2 F-L) and segmented neutrophils (Figure 2 M, N). With fecal peritonitis, the number of immature forms of neutrophils increases rare myelocytes, which are characterized by immature granulation (Figure 2 A, B, C, E), there are young forms of neutrophils (Figure 2 D), the number of banded neutrophils increases. Döhle bodies (Figure 2 G, H) are found in individual neutrophils, which cannot always be distinguished by light microscopy. Among neutrophils there are segmental cells with toxic granularity in the cytoplasm (Figure 2 P, Q), neutrophils with cytoplasmic vacuolation (Figure 2 O, R), nuclear karyorrhexis (signs of nuclear fragmentation are observed) and slight eosinophilia of the cytoplasm (Figure 2 S, T).
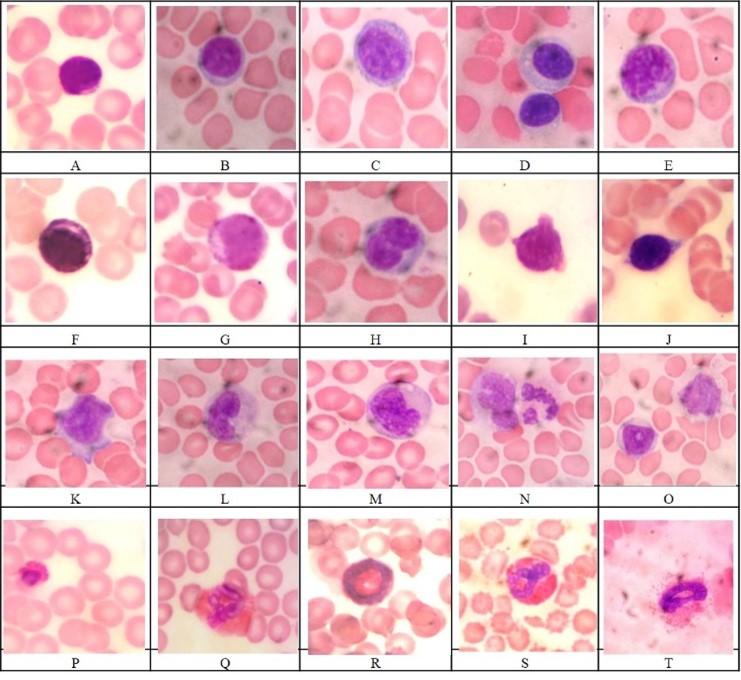
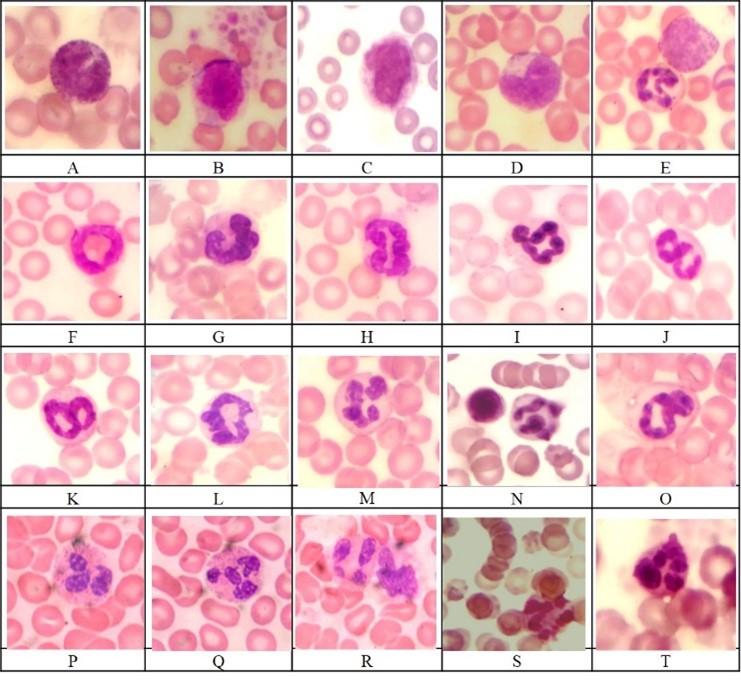
Table 1. Some leukocyte blood indices of intact young, mature laboratory rats (control group) and rats with purulent fecal peritonitis (experimental group), х ̅ ± m.
Table 2. Hematological leukocytes ratio indices of intact young, mature laboratory rats (control group) and rats with purulent fecal peritonitis (experimental group), х ̅ ± m.
Phagocytic activity of blood neutrophils
Evaluation of PAN (Table 1) in young sexually mature rats with fecal peritonitis compared to control group detected inhibition of neutrophils absorption capacity (reduction of PI in 1,56 and PN in 1,41 times at P < 0.05) and an increase of PBV in 3.29 and NAP in 2.97 times (P < 0.05) due to an increase in neutrophils count.
Hematological leukocytes ratio indices
A comprehensive assessment of experimental animals’ organism status carried out using some HLRI (Table 2) represented by intoxication indices (BLSI, МLІІ), indices of nonspecific reactivity (LI, LYMI, ІRІ, NLCR, NMCR, LECR, ELCR, LMCR, sNLCR, MLCR, sNMCR, bNLCR, sNbNCR) and inflammation activity indices (LGI). A statistically significant (P < 0.05) increase, among the analyzed indices in laboratory rats with acute diffuse purulent fecal peritonitis, compared with the control group, observed in BLSI of 4.63, NLCR of 5.21, NMCR of 1.90, sNLCR of 4.87, ELCR of 2.61, MLCR of 2.55, sNMCR of 1.74, bNLCR of 13.0 times and decrease in LI of 4.68, LGI of 4.86, LYMI of 5.12, LECR of 2.80, LMCR of 2.68, ІRІ of 2.53, sNbNCR of 2.72 and МLІІ of 2.24 times were detected (Table 2).
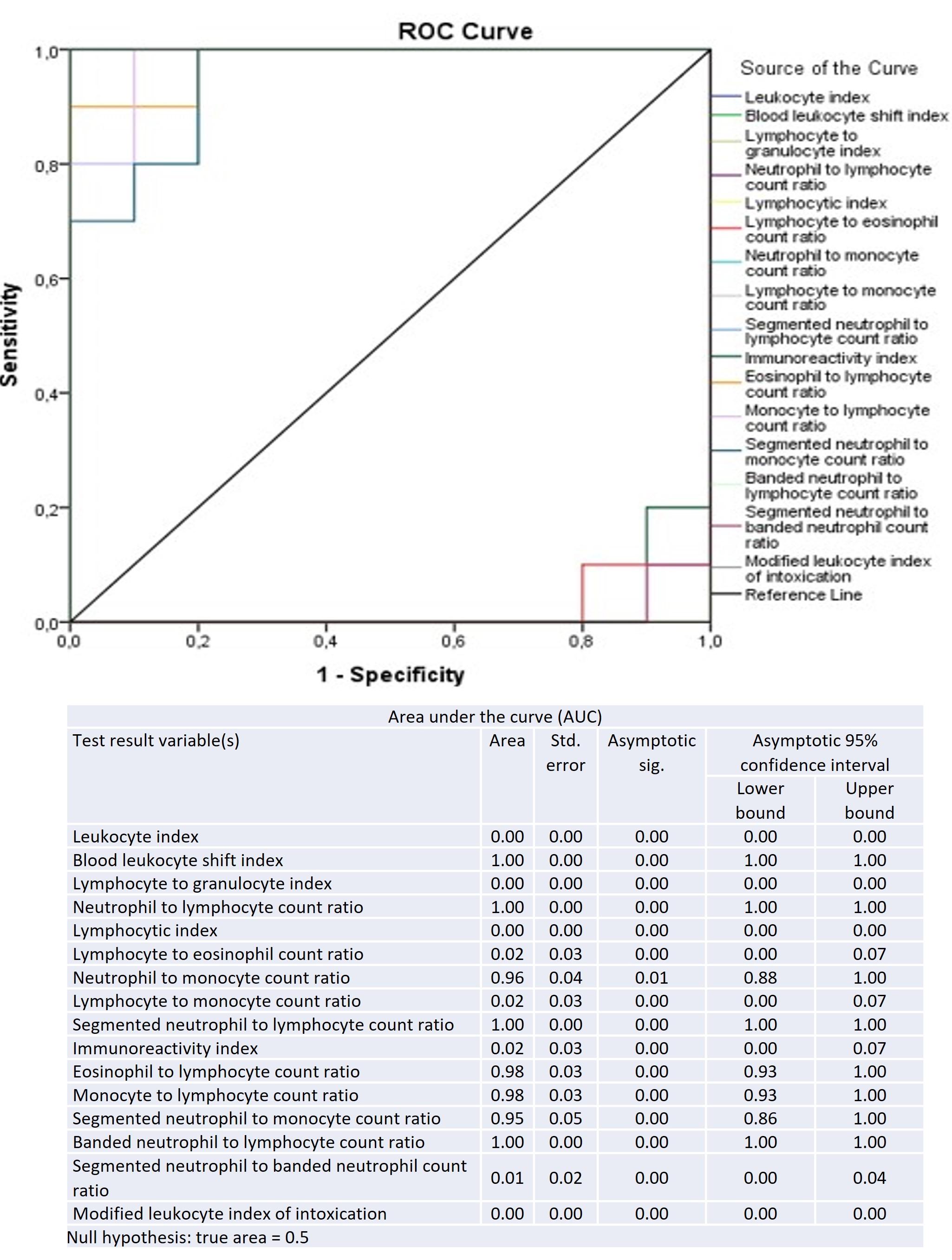
ROC curves of the 16 HLRI for differentiating acute diffuse purulent fecal peritonitis from healthy state are presented in Figure 3. The highest AUC was 1.00 for the following indices: BLSI, NLCR, sNLCR, bNLCR. The AUC of 0.98 (confidence interval = 0.93 to 1.00) was determined in ELCR and MLCR. NMCR and sNMCR had the AUC of 0.96 (confidence interval = 0.88 to 1.00) and 0.95 (confidence interval = 0.86 to 1.00), respectively. All remaining indices (LI, LGI, LYMI, LECR, LMCR, ІRІ, sNbNCR and МLІІ) showed very low levels of specificity and sensitivity.
DISCUSSION
The identified physiological symptoms and laboratory indicators of blood confirm the formation of acute fecal peritonitis in the experimental group of animals. The decrease in the volume of arteriovenous blood obtained after decapitation in experimental animals, compared with the control, indicates the presence of a deficit in the volume of circulating blood [4]. According to the recent published data, peritonitis also causes an imbalance of electrolytes in the blood [21, 22]. According to Mokhber Dezfouli et al. (2012) the concentration of phosphorus increases and the concentration of calcium, magnesium, sodium, potassium and chlorides decreases in animals with peritonitis [21]. During cecal ligation and puncture (CLP) induced sepsis model occurs, multiorgan failure syndrome, metabolic acidosis, electrolyte imbalance and death [22].
The indices of leukocytes count and leukocyte blood formula in healthy young adult laboratory rats corresponded to the normal values for this age. Thus, the normal leukocyte blood formula of nonlinear (outbred) rats has a lymphoid profile: the prevalence of lymphocytes over neutrophils, which is consistent with the literature data [23]. Acute infectious process in experimental group of animals is accompanied by leukocytosis with corresponding changes in leukocyte blood formula (neutrophilia with leukocyte blood formula shift to the left, relative lymphopenia) and inhibition of neutrophils absorption capacity. Such changes could be caused by mixed aerobic-anaerobic infections [4, 6]. It is well known that count and functional activity changes of blood leukocytes have phase character and reflects the reactions of the entire blood system during inflammation: events in the inflammation focus, bone marrow and peripheral blood, the ratio between leukocyte emigration to the site of inflammation and their entry from the bone marrow into the blood, inflammation persistence [24]. An increase in banded neutrophils count apparently indicates an intensification of compensatory mechanisms responsible for inactivation of toxins. It is known that segmentation of neutrophils’ nucleus occurs during maturation, so an increase in the number of ring-shaped nuclei in laboratory rats indicates accelerated granulocytopoiesis [10]. However, young forms of neutrophils are not able to fully perform their functions: they migrate from vessels more slowly because they have fewer chemotaxis receptors, produce less adhesins, their nuclei are more rigid, such cells contain fewer specific granules [25]. Therefore, the predominance of immature forms of neutrophils contributes to a decrease in the efficiency of phagocytosis and chronization of inflammation [15]. A significant decrease in lymphocytes’ count is probably a manifestation of a stress-induced situation and indicates a suppression of immune system functioning and their deposit in the focus of infectious process (in parenchymal organs, where the infectious source accumulates). It is noteworthy that a decrease in PAN indicators is an unfavorable prognostic factor that indicates existence of serious infections and decompensation of anti-infective protection [15].
We found not only quantitative but also morphological changes in blood cells in laboratory rats with fecal peritonitis, compared with controls. When analyzing the morphology of erythrocytes in acute diffuse purulent fecal peritonitis, rouleaux, echinocytes are identified, erythroblasts are rarely found in the blood smear (Figure 1, 2). It is shown that such changes, namely: the presence of echinocytes, erythrocyte aggregates and abnormal erythrocytes from spherostomatocytes to spheroechinocytes, where the erythrocyte membranes are damaged are characteristic of sepsis [26].
Leukocytes also differed by morphological features compared to control, there were blast-transformed and atypical/reactive lymphocytes, vacuolation of monocyte cytoplasm, degranulation of eosinophils, immature forms of neutrophils (myelocytes, young neutrophils), Döhle bodies, signs of neutrophil nucleus karyorrhexis, etc. (Figure 1, 2). Such changes indicate the presence of a pathological process (for example, sepsis), which result from the activation of increased cytokines during bacterial infection [14, 27].
The revealed changes in HLRI in acute infectious process can be explained by the following arguments. LI reflects the relationship between humoral and cellular components of immune system [18]. A decrease in LI during inflammation is a negative factor, since in this case formed a tendency of incomplete immune response [16]. According to Garkavi et al. (1990), the LI is a universal indicator characterizing the type of adaptation reaction to any external intervention in the body. Thus, the well-known “stressful” type of adaptation reaction, where a decrease in LI (less than 0.25 in humans) is present. It reflected relative and/or absolute lymphopenia [28].
A decrease in LYMI that characterizes the lymphocyte to neutrophil count ratio and reflects the relationship between the humoral and cellular components of immune system [18], in laboratory rats with purulent fecal peritonitis indicates predominance of cellular immunity over humoral. As known, IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, MCP-1, macrophage migration inhibition factor (MIF), LIF, G-CSF, IFNɣ and TNF-α are involved in the pathogenesis of sepsis and septic shock, which represent an intense systemic inflammatory response syndrome (SIRS). The evolution of SIRS may have resulted from an imbalance in the endogenous production of cytokines. The production of proinflammatory cytokines at the infection site is important to the recruitment and activation of leukocytes, which mediate local host defenses. However, high levels of the same proinflammatory cytokines in the circulation result in systemic inflammatory response syndrome [29]. IL-1, IL-2, IL -6, IL-10, and TNF-α cytokines are shown to regulate early responses in inflammation linked sepsis [30, 31, 32]. The levels of these cytokines later decrease, but they remain high. A similar dynamic trend is observed in the plasma chemokines, including MIP-1a, MIP-1b, and MIP-2 [31]. Some researchers believe that anti-inflammatory factors (such as IL-4, IL-10, IL-1 receptor antagonist (IL-1RA), and others) are representing a compensatory, anti-inflammatory response, although this is debatable [33].
BLSI reflects the ratio of granulocytopoietic cells to agranulocytes and it is a marker of the body’s reactivity in acute inflammatory process. Its increase indicates the presence of an active inflammatory process and immune reactivity disorders [18]. Increased BLSI indicates the predominance of granulocytes role at this stage of immune response with some delay of lymphocyte-monocyte cells part, which is the central to implementation of immune response to infectious agents. Such a lag in response to entry of the microorganism by monocytes and lymphocytes leads to delay of phagocytosis completion stage and to late activation of lymphocytes, as an effector component of immune response. In this case, conditions for an extra damaging effect on the tissue due to active granulocytes degranulation are created in the inflammation center. Prognostically can expect a protracted illness [16]. A simultaneous increase in BLSI and/or a decrease in LI in acute inflammation are unfavorable changes in the overall reactivity, which may show an insufficient resource of macroorganism adaptation mechanisms. LI considers more informative, since lymphocytes are the main part of adaptation reactions. BLSI considers characterizing the adequacy and timeliness of the immune response of blood cells, and the LI reflects its balance [16].
LGI is a marker of the degree of leukocyte formula shift, which allows identifying the predominant component of intoxication (autoimmune or infectious) [18]. The decrease of this index in experimental group of animals also indicates the predominance of granulocytes’ role at the time of examination and indicates the infectious nature of intoxication.
NLCR is a simplified biomarker of intoxication, which reflects the ratio of cells of non-specific and specific protection [18]. Some studies have reported that an increased NLCR is associated with inflammatory conditions [34] and might be used as an indicator of the subclinical inflammation [35]. Systemic inflammation leads to neutrophilia and lymphopenia that result in increased NLCR [36]. If we consider that neutrophils have a short lifespan and die during phagocytosis, this limits the duration of non-specific immune protection [24]. So, an increase of NLCR in rats with purulent fecal peritonitis indicates the predominance of non-specific defense cells. This is also confirmed by the increase of sNLCR [18]. Generally, a higher NLCR is associated with poor prognosis [37] and high mortality [9]. It has been established that increased NLCR is a sign of infection and could predict the diagnosis and severity of sepsis. Absolute eosinophil count and NLCR came out as better independent biomarker of sepsis compared to C-reactive protein in critically ill patients with infection [38]. It was investigated that lymphocytopenia and NLCR are better predictors of bacteremia than such routine parameters as C-reactive protein, leukocytes and neutrophils count [20]. Also reported that NLCR is a more reliable marker than C-reactive protein and erythrocyte sedimentation rate for evaluating activity of rheumatoid arthritis [39].
bNLCR is used to assess the state of non-specific resistance of the organism [18]. In experimental group of animals bNLCR is increased significantly, that indicates predominance of immature forms of granulocytes.
NMCR allows to check the ratio of components of microphages and macrophages systems [18]; its increase in experimental group of animals indicates predominance of the microphagal system activity over the macrophages. sNMCR also increased.
LECR reflects the ratio of processes of immediate and delayed type hypersensitivity [18]. The detected decrease in this index in laboratory rats with acute purulent fecal peritonitis shows the formation of hypersensitivity reactions predominantly of delayed type due to changes in leukocyte blood formula (decreasing lymphocytes and increasing eosinophils count). This is also confirmed by increase in ELCR, which is a marker of allergization degree.
An association among infection and lymphocyte and monocyte counts was found, as well as specific associations between these two counts [20]. LMCR together with NLCR can be used as factors of the prognosis determination of patients in various clinical situations. However, many differences exist in these markers depending on race, sex, and age [9]. LMCR reflects the relationship between the affector and effector components of immunological process [18]; its decrease in experimental group of animals compared to control group indicates the predominance of effector component of immunological process and the suppression function of affector cells of immunity. LMCR have been reported to measure the degree of systemic inflammation and indicate prognosis in critically ill patients [40].
Increasing MLCR [18] in experimental group of animals is characteristic for the initiation phases of SIRS and immune toxicosis, immune distress. Its reduction to subnormal levels can mean both elimination of the source of aggression and failure of phagocytic mononuclear cells system or “immune paralysis” [41].
ІRІ in general clinical practice is a marker of hyperergic reactivity; it is the ratio of blood cells that synthesize cytokines. In this case, the relative content of cytokine-producing cells reflects a shift in balance towards lymphokines or monokines [42]. Under the action of IL-5 and IL-13, that produced by T-lymphocyte-helper type 2 [43], eosinophils can produce other cytokines (for example, IL-1, IL-3, IL-5, IL-6, IL-8, colony-stimulating factors, adhesion molecules, etc.), which affects the overall cytokine profile and the spectrum of secondary inflammatory mediators [44]. Sukalo et al. (2013) established that ІRІ increases with hyperergic reaction [45]. The decrease of ІRІ in experimental group of laboratory rats indicates an imbalance in functional state of immune system with a shift towards monokines due to decrease of lymphokines’ producers count. It is known that a decrease in IRI is due to a decrease in the relative lymphocytes count and an increase in monocytes count, indicating a lack of inflammatory blockers, and so also a detoxification part in the spectrum of mediators and means an unfavorable dynamic of immune reactions [46]. Furthermore, the hypoergic variant of sepsis, which is characterized by a sharp decrease in IRI, reflects a deficiency of cytokines of lymphocytic origin and limited adaptation reserves, while the hyperergic variant (a significant increase in IRI) indicates cytokine hyperproduction and mediators’ imbalance (“mediator storm”) [42].
Revealed a decrease of sNbNCR in experimental group of animals indicates the predominance of immature granulocytes forms at the time of examination. Decrease in МLІІ [18] indicates existence of infectious intoxication.
Thus, а set of changes of analyzed HLRI in laboratory rats with acute diffuse purulent fecal peritonitis, compared with the control group, reflects next immune system disorders: the predominance of cellular immunity over the humoral (decrease in LI and LYMI), granulocytes prevail and increased activity of the inflammatory process (increase in BLSI and sNLCR), predominance of the microphage system over the macrophage system (increase in NLCR, NMCR, sNMCR), intense non-specific immunity (increase in NLCR and MLCR, decrease in LGI and LMCR), granulocytes and their immature forms prevail (increase in bNLCR, decrease in sNbNCR), the infectious nature of intoxication (decrease in LGI and МLІІ), disturbance of immune system functional state with a shift of balance towards monokines by reducing lymphokines’ producers count (decrease in ІRІ), formation of hypersensitivity reactions mainly of delayed-type (decrease in LECR, increase in ELCR), decrease function of affector cells and predominance of effector part of immunological process (decrease in LMCR, increase in MLCR).
AUC for BLSI, NLCR, sNLCR, bNLCR, ELCR, MLCR, NMCR and sNMCR is in range 0.95-1.00 means that these indices have very high levels of sensitivity and specificity and can be used as diagnostic markers for acute purulent fecal peritonitis.
Prospects for further research are to clarify HLRI informativity in comparison with other methods for assessing reactivity in laboratory rats of different ages in normal and pathological conditions. It is interesting also to analyze correlation of HLRI with laboratory parameters of blood and urine.
CONCLUSIONS
To sum up, acute infectious process in experimental group of animals is accompanies by leukocytosis with corresponding changes in leukocyte blood formula (neutrophilia with leukocyte blood formula shift to the left, relative lymphopenia) and inhibition of neutrophils absorption capacity. A set of changes of analyzed HLRI in laboratory rats with acute diffuse purulent fecal peritonitis reflects such immune system disorders: cellular immunity prevails over the humoral, increased activity of the inflammatory process, microphages system prevail, intense non-specific immunity, granulocytes and their immature forms prevail, the infectious nature of intoxication, disturbance of immune system functional state with a shift of balance towards monokines, formation of hypersensitivity reactions mainly of delayed-type, predominance of effector part of immunological process. Analysis of ROC showed that BLSI, NLCR, sNLCR, bNLCR, ELCR, MLCR, NMCR and sNMCR can be used as predictors of acute purulent fecal peritonitis in nonlinear laboratory rats.
Thus, HLRI give additional information about the state of macroorganism at the time of examination without using additional laboratory tests and can be an informative indicator for early diagnosis, assessment of disease dynamics and the effectiveness of treatment of acute infectious diseases in humans and animals.
ACKNOWLEDGEMENT
The authors received no funding from external sources.
AUTHOR CONTRIBUTIONS
This work is a collaboration among all the authors. ROL and LVM were involved in conception and design of the experiments. ROL contributed to perform the experiments. Both ROL and LVM analyzed and interpreted data, made the illustration. ROL contributed to drafting the article. LVM contributed to revising it critically for important intellectual content, revised the manuscript for necessary changes in grammar and English standard. ROL and LVM made the final approval of the version to be published.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ross JT, Matthay MA, Harris HW. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018; 361: k1407. doi: 10.1136/bmj.k1407.
- [2]Styazhkina SN, Ovechkina IA, Shakirova LCh, Habibullina GF. The problem of generalized peritonitis in modern abdominal surgery. Sinergiya Nauk / Synergy of Science. 2017; 11: 561-566.
- [3]Bensignor T, Lefevre JH, Creavin B, Chafai N, Lescot T, Hor T, et al. Postoperative peritonitis after digestive tract surgery: surgical management and risk factors for morbidity and mortality, a cohort of 191 patients. World J Surg. 2018; 42(11): 3589–3598.
- [4]Krivoruchko IA, Lesovoy VN. (eds). Urgentnaya abdominalnaya hirurgiya [Urgent abdominal surgery]. Schedraya Usadba Plyus: Harkov, Ukraine, 2015. (in Russian).
- [5]Facciorusso A, Antonino M, Orsitto E, Sacco R. Primary and secondary prophylaxis of spontaneous bacterial peritonitis: current state of the art. Expert Rev Gastroenterol Hepatol. 2019; 13(8): 751-759.
- [6]Mascena GV, Figueiredo Filho CA, Lima Júnior M, Oliveira T, Gadelha D, Melo M, et al. Fecal peritonitis in aging rat model. Therapeutic response to different antibiotic strategies. Acta Cir Bras. 2018; 33(5): 446–453.
- [7]Kennedy MA. Feline infectious peritonitis: update on pathogenesis, diagnostics, and treatment. Vet Clin North Am Small Anim Pract. 2020; 50(5): 1001-1011.
- [8]Miesner MD, Reppert EJ. Diagnosis and treatment of hardware disease. Vet Clin North Am Food Anim Pract. 2017; 33(3): 513–523.
- [9]Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore). 2018; 97(26): e11138.
- [10]Campbell TW. Exotic Animal hematology and cytology (4th ed.). Wiley Blackwell: Ames, Iowa, 2015.
- [11]Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PloS One. 2012; 7(8): e42860.
- [12]Lazarenko VA, Lipatov VA, Blinkov YuYu, Skorikov DV. Experimental model of diffuse fecal peritonitis. Kurskij Nauchno-Prakticheskij Vestnik “Chelovek i ego Zdorov’e” / Kursk Scientific and Practical Bulletin “Man and His Health”. 2008; 4: 128-132.
- [13]Gordeev AA, Chetverin AB. Methods for screening live cells. Biochemistry (Moscow). 2018; 83(1): S81-S102.
- [14]Theml H, Diem H, Haferlach T. Color atlas of hematology. Practical microscopic and clinical diagnosis (2nd ed.). Thieme: Stuttgart–New York, USA, 2004.
- [15]Nazarenko GI, Kishkun AA. Klinicheskaya otsenka rezultatov laboratornykh issledovaniy [Clinical assessment of laboratory test results]. Meditsina: Moscow, Russia, 2000. (in Russian).
- [16]Sakovich AR. Haematological leucocytes indexes in cases of acute purulent sinusitis. Medicinskij Zhurnal / Med J. 2012; 4: 88-91.
- [17]Abramovich ML, Ploskireva AA. Characteristics of hematological indices in acute respiratory diseases in children of different age groups. Lechaschiy Vrach. 2015; 11: 59-64.
- [18]Hodlevskyi AI, Savoliuk SI. Diahnostyka ta monitorynh endotoksykozu u khirurhichnykh khvorykh [Diagnosis and monitoring of endotoxicosis in surgical patients]. Nova Knyha: Vinnytsia, Ukraine, 2015.
- [19]Dunn OJ, Clark VA. Basic statistics: a primer for the biomedical sciences (4th ed.). John Wiley & Sons: New York, USA, 2009.
- [20]de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010; 14(5): R192.
- [21]Mokhber Dezfouli MR, Lotfollahzadeh S, Sadeghian S, Kojouri GA, Eftekhari Z, Khadivar F, et al. Blood electrolytes changes in peritonitis of cattle. Comp Clin Path. 2012; 21(6): 1445–1449.
- [22]Liao MH, Chen SJ, Tsao CM, Shih CC, Wu CC. Possible biomarkers of early mortality in peritonitis-induced sepsis rats. J Surg Res. 2013; 183(1): 362–370.
- [23]Bailly Y, Duprat P. Normal blood cell values, rat. In: Jones TC, Ward JM, Mohr U, Hunt RD. (eds). Hemopoietic system. Monographs on pathology of laboratory animals. Springer: Berlin, Heidelberg, 1990, pp. 27-38.
- [24]Serhan CN, Ward PA, Gilroy DW. (eds). Fundamentals of inflammation. Cambridge University Press: New York, USA, 2010.
- [25]Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee M. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018; 11(5): 627–635.
- [26]Bateman RM, Sharpe MD, Singer M, Ellis CG. The effect of sepsis on the erythrocyte. Int J Mol Sci. 2017; 18(9): 1932.
- [27]Segev G, Klement E, Aroch I. Toxic neutrophils in cats: clinical and clinicopathologic features, and disease prevalence and outcome – a retrospective case control study. J Vet Intern Med. 2006; 20(1): 20–31.
- [28]Garkavi LKh, Kvakina EB, Ukolova MA. Adaptatsionnyye reaktsii i rezistentnost organizma [Adaptation reactions and organism resistance] (3rd ed.). Rostov-na-Donu, Russia, 1990. (in Russian).
- [29]Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun. 2002; 70(7): 3602–3610.
- [30]Li JL, Li G, Jing XZ, Li YF, Ye QY, Jia HH, et al. Assessment of clinical sepsis-associated biomarkers in a septic mouse model. J Int Med Res. 2018; 46(6): 2410–2422.
- [31]Bhardwaj M, Rutuja P, Mani S, Malarvizhi R, Vasanthi HR. Refinement of LPS induced sepsis in SD rats to mimic human sepsis. Biomed Pharmacol J. 2020; 13(1): 335–346.
- [32]Skirecki T, Drechsler S, Hoser G, Jafarmadar M, Siennicka K, Pojda Z, et al. The fluctuations of leukocytes and circulating cytokines in septic humanized mice vary with outcome. Front Immunol. 2019; 10: 1427.
- [33]Assinger A, Schrottmaier WC, Salzmann M, Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front Immunol. 2019; 10: 1687.
- [34]Delgado-Miguel C, Muñoz-Serrano AJ, Núñez V, Estefanía K, Velayos M, Miguel-Ferrero M, et al. Neutropthil-to-lymphocyte ratio as a predictor of postsurgical intraabdominal abscess in children operated for acute appendicitis. Front Pediatr. 2019; 7: 424.
- [35]Ahsen A, Ulu MS, Yuksel S, Demir K, Uysal M, Erdogan M, et al. As a new inflammatory marker for familial Mediterranean fever: neutrophil-to-lymphocyte ratio. Inflammation. 2013; 36(6): 1357–1362.
- [36]Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001; 102(1): 5–14.
- [37]Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013; 58(1): 58–64.
- [38]Khajuria R, Jamwal V, Gupta AK, Gupta A. Evaluation of eosinophil count and neutrophil-lymphocyte count ratio versus C-reactive protein levels in patients with sepsis. Int J Res Med Sci. 2017; 5(11): 4754-4760.
- [39]Ghang B, Kwon O, Hong S, Lee CK, Yoo B, Kim YG. Neutrophil-to-lymphocyte ratio is a reliable marker of treatment response in rheumatoid arthritis patients during tocilizumab therapy. Mod Rheumatol. 2017; 27(3): 405–410.
- [40]Li S, Liu K, Zhang R, Gao Y, Fang H, Liu X, et al. Lower lymphocyte to monocyte ratio is a potential predictor of poor outcome in patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol. 2018; 4(3): 148–153.
- [41]Cherniy VI, Nesterenko AN. Narusheniya immuniteta pri kriticheskikh sostoyaniyakh: osobennosti diagnostiki [Immunity disturbances in critical conditions: diagnostic features]. Vnutrennyaya Medicina / Internal Medicine. 2007; 2(2).
- [42]Shabalov NP. Neonatologiya [Neonatology], vol. 2 (3rd ed.). MEDpress-inform: Moscow, Russia, 2004. (in Russian).
- [43]Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15(6): 985–995.
- [44]Weller PF, Lim K. Human eosinophil-lymphocyte interactions. Mem Inst Oswaldo Cruz. 1997; 92(2): 173–182.
- [45]Sukalo AV, Elinevskaya GF, Prilutskaya VA. Allergiya u novorozhdennykh detey [Allergy in newborn children]. Belorusskaya Nauka: Minsk, Belarus, 2013. (in Russian).
- [46]Pakhrova OA, Krishtop VV, Kurchaninova MG, Rumyantseva TA. Changes of blood leukocyte indices under acute experimental cerebral hypoxia in rats with different levels of stress resistance. Modern Problems of Science and Education. 2016; 6.