Potential roles of vitamin D in the treatment of COVID-19 patient and improving maternal and child health during pandemic
Abstract
The coronavirus disease 2019 (COVID-19) pandemic is supposed to cause Vitamin D deficiency in many people by a direct effect of home quarantine in the affected countries. Generally, vitamin D provides the human body with significant health benefits including bone development, specific gene regulation, and protection against different diseases. However, deficiency of the optimal amount of vitamin D inside the human body may result in susceptibility to multiple infectious diseases. Therefore, with vitamin D levels gravely decreased by reduced movement and activity, a number of possible negative outcomes are expected in COVID-19 patients, pregnant women, and children during this ongoing pandemic. Vitamin D has a direct inhibitory effect on post-infection through a number of mechanisms that promise to make vitamin D a future adjunctive therapy for COVID-19 treatment. Besides, clinical evidence also supports its role in preventing pregnancy complications and improving pregnancy outcomes. Consistent with the manifold role of vitamin D, an increasing number of studies suggest its role in improving the mental health of children who have been adversely affected throughout this pandemic. This review article discusses the potential roles of vitamin D on COVID-19 patients, pregnant women, and children focusing its scope to become a supplementary candidate for these vulnerable groups to combat the ongoing pandemic.
INTRODUCTION
The world is now in the merciless clutch of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) outbreak, which is causing exceedingly higher number of deaths of people than any other coronavirus outbreak that the world has witnessed before. SARS-CoV-2 is a novel enveloped RNA beta coronavirus with 79% genetic similarity with SARS-CoV and 50% with MERS-CoV and causes similar clinical manifestations such as pneumonia, fever, dyspnea and acute respiratory distress syndrome (ARDS) as Severe Acute Respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) [1]. The virus has spread so rapidly in almost every country and territories of the world from its origin in Wuhan, Hubei Province, People’s Republic of China, that it has caused more than 2.3 million deaths along with 107 million confirmed infected cases during the time of writing [2].
Being highly transmissible, this contagious virus presents an alarming threat to the people of affected countries. As a result, the infected countries have employed emergency measures like social distancing and lockdown to reduce the spread of the virus which has forced almost two-third of the global people into quarantine. These complementary restrictive measures have dramatically changed the daily routines of people in affected countries which has again raised the concern of alteration of their normal metabolic pattern. For example, vitamin D is primarily produced inside human body upon sunlight exposure. However, due to staying at home for months and consequently having a reduced sunlight exposure, the drop in serum vitamin D level in people who are experiencing the prolonged lockdown or other movement restrictions during this pandemic is expected to be analogous to that seen in winter.
Vitamin D plays significant roles in maintaining body health in different ways such as by preventing cardiovascular diseases, cognitive deterioration, Type 1 and Type 2 diabetes, by improving the development of autoimmune diseases as well as by participating in immune regulations [3,4]. Likewise, low levels of vitamin D has been linked to hypertension, cognitive impairment, glucose intolerance, increased autoimmune and infection rate [3,4]. Vitamin D has been observed to limit rhinovirus replication, attenuate Respiratory Syncytial virus (RSV) and lessen the risk of developing influenza, advocating its role against respiratory RNA viruses [5, 24]. Regarding COVID-19 susceptibility and maternal health, the immunomodulatory effects of vitamin D are well acknowledged [6,7]. Moreover, low levels of vitamin D have been linked with many pregnancy complications in different studies [7]. Therefore, any imbalance in vitamin D profile caused by prolonged lockdown in COVID-19 patients and pregnant women may lead to undesirable consequences. Additionally, months of social isolation and restricted out-of-home activities have left many children struggling with their mental health. Children are constantly exposed to COVID-19 updates and have to battle with their hurled-up emotions over a number of reasons like loss of a close one. Consequently, there is a sharp incline in mental health issues among children and adolescents following the COVID-19 outbreak [8]. Different studies have also suggested the potential roles of vitamin D in improving mental health [9]. With only a few globally approved, licensed vaccines and specific antiviral drugs, this pandemic calls for effective adjunctive therapy. As the virus spreads in new geographical areas and a potential vaccine being available to the mass population is both time consuming and costly, cost-effective supplementary therapeutic candidates such as vitamin C and honey have already been recognized for their pharmacological effects against SARS-CoV-2 [10,11]. Recent epidemiological data on COVID-19 and a century worth of research behind vitamin D suggest that it could be a potential, complementary therapeutic agent in mitigating COVID-19 [24,26,27,30].
Here, we sought to explore the possible roles of vitamin D in the treatment of COVID-19 patient and improving maternal health. Herein, we also discuss the beneficial aspects of vitamin D on children’s mental health which is at risk or adversely affected by this continuing global crisis.
GENERAL ROLES OF VITAMIN D IN HUMAN
Vitamin D is classically recognized for its role in calcium absorption and in bone mineralization. Deficiency of vitamin D is known to cause myopathy, osteoporosis, osteomalacia, sarcopenia, rickets, juvenile as well as rheumatoid arthritis [12,13,3].
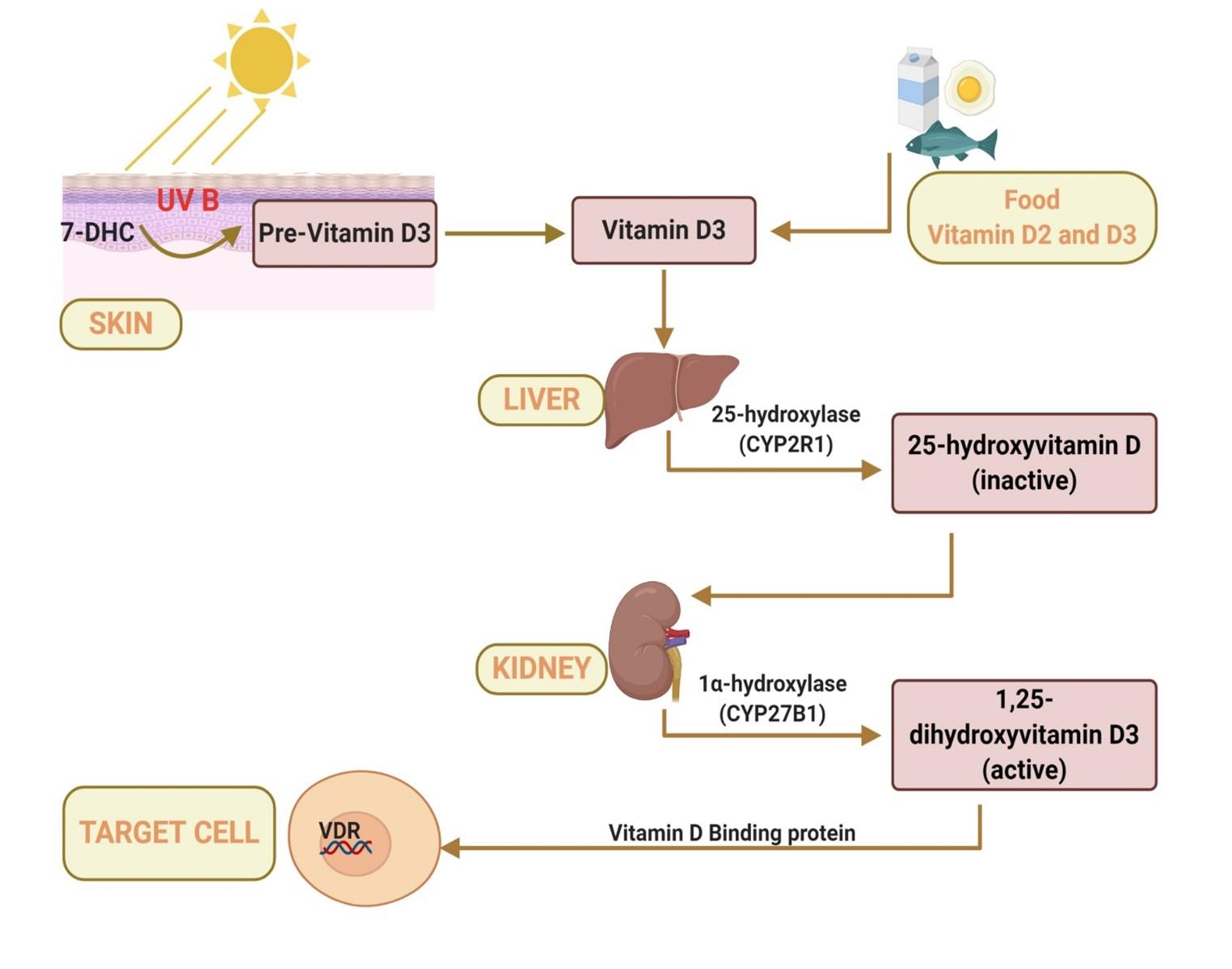
The largest organ, skin, is the site of synthesis of vitamin D. Ultraviolet B (UVB) rays of wavelength 290 to 315 nm drive the cutaneous synthesis of pre-vitamin D3 from 7-dehydrocholesterol (7-DHC) (Figure 1). Vitamin D3 formed from thermal isomerization of pre-vitamin D3 is hydroxylated once by 25-hydroxylase in the liver to form the relatively inactive metabolite, 25-hydroxyvitamin D (calcifediol). The second hydroxylation is mediated by 1α-hydroxylase in the kidney to form the active hormone, 1,25-dihydroxyvitamin D (calcitriol) [5]. While cutaneous synthesis accounts for most of the vitamin D, diet provides only a minor portion of the required amounts of vitamin D in the human body. Moreover, except for a few dietary sources such as fatty fish, egg yolk and mushrooms, most of the common foods do not contain vitamin D. Afterwards, the vitamin D metabolites bind to its primary transport protein called Vitamin D binding protein (DBP) present in the blood plasma for systemic transport to the target organs. Ultimately, all biological actions of the hormonal form of vitamin D (calcitriol) in the target cells are mediated by a transcription factor called Vitamin D receptors (VDRs) present on the nuclei of most cells. Binding of the ligand calcitriol to VDR on the nucleus modulates cell-specific gene expression. Most cells in the body, including skin, heart, stomach, brain, pancreas and activated B and T cells have nuclear VDR [14]. Upon binding of Calcitriol with VDRs, the retinoic receptor of the heterodimer VDR subsequently binds to the Vitamin D response element (VDRE) in the promoter of vitamin D regulated genes [5]. This modulates 3–5% of the human genome, enabling vitamin D to exhibit pleiotropic effects [15].
Despite utilizing the largest organ with large surface area for UVB exposure, vitamin D synthesis is strictly dependent on both the amount of UVB reaching the dermis and the amount of 7-DHC present in the skin. This is because dietary vitamin D intake does not suffice the recommended optimal vitamin D levels as does synthesis of endogenous vitamin D. Epidermal concentration of 7-DHC declines with age, placing elderly population under an increased risk of developing vitamin D deficiency [16]. Co-incidentally, the severity of COVID-19 also increases with aging. The amount of UVB penetrating the dermis is hindered by the type of clothing, sunscreen, season, altitude and type of day-to-day activity, time spent away from windows, or outside as well. As a result, being house bound for long durations greatly suppresses cutaneous synthesis of vitamin D.
The presence of VDR in most cells of the body indicates that the extra skeletal effects stretch far beyond calcium absorption and bone health. Among many pathways that calcitriol regulates, a significant number is devoted to antineoplastic actions and thus plays a crucial role in preventing cancer progression [15]. Besides these, different studies have revealed the association of vitamin D deficiency with variety of diseases including cardiovascular diseases, type 1 and type 2 diabetes mellitus and multiple sclerosis [3].
Additionally, vitamin D plays crucial roles for different groups of people like children, adolescents and pregnant women who are the center of attention during the ongoing pandemic. Expression of VDR by almost all cells and the enzyme that converts calcifediol to calcitriol by some immune cells indicate vitamin D’s role in modulating both the innate and adaptive immune system [4]. This key enzyme and VDR are also expressed by the cells present in placenta reflecting its potential roles during pregnancy [17]. Vitamin D, being able to bind to VDR in neuronal and glial cells, highlights its neurosteroid function in the central nervous system and hence in mental wellbeing [18]. Above all, the multiple roles of vitamin D in human body seem to be seemingly important with other dietary metabolites in order to maintain good body health. And therefore, the lack of synthesis of vitamin D due to reduced activity during this lockdown may leave one individual unhealthy and increase the risk of disease. With the general roles of vitamin D being described so far, the later part of this article sheds light on the necessity of vitamin D supplementation for regulating the immune system and for the mental wellbeing of children and women during pregnancy.
The half-life of circulating calcitriol is only 12 hours whereas it is about 2 weeks for 25(OH) D (Calcifediol) [19]. Therefore, serum 25(OH) D level instead of calcitriol is a reliable indicator of blood vitamin D levels. And thus, serum level of 25(OH) D above 30 ng/ml in serum is considered as ideal whereas less than 15 ng/ml is an indicator of vitamin D deficiency [4]. As long as blood level of 25(OH) D does not exceed 150 ng/ml, vitamin D intoxication is very unlikely to occur. Since blood level of 25(OH) D increases only by 1ng/ml for every 100 International Units (IU) vitamin D ingested, the daily recommended vitamin D intake for infants should be at least 400 IU, for children at least 600 IU according to both Endocrine Society and Institutes of Medicine and between 1500–2000 IUs for adults according to Endocrine Society [20,21]. Considering the limited amount of sunlight exposure in this pandemic, these should be the minimum doses of every age range.
POTENTIAL ROLES OF VITAMIN D ON COVID-19 PATIENTS
In light of different epidemiological findings on COVID-19, a number of studies have discovered the correlations between the disease susceptibility and vitamin D levels. Assessing the prevalence of COVID-19 cases worldwide, it is revealed that people in countries with high latitude and colder temperatures and with low means of vitamin D are most affected [22]. In fact, the major coronavirus epidemics, SARS and COVID-19, all thrived in colder temperatures and disproportionately affected the people in these regions compared to those in comparatively warm and hot atmosphere [23].
A number of observational and clinical trials have shown that vitamin D supplementation could reduce the risk of respiratory diseases like influenza and direct correlations of low vitamin D levels and COVID-19 severity exist [24]. COVID-19 and influenza are both caused by respiratory tract viruses and both were very likely to reach their peaks in winter. Promising clinical trials with vitamin D supplements against influenza could also be extended to COVID-19. Moreover, people with tuberculosis share similar symptoms with COVID-19 patients. Cod liver oil, a rich source of vitamin D was used unknowingly as a remedy for Tuberculosis for its remarkable health-restoring ability [25]. Higher levels of calcitriol have shown to be effective in alleviating severe pneumonia [26]. Low 25(OH) D levels have also been related to high COVID-19 case-fatality rates, while increasing serum 25(OH) D levels have shown to abate the disease severity [27] indicating that vitamin D might play pivotal roles in reducing COVID-19 severity and death rates as well. Additionally, high doses of vitamin D3 on mechanically ventilated intensive care unit (ICU) patients have helped them get discharged from the hospital considerably earlier than planned in one study and have shown to increase mRNA expression of the Human Cationic Antimicrobial Protein (hCAP18) in critically ill, ventilated patients in another study, suggesting the effective role of vitamin D in post SARS-CoV-2 infection treatment [28,29]. Thus, suppressing severe pneumonia and disease severity could save patients on the brink of death. Moreover, reduced hospital stays and expedited recovery time is not only a relief for the patient but also allows new patients to be admitted. This is particularly essential in this COVID-19 crisis as hospitals are brimming with patients and the healthcare system is facing challenges.
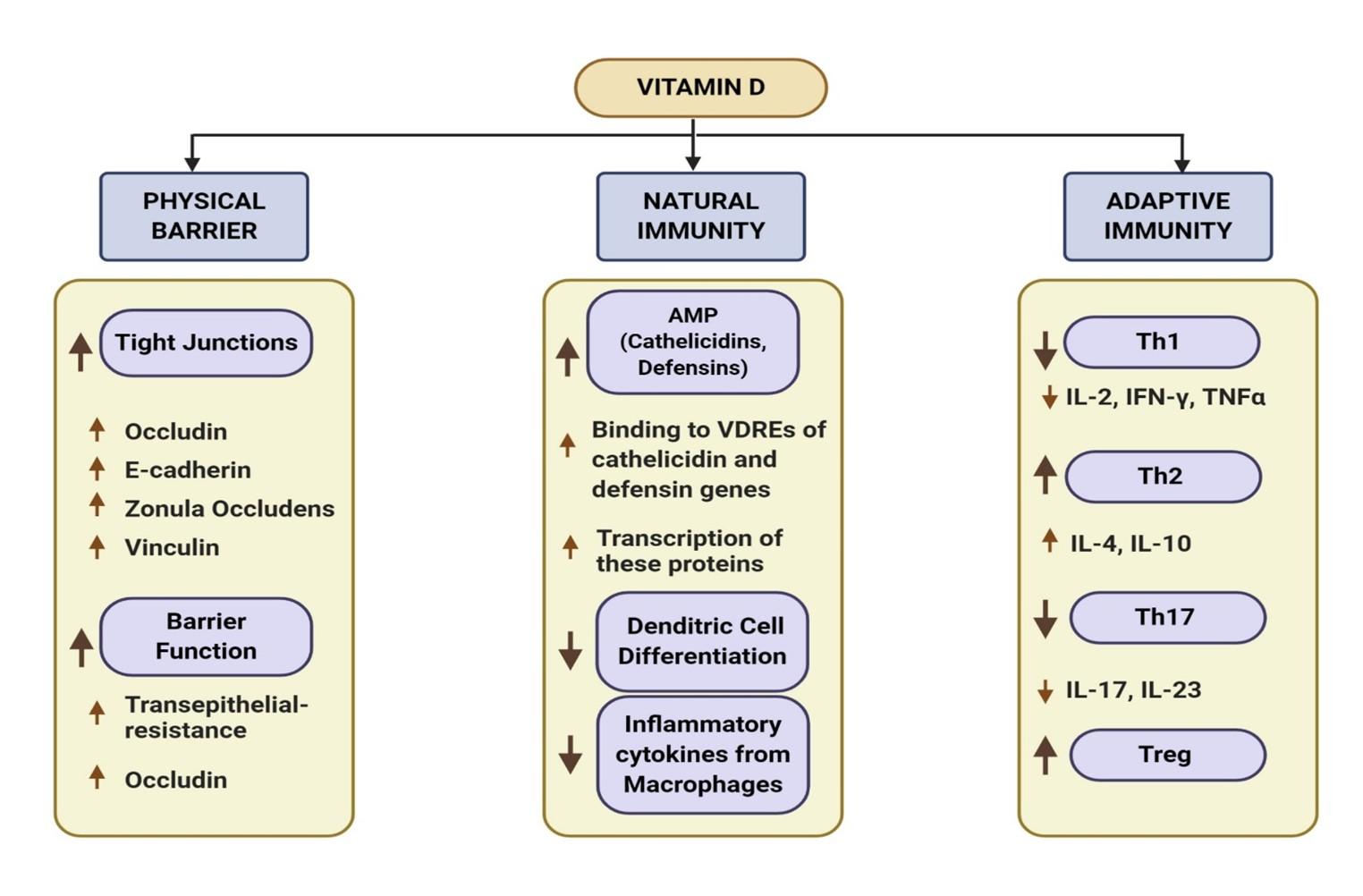
Besides the classical roles of vitamin D in maintaining homeostasis of calcium and bone metabolism, the immunomodulatory effects of vitamin D have become clearer in last few decades. Hence, the deficiency of vitamin D can increase the susceptibility of a number of infections. For example, it is known to modulate 3 pathways during episodes of common cold i.e., physical barrier, cellular natural immunity and adaptive immunity (Figure 2) [30].
Calcitriol upregulates occludin, E-cadherin, vinculin and promotes translocation of “zonula occludens” that are essential for maintaining tight junction between epithelial cells [31]. It has also shown to contribute to epithelial barrier function by increasing trans epithelial resistance. As epithelial tissue is the entry site of SARS-CoV-2, barrier integrity is crucial which is oftentimes disrupted upon viral infection [32].
VDR, the receptor of calcitriol, is expressed in many immune cells of myeloid and lymphoid lineage, suggesting its role as an immunomodulator both in autocrine and paracrine manner [33,25]. Following infection, macrophages use Toll-like receptors (TLR) to recognize foreign antigens. Binding of TLR causes enhanced expression of both VDR and the 1-α-hydroxylase, the enzyme that synthesizes calcitriol from serum 25-hydroxyvitamin D [34]. Metabolically active calcitriol binds to VDR which then recognizes and binds to VDREs in the promoters of genes that code for antimicrobial peptide (AMP) [35]. As a result, enhanced transcription of the human cathelicidin antimicrobial peptide (camp) and defensin β2 (defB2) occur. Defensins and cathelicidins are body’s host defense peptides which act against viruses. Multi antiviral mechanisms of defensins target viral envelopes, capsids and inhibit viral replication, while cathelicidin have shown to inactivate virus and reduce influenza A viral replication in infected mice [36,37]. Moreover, vitamin D also improves innate immunity by acting as a potent inhibitor of dendritic cell differentiation and pro-inflammatory cytokine secretion from macrophages to prevent uncontrolled inflammation [38]. This is essential because infection by SARS-CoV-2 triggers a T helper1 (Th1) cell response leading to a dysfunctional immune response referred to as “cytokine storm” which is injurious to lung health [39]. In fact, COVID-19 severity is fueled by this pro-inflammatory “cytokine storm” in the lung lining leading to severe pneumonia and COVID-19 associated Acute Respiratory Distress Syndrome (ARDS).
As a modulator of adaptive immunity, vitamin D inhibits production of inflammatory cytokines and enhances cytokine production by the T helper type 2 (Th2) cells and thus causing the shift from Th1 to Th2 response [25,38]. It also suppresses the inflammatory Th17 response and induces Treg response and help preventing further havoc in the cytokine storm [25,40].
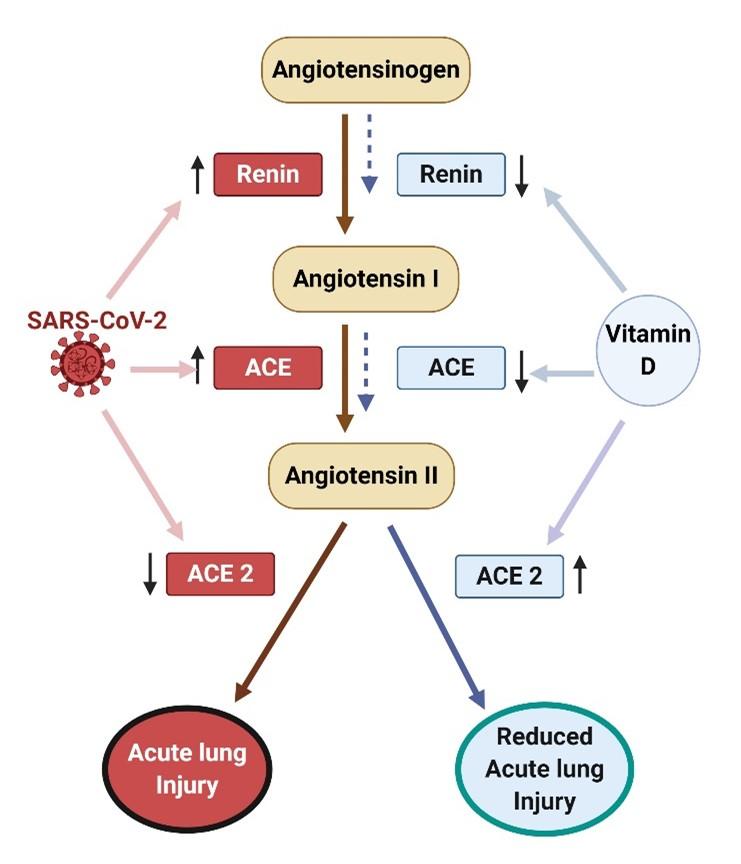
Vitamin D may also protect against COVID-19 severity by increasing the expression of angiotensin-converting enzyme 2 (ACE2) by the lung cells [41]. SARS-CoV-2 uses ACE2 receptor for entry into cells, a receptor that is found in most body tissues but with more prominent expression in respiratory, oral epithelial and alveolar cells [41]. Binding of the virus with ACE2 causes down regulation of the ACE2 receptors. As a result, less ACE2 remains that can degrade Angiotensin II known to increase blood pressure, cause ARDS and even myocarditis [41]. To prevent these adverse effects and multiorgan failure from COVID-19, the therapeutic approach of vitamin D is that it increases ACE-2 that then breaks down angiotensin II (Ang II) and suppresses ACE and renin expression that are essential for Ang II formation
(Figure 3) [41,42].
Furthermore, an in vitro study demonstrated calcitriol’s effectiveness in reducing SARS-CoV-2 viral load in African green monkey Vero E6 cells [43]. The same study also reported that post-infection treatment of human nasal epithelial cells, the primary target of SARS-CoV-2, with calcitriol proved to be more beneficial than most other prophylactic compounds. This study showed a direct inhibitory effect of vitamin D against SARS-CoV-2. Thereby, with only limited and nonspecific current therapeutic agents, vitamin D may be an effective prophylactic combination of vitamin D3 and primaquine significantly reduces lung inflammation and alleviates inflammatory cytokines in pneumonia than primaquine alone, proposing its potential in treating pneumonia associated with COVID-19 [44]. Patients with ARDS often have damaged alveoli as the cells undergo apoptosis upon infection, greatly reducing their oxygen saturation. One study showed that vitamin D deficient mice had greater bronchoalveolar lavage fluid (BALF) cellular inflammation and hypoxia [45] suggesting that vitamin D could have positive impacts on severe ARDS patients. Overall, a notable number of in vitro and in-vivo studies are emerging that relate and show how vitamin D reduces post-infection viral load in infected cells and COVID-19 associated lung damage and examine more effectively than other prophylactic candidates. Although no animal studies have been conducted till now to directly state that vitamin D could prevent the infection of COVID-19 in the first place, epidemiological trends of countries with high mean vitamin D levels and their corresponding low number of cases and the immunomodulatory effects of vitamin D can be considerable factors for its protective role.
ESSENTIAL ROLES OF VITAMIN D ON PREGNANT WOMEN AND INFANTS
Natural disasters and viral outbreaks affect the world in a similar fashion and thus most of them involve a common vulnerable group. For example, the pregnant women were hardest hit during the Spanish flu, the deadliest outbreak of the world so far but the reason behind it still remains a subject of debate [46]. The outbreak of Severe Acute Respiratory Coronavirus (SARS-CoV) was also linked to many pregnancy complications [47] and hence since most about the COVID-19 is still unknown therefore, the impact of the SARS-CoV-2 on pregnant woman cannot be entirely ignored. As a result, the pregnant women still remain another center of great attention and care during COVID-19 pandemic. Different studies have suggested that the influences of vitamin D in the maintenance of good maternal health during pregnancy and healthy infants travel beyond intestinal calcium absorption and bone metabolism. However, maternal 25-hydroxyvitamin D is significantly lower in black and Hispanic women than in white women as melanin competes with 7-DHC in absorbing UVB [48,49]. Although skin pigmentation and season are important determinants of vitamin D synthesis, it has been seen 84% of pregnant women in India had vitamin D insufficiency and 95.7% of neonates had hypovitaminosis D which is much unexpected in a tropical country with abundant sun exposure [50]. It can thus be reasoned that vitamin D insufficiency might be quite common during pregnancy all around the world [51].
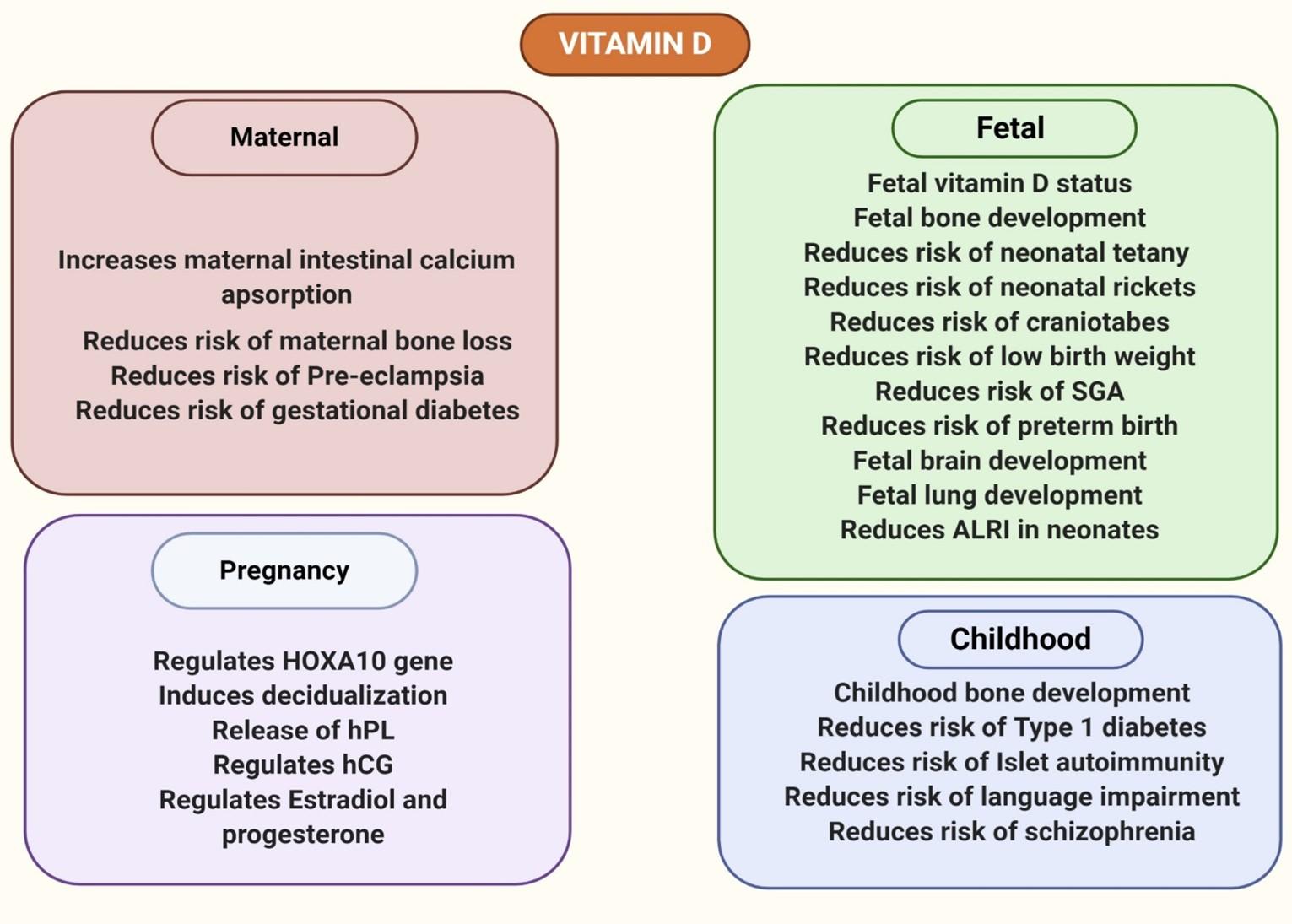
Spending months inside a home in quarantine further diminishes serum 25(OH) D levels, the demand for which escalates as increasing levels of calcitriol is made throughout pregnancy [52]. This increased synthesis of calcitriol during pregnancy is not only to increase calcium absorption for fetal growth but also to provide the required amount of vitamin D for the fetus (Figure 4). Again, the fetal vitamin D levels are so strongly associated with maternal levels that vitamin D supplementation of pregnant women prevents neonatal vitamin D deficiency as evidenced by cord blood vitamin D level measurement [53]. Both placental and decidual tissues are able to synthesize VDR and 1α-hydroxylase [54]. The expression of these vitamin D signaling components increases during the first trimester and thus again suggests the increased synthesis of calcitriol and its potential roles in fetal development [54].
Calcium is indispensable for fetal bone mineralization and accounts for 30g of calcium in the fetal skeleton at term, most of which is derived from maternal nutrition [55]. If maternal intestinal calcium absorption is restricted, the fetus might derive its necessary calcium from maternal bone resorption resulting in maternal bone loss [56]. To prevent bone loss of the mother and to ensure proper skeletal mineralization of the fetus, the classical role of calcium absorption of vitamin D increases to double maternal intestinal calcium uptake (Figure 4) [57]. While some animal models demonstrate the lack of function of the components of vitamin D signaling in active transport of calcium and phosphorus across the placenta [58,59], another study suggests the possible role of VDR in placenta involved in transplacental calcium transfer as hypothesized from positive correlation between placental VDR and fetal femur length [60].
Moreover, maternal vitamin D deficiency can result in enamel defects, congenital rickets as well as craniotabes in neonates [61-63]. In addition to that, vitamin D has also been shown to be associated with fertility in mice [64] and in the regulation of HOXA10, a gene involved in embryogenesis and implantation [65,66]. Tolerance of the semi-allogeneic fetus possessing half of the genes from the father- throughout pregnancy might require the suppression of the adaptive immune system and enhanced stimulation of the innate immune system to compensate for the compromised immunity [54,67]. The immunomodulatory and immunosuppressive effects of vitamin D explained earlier could suggest a role in implantation tolerance. Calcitriol also induces the differentiation of endometrial cells into decidual cells, synthesis and secretion of human placental lactogen and regulates human chorionic gonadotropin, progesterone and estradiol secretion in trophoblasts, all of which are essential during pregnancy and any dysregulation may give rise to a number of complications [68-70].
Besides, vitamin D deficiency is correlated with a number of different adverse pregnancy outcomes. Pre-eclampsia and eclampsia are directly related to 10% to 15% of maternal deaths, with early onset preeclampsia increasing risk of maternal mortality by 20 folds [71,72]. Vitamin D deficiency is a significant risk factor of severe and mild forms of preeclampsia in pregnant women [73-76]. In fact, a 10 ng/mL increase in 25-hydroxyvitamin D in pregnant women has shown to cause a 63% decrease in risk of early onset of severe preeclampsia [76]. Vitamin D is also associated with an increased risk of low birth weight, Small for Gestational Age (SGA) babies and preterm birth [77-81]. Again, supplementation of vitamin D has been shown to reduce the risk of gestational diabetes [82]. Moreover, optimal level of vitamin D during gestation is crucial for prenatal brain development and proper alveolarization. Mice progeny born to mothers with low 25(OH)D levels have unusual brain sizes and shapes due to uncontrolled neuronal proliferation (Figure 4) [83]. This backs up vitamin D’s role in maintaining an orderly brain development, morphology and cellular proliferation in embryos.
Maternal vitamin D also regulates proteins involved in surfactant synthesis and alveolar inflation in the fetus, a deficiency of which leads to low lung volume and stiffness in mice [84]. Lower surfactant levels and increased collagen deposition during alveolar development as shown in this study suggests its importance in pregnancy to prevent babies born with impaired lung function and incidents like this during respiratory disease pandemic could be fatal. This is particularly important as hospital transmission poses a risk factor to newborn babies, putting them in an increased risk of respiratory morbidity and debilitation if they are born with impaired lungs. Study has also linked low vitamin D status to increased risk in acute lower respiratory infection in neonates [85]. Complication due to hypovitaminosis D during pregnancy does not end but even extends to health problems later in life of child. Vitamin D insufficiency of the mother during pregnancy does not only affect bone development of the child during childhood but also increases risk of developing type 1 diabetes and islet autoimmunity [86-90]. Language impairments in the child and schizophrenia have also been positively associated with maternal and neonatal vitamin D status [91-93].
Although little is known about the effects of COVID-19 in pregnant women, lessons learned from pregnancy complications of SARS-infected mothers indicate the emergency implication of necessary measures to improve maternal health in this pandemic. Miscarriage, preterm delivery and intrauterine growth restriction was seen among 12 patients who contracted SARS-CoV [47]. Mouse hepatitis virus, a species of coronavirus, has shown to be transmitted in utero from the infected mother mice to the mice fetus [94]. In humans, placental infection with SARS-CoV-2, miscarriage, maternal vascular malperfusion has been observed in pregnant women with COVID-19 [95-97].
Vitamin D deficiency is widely prevalent in pregnant women despite taking prenatal vitamins [98]. This, combined with only minimal exposure to sunlight during quarantine is supposed to greatly deplete serum 25(OH) D levels in pregnant women. In this case,120,000 IU doses given at 20, 24, 28, and 32 weeks of gestation will be sufficient and safe for pregnant women with inadequate sun exposure [98]. Existing literature provide various implications of vitamin D in preventing pregnancy complications and improving postnatal outcomes. Taken together, the supplementation of vitamin D could be an alternative remedy to ensure good maternal and child health during this pandemic. However, little is known if maternal vitamin D has genetic or epigenetic effects on the developing fetus which offers new areas to be addressed to more effectively demonstrate the pivotal roles of vitamin D on pregnant woman, fetus and infant.
VITAMIN D SUPPLEMENTATION IN IMPROVING CHILDREN’S MENTAL HEALTH DURING COVID-19 PANDEMIC
To prevent the transmission of the highly contagious COVID-19, government of nearly all countries implemented disease containment measures. Along with wearing masks, washing hands, maintaining social distance and avoiding public gatherings, the government has also ordered emergency school closure and home quarantine. All previous viral outbreaks of the world were reported to have psychological impacts on affected people i.e., post-outbreak depressive symptoms following 2003’s SARS outbreak, high level of concern, worry and anxiety due to swine flu (H1N1) outbreak, post-traumatic stress disorder (PTSD) and anxiety-depression following the Ebola outbreak [99-102]. Although these quarantine policies are commendable to bring down COVID-19 infection rates, lack of social contact, and separation from school friends further threaten the mental health of children. Not only do these outbreaks cause panic and worry, but restrictive measures such as quarantine and isolation have caused PTSD in 30% children, boredom, isolation frustration, loneliness and depression in people who had been quarantined [103-106].
Many education institutions have shifted to online education providing remote and pre-recorded classes. Lack of in-person contact with peers, teachers and friends, separation from an infected family member, grief of a lost family member, fear of their parents losing their job or suffering financial crisis, exposure to endless reports of deaths is likely to worsen the mental health of a child. COVID-19 trauma might have similar psychological effects as childhood emotional abuse in children Childhood trauma and emotional abuse lead to heightened stress in later life [107]. Many parents and teachers have reported seeing children become withdrawn and depressed especially due to at home classes [108]. Their inability to focus and learn are signs of depression that could prevail even later in life.
Besides quarantine, vitamin D status is also associated with psychological disorders. Evidence suggests that low vitamin D status is associated with depression and depressive disorder, attention deficit hyperactivity disorder (ADHD) in children, PTSD, low mood and impairment of cognitive performance [109-113]. Moreover, Vitamin D supplementation has been shown to lower the depressive symptoms in people with depressive symptoms [114,115]. COVID-19 trauma and vitamin D deficiency due to quarantine could have integrated negative effects on the mental health of children. Additionally, high pro-inflammatory cytokines, as in the cytokine storm induced by SARS-CoV-2, are involved in the pathogenesis of severe post-traumatic psychiatric symptoms [116]. Prolonged imbalance of pro- and anti-inflammatory cytokines could have potential negative impacts in physical health. Suppressing CD4+ T cell cytokines and pro-inflammatory response mentioned previously, vitamin D aids in rectifying the imbalance.
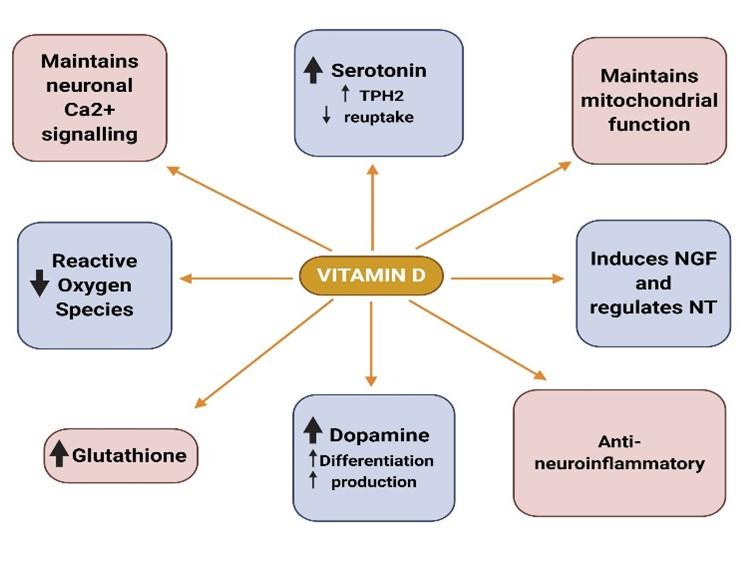
The presence of VDR and 1α-hydroxylase (CYP27B1) in both neurons and glial cells highlights neurosteroid function of vitamin D in the central nervous system and hence in mental wellbeing [117]. Calcitriol also might have an anti-neuroinflammatory activity as seen by its potential of reducing the production of inflammatory molecules in neuron-glia cultures [118]. It has also shown its anti-oxidative properties against reactive oxygen species to protect dopaminergic neurons [119]. Moreover, in vitro studies have revealed its role in the synthesis of nerve growth factor (NGF) and regulation of neurotrophin (NT) [120-122]. VDR is not only expressed in the brain micro vascular endothelial cells of the blood brain barrier [123] but is extensively distributed in many regions of the brain, namely in the cingulate cortex, limbic system, hypothalamus, cerebellum, hippocampus, and cerebral cortex [124,125], with many of the regions showed co-expression of both VDR and 1α-hydroxylase [117]. VDR mutant mouse models exhibit anxiety-like behavior and vitamin D deficient mice have depression-like behavior, both of which have risen during the past couple of months in children [126,127]. This demonstrates how vitamin D could be a sole factor behind anxiety and depression.
Although many factors are thought to interplay in the pathogenesis of depression and have yet to be fully understood, advances have been made that have found potential links in the etiology of depression. Dysregulation of neuronal calcium signaling is linked to major psychiatric and neuronal diseases [128,129]. Vitamin D maintains neuronal calcium homeostasis by controlling the expression of genes involved in neuronal calcium signaling [130]. The long-discussed neurotransmitter, serotonin for its role against depression [131] is up regulated by vitamin D via inducing tryptophan hydroxylase 2 (TPH2), the gene responsible for synthesizing serotonin [132,133]. It has not only proved to enhance serotonin as well as repress serotonin reuptake in in-vitro serotonergic neuronal cells, exhibiting its antidepressant properties [134]. Thus, the ability of vitamin D in neuroplasticity and potentiation opens new insight in its role as alternative Selective Serotonin Reuptake Inhibitor (SSRI) antidepressants. Another neurotransmitter, dopamine, is also well known for its role against the physiology of depression [135-137]. Calcitriol has shown increased dopaminergic neuron differentiation and the production of dopamine [138]. It also stimulates the expression of the antioxidant, glutathione and inhibits the expression of gamma-glutamyl transpeptidase, key enzyme of glutathione metabolism to prevent glutathione depletion and thus has a protective role against neurodegeneration [129,140].
Mitochondrial dysfunction, such as impaired oxidative phosphorylation and membrane polarity are linked to the onset of depression, whereas vitamin D has shown to increase oxidative function [141,142]. By reduction of Ca2+ level in the brain, increasing glutathione, inhibiting the toxicity of reactive oxygen species, inducing nerve growth factors, vitamin D exhibits its neuroprotective effect (Figure 5) [140].
Childhood and adolescence are considered as the window of opportunity for cognitive development and prolonged home quarantine and other restrictive measures may retard the developmental process. Consequently, these may give rise to many short terms and long-term psychological effects. Therefore, preventing negative psychological outcomes of COVID-19 trauma is crucial to prevent predisposed children from developing depression and other disorders. Children dealing with forgetfulness, distraction and restlessness, the prominent signs of stress and anxiety could see changes in their mood and mental health by raising their 25(OH) D levels as shown in different studies. However, lack of direct clinical trials and human studies on exploring the roles of vitamin D on children mental health may require further interventions to establish vitamin D as protective candidate for children suffering from psychological disorders.
FUTURE PERSPECTIVES
Above all, the vitamin D status seems to be an individual risk factor for everyone as evidenced in different epidemiological and observational studies discussed earlier. But there is still lack of enough clinical experiments that can significantly draw the functional roles of vitamin D in preventing COVID-19 severity and reducing mortality. However, depending on the general roles of vitamin D and its function in maintaining good lung health and preventing infection of respiratory tract diseases, people can maintain balanced serum vitamin D level during this pandemic. Hence, it should be advisable for everyone to intake vitamin D supplements or consume fatty fish, or food fortified with vitamin D. Loading doses of 200,000-300,000 IU for vitamin D repletion and subsequent smaller doses according to age, gender and lifestyle is recommended to maintain vitamin D above 30ng/ml [32].
The effect of vitamin D on the cardiovascular and respiratory health in pregnant women suffering from respiratory diseases still remains poorly understood. The lack of studies of mental disorder in children should also be briefly mentioned. On the contrary, the importance of vitamin D in the nervous system is underappreciated, till now. The role of vitamin D in mental disorders is only extensively studied in older adults. Yet again, association between vitamin D deficiency and mental health is largely unexplored in children. More animal models and clinical trials should be conducted so the results can be extrapolated to children and pregnant women as well. Nonetheless, based on the extant literature, this article suggests that vitamin D could be a potential candidate for COVID-19 prophylaxis and complementary treatment. However, more animal studies and clinical trials are needed to study the preventive effects of vitamin D against COVID-19. Moreover, it also appears to be a promising agent for combatting pregnancy comorbidities and ameliorating built-up anxiety and depression in children during hard times like the ongoing pandemic which again requires further investigations to confirm its effectiveness.
CONCLUSION
Although the confirmatory and clinical data to postulate the direct impact of vitamin D on COVID-19 patients, pregnant woman and children’s mental health are scarce, given the strong biological evidence, it seems logical to take vitamin D supplementation especially by the people in the regions where its deficiency predominates. Again, the safeness of its use makes it indispensable to advocate in order to maintain a sound body health. Therefore, uncertain dietary intake of vitamin D to prevent COVID-19 severity should benefit the consumers a much by the time the true correlation establishes. Moreover, the observational and epidemiological studies involving COVID-19 and vitamin D suggest immediate further investigations on vitamin D and its roles in respiratory tract diseases not only for combating COVID-19 but also for any other forthcoming severe respiratory virus outbreak.
ACKNOWLEDGEMENT
Authors are thankful to the members of Community of Biotechnology and Swift Integrity Computational Lab, Dhaka, Bangladesh for the supports during the preparation of the manuscript.
AUTHOR CONTRIBUTIONS
YA conceived the study. MAU designed the study and refined the outline. NA conducted the complementary literature searches and reviews. NA and YA wrote the initial draft. MAU and NA edited and revised the final draft.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Rahman MH, Zahan MS, Al Hasib T, Ahmed KA, Khanam M, Omit MS, Moni A, Uddin MJ. Current knowledge on mechanisms involved in SARS-CoV-2 infection and kidney diseases. J Adv Biotechnol Exp Ther. 2020;3: 30-35.
- [2]Coronavirus Update (Live): 107,982,232 Cases and 2,368,183 Deaths from COVID-19 Virus Pandemic – Worldometer [Internet]. Worldometers.info. 2021 [cited 11 February 2021]. Available from: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdUOA?Si
- [3]Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018;175:60-81.
- [4]Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482-96.
- [5]Lee C. Controversial Effects of Vitamin D and Related Genes on Viral Infections, Pathogenesis, and Treatment Outcomes. Nutrients. 2020;12(4).
- [6]Vyas N, Kurian SJ, Bagchi D, Manu MK, Saravu K, Unnikrishnan MK, et al. Vitamin D in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. J Am Coll Nutr. 2020:1-14.
- [7]Barrett H, McElduff A. Vitamin D and pregnancy: An old problem revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(4):527-39.
- [8]The Coronavirus Seems to Spare Most Kids From Illness, but Its Effect on Their Mental Health Is Deepening [Internet]. Time. 2021 [cited 11 February 2021]. Available from: https://time.com/5870478/children-mental-health-coronavirus/
- [9]Focker M, Antel J, Ring S, Hahn D, Kanal O, Ozturk D, et al. Vitamin D and mental health in children and adolescents. Eur Child Adolesc Psychiatry. 2017;26(9):1043-66.
- [10]Farjana M, Moni A, Sohag AA, Hasan A, Hannan MA, Hossain MG, Uddin MJ. Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19. Infect Chemother. 2020;52(4):461.
- [11]Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon. 2020;6(12):e05798.
- [12]Yuen AW, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74(1):39-44.
- [13]Sengler C, Zink J, Klotsche J, Niewerth M, Liedmann I, Horneff G, Kessel C, Ganser G, Thon A, Haas JP, Hospach A. Vitamin D deficiency is associated with higher disease activity and the risk for uveitis in juvenile idiopathic arthritis-data from a German inception cohort. Arthritis Res Ther. 2018;20(1):276.
- [14]Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-71.
- [15]Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342-57.
- [16]Chen TC, Lu Z, Holick MF. Photobiology of vitamin D. InVitamin D 2010 (pp. 35-60). Humana press.
- [17]Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888-94.
- [18]Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus. 2018;10(7):e2960.
- [19]Vieth R. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. 2020;74(11):1493-7.
- [20]Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for vitamin D. InVitamin D 2010 (pp. 3-33). Humana Press.
- [21]Vieth R, Holick MF. The IOM—endocrine society controversy on recommended vitamin D targets: in support of the endocrine society position. InVitamin D 2018 Jan 1 (pp. 1091-1107). Academic Press.
- [22]Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195-8.
- [23]Chan KH, Peiris JS, Lam SY, Poon LL, Yuen KY, Seto WH. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv Virol. 2011;2011:734690.
- [24]Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988.
- [25]Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881-6.
- [26]Pletz MW, Terkamp C, Schumacher U, Rohde G, Schutte H, Welte T, et al. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res. 2014;15:53.
- [27]Alipio M. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-19). Available at SSRN 3571484. 2020.
- [28]Han JE, Jones JL, Tangpricha V, Brown MA, Brown LAS, Hao L, et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J Clin Transl Endocrinol. 2016;4:59-65.
- [29]Han JE, Alvarez JA, Jones JL, Tangpricha V, Brown MA, Hao L, et al. Impact of high-dose vitamin D3 on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition. 2017;38:102-8.
- [30]Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, et al. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid Based Complement Alternat Med. 2018;2018:5813095.
- [31]Zhang YG, Wu S, Sun J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers. 2013;1(1).
- [32]Short KR, Kasper J, van der Aa S, Andeweg AC, Zaaraoui-Boutahar F, Goeijenbier M, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 2016;47(3):954-66.
- [33]Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123-39.
- [34]Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-3.
- [35]Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-12.
- [36]Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol. 2013;425(24):4965-80.
- [37]Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6(10):e25333.
- [38]Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69-87.
- [39]Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32.
- [40]Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285(50):38751-5.
- [41]Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin‐converting enzyme 2: Implications for a potential treatment for COVID‐19. Rev Med Virol. 2020;30(5):e2119.
- [42]Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1, 25-Dihydroxyvitamin D 3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229-38.
- [43]Mok CK, Ng YL, Ahidjo BA, Lee RC, Loe MW, Liu J, Tan KS, Kaur P, Chng WJ, Wong JE, Hao EW. Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis. bioRxiv. 2020.
- [44]Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob Agents and Chemother. 2017;61(10).
- [45]Dancer RC, Parekh D, Lax S, D’Souza V, Zheng S, Bassford CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617-24.
- [46]Gottfredsson M. [The Spanish flu in Iceland 1918. Lessons in medicine and history]. Laeknabladid. 2008;94(11):737-45.
- [47]Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292-7.
- [48]Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(01):007-12.
- [49]Gokhale S, Bhaduri A. Provitamin D3 modulation through prebiotics supplementation: simulation based assessment. Sci Rep. 2019;9(1):19267.
- [50]Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81(5):1060-4.
- [51]Agudelo-Zapata Y, Maldonado-Acosta LM, Sandoval-Alzate HF, Poveda NE, Garcés MF, Cortés-Vásquez JA, Linares-Vaca AF, Mancera-Rodríguez CA, Perea-Ariza SA, Ramírez-Iriarte KY, Castro-Saldarriaga CA. Serum 25-hydroxyvitamin D levels throughout pregnancy: a longitudinal study in healthy and preeclamptic pregnant women. Endocr Connect. 2018;7(5):698-707.
- [52]Barrera D, Diaz L, Noyola-Martinez N, Halhali A. Vitamin D and Inflammatory Cytokines in Healthy and Preeclamptic Pregnancies. Nutrients. 2015;7(8):6465-90.
- [53]Rodda CP, Benson JE, Vincent AJ, Whitehead CL, Polykov A, Vollenhoven B. Maternal vitamin D supplementation during pregnancy prevents vitamin D deficiency in the newborn: an open-label randomized controlled trial. Clin Endocrinol (Oxf). 2015;83(3):363-8.
- [54]Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11(5):263-71.
- [55]Mahadevan S, Kumaravel V, Bharath R. Calcium and bone disorders in pregnancy. Indian J Endocrinol Metab. 2012;16(3):358-63.
- [56]Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10(2):105-18.
- [57]Kirby BJ, Ma Y, Martin HM, Buckle Favaro KL, Karaplis AC, Kovacs CS. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J Bone Miner Res. 2013;28(9):1987-2000.
- [58]Kovacs CS, Woodland ML, Fudge NJ, Friel JK. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab. 2005;289(1):E133-44.
- [59]Brommage R, DeLuca HF. Placental transport of calcium and phosphorus is not regulated by vitamin D. Am J Physiol. 1984;246(4 Pt 2):F526-9.
- [60]Young BE, Cooper EM, McIntyre AW, Kent T, Witter F, Harris ZL, O’Brien KO. Placental vitamin D receptor (VDR) expression is related to neonatal vitamin D status, placental calcium transfer, and fetal bone length in pregnant adolescents. The FASEB J. 2014;28(5):2029-37.
- [61]Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398-417.
- [62]Paterson CR, Ayoub D. Congenital rickets due to vitamin D deficiency in the mothers. Clin Nutr. 2015;34(5):793-8.
- [63]Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, et al. Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency. J Clin Endocrinol Metab. 2008;93(5):1784-8.
- [64]Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-76.
- [65]Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19(9):2222-33.
- [66]Zanatta A, Rocha AM, Carvalho FM, Pereira RM, Taylor HS, Motta EL, et al. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet. 2010;27(12):701-10.
- [67]Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241-6.
- [68]Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166(5):765-78.
- [69]Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, et al. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol. 2008;6:3.
- [70]Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, et al. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103(3-5):529-32.
- [71]Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130-7.
- [72]Von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143-8.
- [73]Zhao X, Fang R, Yu R, Chen D, Zhao J, Xiao J. Maternal Vitamin D Status in the Late Second Trimester and the Risk of Severe Preeclampsia in Southeastern China. Nutrients. 2017;9(2).
- [74]Benachi A, Baptiste A, Taieb J, Tsatsaris V, Guibourdenche J, Senat MV, Haidar H, Jani J, Guizani M, Jouannic JM, Haguet MC. Relationship between vitamin D status in pregnancy and the risk for preeclampsia: A nested case-control study. Clin Nutr. 2020;39(2):440-6.
- [75]Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366 e1-6.
- [76]Baca KM, Simhan HN, Platt RW, Bodnar LM. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann Epidemiol. 2016;26(12):853-7 e1.
- [77]Song SJ, Si S, Liu J, Chen X, Zhou L, Jia G, et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013;16(4):687-92.
- [78]Chen YH, Fu L, Hao JH, Yu Z, Zhu P, Wang H, et al. Maternal vitamin D deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in Chinese population. J Clin Endocrinol Metab. 2015;100(5):1912-9.
- [79]Chen Y, Zhu B, Wu X, Li S, Tao F. Association between maternal vitamin D deficiency and small for gestational age: evidence from a meta-analysis of prospective cohort studies. BMJ Open. 2017;7(8):e016404.
- [80]Bodnar LM, Platt RW, Simhan HN. Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet Gynecol. 2015;125(2):439-47.
- [81]Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does maternal vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients. 2016;8(5):301.
- [82]Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125(7):784-93.
- [83]McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol. 2004;89-90(1-5):557-60.
- [84]Chen L, Wilson R, Bennett E, Zosky GR. Identification of vitamin D sensitive pathways during lung development. Respir Res. 2016;17:47.
- [85]Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63(4):473-7.
- [86]Zhu K, Whitehouse AJ, Hart PH, Kusel M, Mountain J, Lye S, et al. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res. 2014;29(5):1088-95.
- [87]Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36-43.
- [88]Sorensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175-8.
- [89]Stene LC, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia. 2000;43(9):1093-8.
- [90]Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, Hoffman M, et al. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237-42.
- [91]Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129(3):485-93.
- [92]Eyles DW, Trzaskowski M, Vinkhuyzen AA, Mattheisen M, Meier S, Gooch H, Anggono V, Cui X, Tan MC, Burne TH, Jang SE. The association between neonatal vitamin D status and risk of schizophrenia. Sci Rep. 2018;8(1):1-8.
- [93]Kinney DK, Teixeira P, Hsu D, Napoleon SC, Crowley DJ, Miller A, et al. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections? Schizophr Bull. 2009;35(3):582-95.
- [94]Baergen RN, Heller DS. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr Dev Pathol. 2020;23(3):177-80.
- [95]Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947-53.
- [96]Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, et al. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA. 2020;323(21):2198-200.
- [97]Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23-32.
- [98]Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf). 2015;83(4):536-41.
- [99]Liu X, Kakade M, Fuller CJ, Fan B, Fang Y, Kong J, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23.
- [100]Jones JH, Salathe M. Early assessment of anxiety and behavioral response to novel swine-origin influenza A(H1N1). PLoS One. 2009;4(12):e8032.
- [101]Goodwin R, Gaines SO, Jr., Myers L, Neto F. Initial psychological responses to swine flu. Int J Behav Med. 2011;18(2):88-92.
- [102]Jalloh MF, Li W, Bunnell RE, Ethier KA, O’Leary A, Hageman KM, Sengeh P, Jalloh MB, Morgan O, Hersey S, Marston BJ. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Glob Health. 2018;3(2):e000471.
- [103]Sprang G, Silman M. Posttraumatic stress disorder in parents and youth after health-related disasters. Disaster Med Public Health Prep. 2013;7(1):105-10.
- [104]Reynolds DL, Garay JR, Deamond SL, Moran MK, Gold W, Styra R. Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiol Infect. 2008;136(7):997-1007.
- [105]Yoon MK, Kim SY, Ko HS, Lee MS. System effectiveness of detection, brief intervention and refer to treatment for the people with post-traumatic emotional distress by MERS: a case report of community-based proactive intervention in South Korea. Int J Ment Health Syst. 2016;10(1):51.
- [106]Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-12.
- [107]Muller N, Krause D, Barth R, Myint AM, Weidinger E, Stettinger W, et al. Childhood Adversity and Current Stress are related to Pro- and Anti-inflammatory Cytokines in Major Depression. J Affect Disord. 2019;253:270-6.
- [108]Bracho-Sanchez D. The full toll of Covid-19 on children’s mental health won’t be known for years [Internet]. CNN. 2021 [cited 12 February 2021]. Available from: https://edition.cnn.com/2020/05/28/health/coronavirus-mental-health-children/index.html
- [109]Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.
- [110]Jaddou HY, Batieha AM, Khader YS, Kanaan SH, El-Khateeb MS, Ajlouni KM. Depression is associated with low levels of 25-hydroxyvitamin D among Jordanian adults: results from a national population survey. Eur Arch Psychiatry Clin Neurosci. 2012;262(4):321-7.
- [111]Milaneschi Y, Hoogendijk W, Lips PT, Heijboer AC, Schoevers R, Van Hemert AM, Beekman AT, Smit JH, Penninx BW. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2014;19(4):444-51.
- [112]Terock J, Hannemann A, Van der Auwera S, Janowitz D, Spitzer C, Bonk S, Völzke H, Grabe HJ. Posttraumatic stress disorder is associated with reduced vitamin D levels and functional polymorphisms of the vitamin D binding-protein in a population-based sample. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;96:109760.
- [113]Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-40.
- [114]Högberg G, Gustafsson SA, Hällström T, Gustafsson T, Klawitter B, Petersson M. Depressed adolescents in a case‐series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-83.
- [115]Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
- [116]Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13(1):40.
- [117]Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
- [118]Huang YN, Ho YJ, Lai CC, Chiu CT, Wang JY. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J Neuroinflammation. 2015;12:147.
- [119]Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40(6):761-71.
- [120]Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139-43.
- [121]Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-5.
- [122]Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117-41.
- [123]Takahashi S, Maeda T, Sano Y, Nishihara H, Takeshita Y, Shimizu F, Kanda T. Active form of vitamin D directly protects the blood–brain barrier in multiple sclerosis. Clin Exp Neuroimmunol. 2017;8(3):244-54.
- [124]Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
- [125]Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-45.
- [126]Lardner AL. Vitamin D and hippocampal development-the story so far. Front Mol Neurosci. 2015;8:58.
- [127]Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120-31.
- [128]Berridge MJ. Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res. 2014;357(2):477-92.
- [129]Berridge MJ. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. 2013;7(1):2-13.
- [130]Berridge MJ. Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol Rev. 2017;69(2):80-92.
- [131]Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123.
- [132]Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398-413.
- [133]Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29(6):2207-22.
- [134]Sabir MS, Haussler MR, Mallick S, Kaneko I, Lucas DA, Haussler CA, et al. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018;13:19.
- [135]Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327-37.
- [136]Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32(1):1-17.
- [137]Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect. 1993;91(2-3):75-109.
- [138]Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193-203.
- [139]Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437(1):7-11.
- [140]Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3). Biochemistry (Mosc). 2004;69(7):738-41.
- [141]Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front Neurosci. 2018;12: 386.
- [142]Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab. 2013;98(3): E509-13.