Evaluation of optimum dietary inclusion level of probiotics for potential benefits on intestinal histomorphometry, microbiota, and pH in Japanese Quails
Abstract
Among the alternative options of antibiotics as growth promoters (AGP) to reduce the antimicrobial resistance, probiotics are the attractive alternative which needs to compare at different doses with AGP on the intestinal health of Japanese quail. For this, a total 75 Japanese quails were equally assigned to five treatment groups having three replicates in each group (n=5). In addition to basal diet (control), four other groups were supplemented by AGP and probiotics at the dose of 0.015 gm/bird, 0.03 gm/bird, and 0.045 gm/bird. The results revealed, 0.03 gm/bird probiotics group had significantly (p<0.05) lower mean on gizzard and intestine relative weights (gm/kg) of 23.68 and 35.61; and the relative length (cm/kg) of duodenum, jejunum and ileum were 51.06, 137.30 and 101.95, respectively. Additionally, the villus height (VH) of jejunum and ileum had significantly (p<0.01) higher mean in 0.03 gm/bird probiotics group of 599.25 and 417.25 µm, respectively. Although, there was a quadratic relationship in VH of jejunum (p<0.001) and ileum (p<0.01), CD (p<0.01) and VH:CD (p<0.05) of duodenum with the probiotics dose, but only VH of jejunum and ileum (p<0.001) showed a linear interaction. The enumeration of intestinal bacteria was lower in AGP group but did not differ significantly (p>0.05) with 0.03 gm/bird probiotics group in which the E. coli, Salmonella, Staphylococcus and TBC mean was 5.160 log10, 4.440 log10, 2.923 log10 and 6.972 log10 CFU/gm, respectively. However, the highest pH was recorded in ileum in each group without any significant differences. In a short of, probiotics are effective substitute to AGP and having the potential effects on intestinal health especially for 0.03 gm/bird.
INTRODUCTION
Quail (Coturnix coturnix japonica) farming is the lucrative addition in the poultry industry that the fastest growing sector across the world. During the last few decades, antibiotics as growth promoters (AGP) had been used in the poultry industry to improve feed efficiency and reduce mortality [1]. However, the successive infliction of AGP has driven towards the acquired resistance and residual agents are now one of the major growing concerns [2]. Therefore, embargoes on the use of AGP in many countries have created a gap in preventing poultry against the common pathogens [3]. Consequently, the poultry researchers focused on alternative approaches to improve broiler performance and optimize intestinal health [4]. Among the available options, probiotics are the attractive alternatives which markedly improve performance in comparison to diets without AGP [5]. Probiotics are single or mixed cultures of live microorganisms which balance the intestinal flora as well as leave no residues in animal originated food therefore have no antimicrobial resistance (AMR) properties [6]. The probiotics are mainly composed of some beneficial microorganisms and the most common probiotics containing micro-organisms are Lactobacillus acidophilus, Lactobacillus casei, Bifidobactuium bifidum, Aapergillus oryzae and Torulopsis sp. [7]. Interestingly, these probiotics bacteria prevent the colonization in the gut by reducing the load of various harmful pathogens, such as Escherichia coli, Salmonella spp, Streptococcus spp. and Staphylococcus spp [8]. Besides this, probiotics decrease the GIT pH level and release bacteriocins that hinder the growth of these harmful pathogens [9]. Afterwards, the activity of probiotics improves digestibility of dry and organic matters in the diets [10] and this diet of poultry greatly enhance the development of intestinal morphometric [11]. The morphology of intestinal villi and epithelial cells are related to intestinal functions and the huge villi height indicate active intestinal functions [12]. However, several studies also determined the impact of probiotics on intestinal histology and also on intestinal microbial population but limited were in Japanese quail along with pH measurements of the small intestine [13].
Therefore, update information is always necessary to enhance the performance of poultry. Hence, this study was designed to assess the effects of market available probiotics at different doses in comparison to AGP and basal diets on small intestinal histomorphology, microbial counts and pH of Japanese quail.
MATERIALS AND METHODS
Ethical approval
In this study, all efforts were made to minimize the suffering of the experimental birds in considering with animal welfare policies. Therefore, this study was approved by the Animal Welfare and Ethical Committee, Faculty of Veterinary Science, Bangladesh Agricultural University and the approval number is AWEEC/BAU/2020(29).
Study site, experimental birds, and management
This study was conducted at the Department of Anatomy and Histology, Bangladesh Agricultural University during the period of 3rd January to 2nd February 2019 and Microbial study was performed at the laboratory of department of Microbiology and Hygiene of the same university. In this study, a total of 75 overtly healthy one-day-old Japanese quails (Coturnix coturnix japonica) were purchased from a local hatchery located in Mymensingh Sadar of Bangladesh. After purchase, the birds were transferred to the experimental house under the department of Anatomy and Histology, Bangladesh Agricultural University. This experimental house has 16 hours continuous light facilities for birds both of natural and artificial (light on from 6 a.m. to 10 p.m.). The birds rose with ad-libitum of safe drinking water and mash feed. The basal diet was containing the ingredients without probiotics and antibiotics as per described by Razee et al., in 2016 [14]. Moreover, proper hygiene and sanitation were maintained.
Experimental design
A total of 75, day-old quail chicks irrespective of sex were randomly assigned to 5 experimental groups with the similar average body weight. All experimental groups included 3 replicates with 5 quail chicks in each. Among the five groups, the first was control and maintained only with basal diet but the 2nd, 3rd and 4th groups were supplemented by probiotics additionally with the basal diet at the dose rate of 0.015 gm/bird, 0.03 gm/bird and 0.045 gm/bird, respectively. And the last, 5th group was supplemented by antibiotics growth promoter (AGP) with the basal diet. The commercially available probiotic having the strength of minimum 5×l012 colony forming units (CFU)/gm were supplemented. This product is a freeze dried preparation containing the following live viable strains of naturally occurring microorganisms:
| Bacteria | CFU/gm | Active ingredient (%) |
| Lactobacillus plantarum | 1.26×108 | 6.3 |
| Lactobacillus bulgaricus | 2.06×108 | 10.3 |
| Lactobacillus acidophilus | 2.06×108 | 10.3 |
| Lactobacillus rhamnosus | 2.06×108 | 10.3 |
| Bifidobacterium bifidum | 3.69×108 | 10.0 |
| Pediococcus sp. | 3.69×108 | 18.48 |
| Enterococcus faecium | 5.32×107 | 29.00 |
The AGP was sourced from commercially available broad-spectrum antibiotic (Ciprofloxacin) @ 0.5 gm per 50 kg basal feed. At the day of 49, all birds were weighed individually and recorded. All the birds of each replicate belong to the different groups were maintained under similar management and housing conditions.
Sample collection
On the day 49 (Seven weeks) of study, two quails per replication of each group were randomly selected and killed by cervical dislocation. Then the digestive organs were separated for further morphometric and histological study. Before removing the ingesta from the digestive tract, samples were taken from intestinal content for microbial analysis.
Intestinal morphometric study
In order to estimate the intestinal morphometry of the quail at 49 days of age in each treatment groups, the gastrointestinal tracts were collected, and its contents were removed and cleaned immediately for recording the empty gizzard weight (without fat), intestinal weight along with the length of small intestinal segments (duodenum, jejunum and ileum). The weights were expressed relative to live body weight (BW) (gm/kg) and similarly, the relative lengths to live BW (cm/kg) were measured [15]. A measuring tape and RADWAG balance and scales (Model AS 220.R2) were used to measure the lengths and weights, respectively.
Intestinal histological analysis
Two tissue Samples approximately 2cm from the duodenum (midpoint of the gizzard to bile duct), jejunum (midpoint of the bile ducts to Meckel’s diverticulum) and ileum (midway of the Meckel’s diverticulum to the ileo–caecal junction) of each group per replication were taken for microscopic assessment according to the methods described by Afrin et al., in 2016 [16]. The histological indices from these segments were prepared where the 10% formalin fixed tissues were dehydrated in the series of ascending grade of alcohol. The clearing was done by several changes of xylene and immersing samples into it for 2 hours. Then, different graded of melted paraffin (60 °C and 62 °C) were used to impregnation of the tissues at 30 minutes interval. The samples were thoroughly embedded with 62 °C melted paraffin into a block and finally the tissues were sectioned at 6µm thickness using sliding microtome (MIC 509, Euromex, Japan). The sections were allowed to spread on warm water bath (45°C). The sections were taken on the glass slides after putting 1-2 drops adhesive (Mayer’s egg albumin) onto the slides. After that the slides were allowed to dry at 40°C on a slide warmer for 8 hours. The sections were then stained using Mayer’s Hematoxylin and Eosin (H & E). Finally, a cover slip was mounted over the tissue samples on the slide, using optical grade non-aqueous mounting medium DPX. Necessary photographs were taken with light microscope (Olympus, BX 51, Japan) under low (×10) and high (×40) magnifications. Then the biometric measurements of different histological structures of the intestinal tissues were performed. Villus height (VH) and crypt depths (CD) of the segments were measured by using calibrated eyepiece micrometer (Olympus, U-OCMC10/ 100XY, Japan). Then the villus height and crypt depths ratio (VH: CD) was calculated [17].
Enumeration of intestinal bacteria
To investigate the bacteriological status, 1 gm of intestinal content was collected immediately after sacrificing the two quails from each replication of all experimental groups and processed immediately for microbiological studies. The intestinal contents were serially diluted, whereby 0.1 ml per dilution were inoculated on standard plate count agar for total bacterial count (TBC), Mac Conkey agar for E. coli, Salmonella Shigella (SS) agar for Salmonella and Mannitol salt agar for Staphylococci. After 24 hours incubation, the total numbers of bacterial colonies were counted by a digital colony counter. All the bacteriological culture was maintained according to the methods described by Merchant and Packer in 1967 [18]. Before statistical analysis, the number of bacteria was transferred to Log10 numbers and expressed as arithmetical means ± SE (Log10 CFU/gm).
Intestinal pH measurement
At the end of the study two quails from each group per replication were randomly chosen, slaughtered and pH values of the intestinal segments (duodenum, jejunum, and ilium) were measured by direct probe of pH meter (Lutron PH-208). The intestinal pH measurement was taken five minutes after placing the electrode.
Statistical analysis
The statistical Package for Social Sciences (SPSS) version 25.0 was used for analysis of data. The obtained data from the treatments were subjected to One-Way Analysis of Variance (ANOVA) and followed by Duncan’s multiple range tests was applied to determine the statistically significant differences among the treatment groups. Additionally, the orthogonal polynomial contrast test was considered to ascertain the linear and quadratic effects of increasing probiotics supplementation (gm) in diets on each parameter. The intestinal pH level was analyzed through a boxplot to show the distribution and the level in different treatment groups. However, before considering the statistical test, the assumptions of the performed statistical test were assessed and none found violated. The significant value of the entire test was set, p ≤ 0.05.
RESULTS
Intestinal morphometric study
Commercial strains of probiotic at different doses and AGP were used for experimental challenges in Japanese quails. The results (Table 1) showed that dietary inclusion of commercial probiotic @ 0.03g/bird had significantly increased the relative weights of gizzard (p<0.01) and intestine (p<0.05) over the control (16.94 and 29.09 gm/kg) and AGP (17.43 and 30.27 gm/kg) groups respectively. However, AGP and among the probiotic groups the relative length of intestinal segments (duodenum, jejunum, and ileum) was not statistically significant (p˃0.01) than that of those fed only basal diet (control). Interestinly, the increasing dose of probiotics had a linear effect on relative weight of intestine (p<0.01) and relative length of the duodenum (p<0.05) of Japanese quail. On the other hand, quadratic effect was on relative weight of gizzard (p<0.01) and intestine (p<0.05) of Japanese quail (Table 1).
Table 1. Effects on digestive tract morphometric indices (Mean ± SEM) of control, probiotics and AGP groups in Japanese Quail (Coturnix coturnix japonica).
Intestinal histoarchitecture study
Table 2 shows, the mean data of different treatment groups on intestinal histology, where the villus height (VH), Crypt depth (CD) and VH:CD were measured. In duodenum, the CD had significant (p<0.01) difference among the dietary treatment groups where highest mean was 117.45 µm in group treated with 0.03 gm/bird of probiotics (Figure 1) but had no significant difference with 0.045 gm/bird probiotics diet. Likewise, the VH:CD of duodenum had found significant (p<0.05) difference among all of the groups but the highest value was noted in control group (11.59 µm). Villus height of the jejunum in control and treated groups, the mean had significant (p<0.01) difference and highest was 599.25 µm in diet containing 0.03 gm/bird of probiotics (Figure 2). The last segments of small intestine i.e. ileum of Japanese quails of different treatment groups had significant (p<0.01) difference on VH where the highest was 417.25 µm recorded in group fed by 0.03 gm/bird of probiotics (Figure 3). Although, the quadratic relationship was observed in VH of jejunum (p<0.001) and ileum (p<0.01), CD (p<0.01) and VH:CD (p<0.05) of duodenum with the probiotics dose, but a linear interaction was found only in VH of jejunum (p<0.001) and ileum (p<0.001).
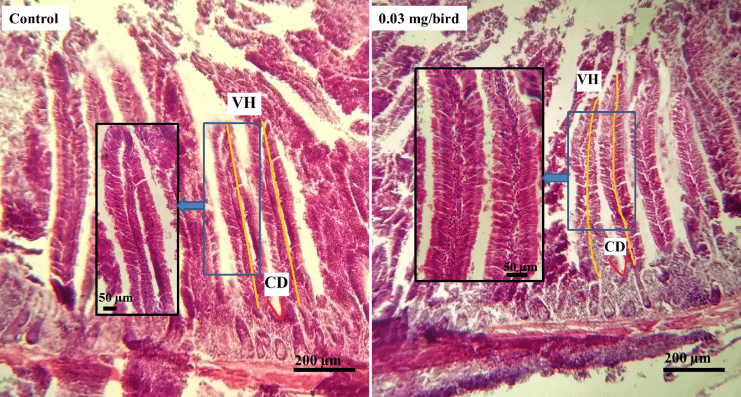
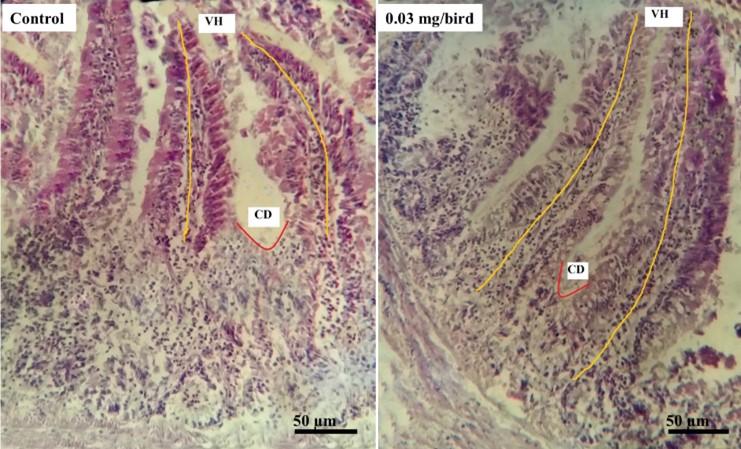
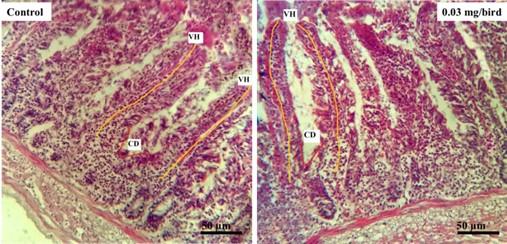
Table 2. Effects on small intestine histological indices (Mean ± SEM) of control, probiotics, and AGP groups in Japanese Quail (Coturnix coturnix japonica).
Enumeration of intestinal microbiota
The enumeration of intestinal microbial population (E. coli, Salmonella spp and Staphylococcus spp) in Japanese quails of different treatment groups were performed and is presented in Table 3. The count of E. coli, Salmonella spp Staphylococcus spp and total bacterial count (TBC) in Japanese quails of 0.03 gm/bird probiotics supplemented group was found lower mean of 5.160 log10, 4.440 log10, 2.923 log10 and 6.972 log10 CFU/gm respectively while significantly (p<0.01) lower mean was also found in AGP treated group but had no significant (p>0.05) difference to 0.03 gm/bird supplemented probiotics group. Interestingly, the higher reduction rate was observed in 0.03 gm/bird probiotics treated group than the other doses of probiotics and control groups. Nevertheless, the highest count of this certain bacteria was in quails of control group, had no additional dietary supplementation. Both linear (p<0.001) and quadratic (p<0.05) effect of probiotics supplementation was observed on intestinal microbial count.
Table 3. Effects on intestinal microbial populations (log10 CFU/gm of intestinal content) of control, probiotics and AGP groups (Mean ± SEM) in Japanese Quail (Coturnix coturnix japonica).
Intestinal pH assessment
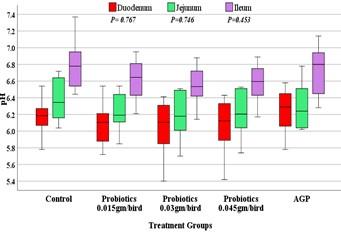
The pH of intestinal segments (duodenum, jejunum and ileum) is reported in boxplot and presented in Figure 4. The boxplot indicated that a higher trend of pH observed in the groups of ileum and lower trend in duodenum. The highest median of pH was found in jejunum of AGP treated quails (6.80) whereas the lowest median was in duodenum at different doses of probiotics supplemented quails i.e. approximately 6.10. However, the pH of duodenum, jejunum and ileum of several groups had no significant (p>0.05) differences.
DISCUSSION
The study revealed that the dietary supplementation of probiotics in comparison to AGP had some extent of potential effects on intestinal properties of Japanese quails. Although Inborr in 2000 [19] reported that probiotics and antibiotics markedly improved the general health status of the poultry. But the effects of antibiotics as growth promoter have been omitted regarding to its toxicity, residues in food and transferable antibiotic resistance after long term administration at low doses in Japanese quails.
The significant positive impacts of probiotics on poultry performance and health were well established. The main assumed health benefits of probiotic bacteria include improving the balance of commensal and pathogenic gut microflora, immunomodulation, producing digestive enzymes, enhancing nutrients bioavailability and digestibility as well as carcass yield and quality of Japanese quails [20]
In our study, the relative weight of gizzard and GIT were significantly higher in 0.03 gm/bird probiotics supplemented group while Hetland et al., in 2005 claimed that the more muscular and enlarged gizzard can improve digestion and unable to affect the digesta movements when lacking feed stimuli [21]. The present study is in agreement with previous studies where the gizzard [22] and intestinal [23] weight of broiler was higher in probiotics treated group but without any significant differences. In contrast, Awad et al., in 2006 [24] observed that the probiotics had no significant effects on gizzard and intestine weight of broiler. These variations could be for differences in sample sizes and species. In case of, the relative length of individual segments of the small intestine (duodenum, jejunum, ileum), Stęczny and Kokoszyński in 2020 [25] found no significant differences in broiler raised with or without probiotics supplement, which suggest our findings in Japanese quails.
The histological study of the intestinal mucosa and the state of microscopic structures can be good indicators for determining the health status of quail provided by active substances in feed [26]. It is assumed that the optimum development and the morphology of the intestinal tract are dependable on the first exposure of microbiota as long as the host matures [27]. The use of probiotics to quail feed is one of the key strategies to enhance the intestinal health for digestion and absorption of nutrients can be assessed by measuring the VH and CD [28]. In our study, the probiotics treatment had the trends to have longer villus in both jejunum and ileum which is in accordance with findings of Hidayat et al., in 2018 [29] who stated that the broiler fed by probiotics can significantly increase the VH of jejunum and ileum other than the duodenum. Subsequently, the inreased ileal villus height was observed with addition of E. faecium [30], and also increased jejunal villus height was found with addition of a probiotic containing Lactobacilli, B. thermophilum, and E. faecium in broiler diet [31]. Longer VH reflected the better gut epithelial cell proliferation after probiotic supplement [32]. Specifically, the longer intestinal villi indicate an increased surface area for enhancing capability of intestine to absorb nutrients [24] and also associated with activated cell mitosis [33]. It is agreed that greater villus height is a sign that the function of intestinal villi is activated [34]. Additionally, the concentrations of amylase in broiler intestine were increased after supplementation of diet with either a single strain of Lactobacillus acidophilus or a mixture of Lactobacillus strains which is responsible for longer villi [35]. However, amylase concentrations were not estimated in the present study and further experiments are needed to verify this effect. In the duodenum of 0.045 gm/bird probiotics supplemented group, the significantly higher value on crypt depth (CD) and some extent longer VH was reflected. Deeper intestinal villi crypts allow the renewal of the intestinal villi by rapid metabolism of tissue when its regeneration is required [36]. Thereafter, a decrease in intestinal villi height or shorter CD may diminish the absorption of nutrients and/or increase the energy requirement to maintain the functions of the intestine [36]. Furthermore, it is assumed that the epithelium of intestinal villi can act as a natural barrier against commonly existent infectious bacteria and toxic substances in the intestinal lumen [37].
Probiotics are living bacteria having the beneficial effects on the host body by improving the balance of intestinal microflora [6]. In our findings, the supplementation of 0.03 gm/bird probiotics had higher beneficial effects on quails by increasing the reduction of intestinal microbial population (E. coli, Salmonella spp, Staphylococcus spp and TBC) which is in agreement with the findings of Manafi et al., in 2016 [38] who narrated that feeding of Bacillus subtilis in Japanese quails markedly reduced the populations of Salmonella, coliforms and E. coli. Similarly, the other authors Siadati et al., in 2017 [39] reported the lowest E. coli populations in Japanese quails under the treatments of the native probiotics. On the other side, though Strompfova et al., in 2012 [40] noted that there were no significant differences in the counts of Staphylococci spp, but the count was lower in caecal content in probiotics treated group than control. This is in line with some studies representing that the administration of probiotics do appear to have only minor and temporary measurable effects on fecal microbiota as assessed by qPCR assays or sequencing of 16S rRNA genes [41]. Surprisingly, the lower count of potentially pathogenic bacteria indicated that the probiotics suppressed the growth of several harmful bacterial species [42]. Those effects are caused by activating the metabolism of one or a partial number of health-promoting bacteria or by collectively stimulating their growth, which improved the health of the host, or both [43]. Particularly, the probiotics that contain Lactobacillus spp. accomplished to produce lactic acid and antimicrobial compounds (organic acids, hydrogen peroxide, diacetyl and bacteriocin) that can kill pathogenic bacteria [44]. Moreover, the probiotic Lactobacillus casei hinders the increase in paracellular permeability normally induced by enteropathogen E. coli [45]. Subsequently, supplementation of probiotic does favor the enterocytes to act as phagocytic cells or as antigen-presenting cells in the same way as M-cells in higher vertebrates that possess degraded bacteria within phagolysosome-like vesicles in its cytoplasm [46]. Hansen and Olafsen in 1999 [47] also observed endocytosis of bacteria by enterocytes in herring (Clupea harengus L.) larvae. The reason behind this has not been interpreted yet. To some extent, this might be attributable to that the pathogen outcompeting the probiotic bacteria due to administration of appropriate levels of probiotic bacteria in quail diets.
On the other side, in our study, the pH values of small intestinal contents did not differ significantly. Likewise, the authors Fonseca et al., in 2010 [48] did not found any significant differences in intestinal pH between the broilers of supplemented and not supplemented with probiotics groups. Additionally, Alam and Ferdaushi in 2019 [22] also observed that pH values of broiler breast meat did not differ significantly among the probiotics, antibiotics, and control groups. However, Eizaguirre et al., in 2002 [49] stated that probiotics reduced intestinal pH in humans, improving the absorption of minerals by enhancing their solubility. Probably, a quantitative proportion of L (+) lactate isomer and D (–) lactate isomer produced by the applied strain influence the pH values [40].
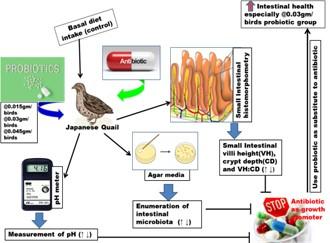
The present study concerning the dose and exposure protocol of probiotics for uttermost protective shield on the intestine of Japanese quails. This study elicited that supplementation of probiotics in quail’s diet can increase the relative weight of gizzard and GIT as well as increase the VH of Jejunum, ileum, and also duodenum CD as well as VH:CD significantly compared to control and AGP. Moreover, the effects of probiotics especially at 0.03 gm/bird reflected on pathogenic intestinal bacteria count where a decrease count was observed in relation to AGP. Although the lactobacillus containing probiotics were supplemented but no significant changes appeared in intestinal pH. However, the aforementioned findings of this study imply that the probiotics, especially at the dose rate of 0.03 gm/bird offers a good alternative to AGP to improve intestinal health in Japanese quail (Figure 5). Furthermore, in future, the probiotics performance in Japanese quails can be assessed in consideration to the larger sample size along with haematological, biochemical, and molecular analysis.
ACKNOWLEDGEMENT
The authors are gratefully acknowledged to the financially support of the BAURES of Bangladesh Agricultural University (BAU), Mymensingh-2202, Bangladesh (Project Number: 2018/559/AU-GC). The authors are also grateful to Professor Dr. Sukumar Saha, Department of Microbiology and Hygiene, BAU for his advice and technical supports during microbiological study.
AUTHOR CONTRIBUTIONS
MA and MS were involved in conception and design of the experiments. MA and NJ contributed to perform the experiments. MMM analyzed data statistically and contributed to drafting the article. MA and MMM contributed to revising it critically for important intellectual content. All authors read the article and approved the final version to be published.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ Sci Pollut Res. 2016; 23(23): 24151–7.
- [2]Bajpai VK, Baek KH, Kang SC. Control of Salmonella in foods by using essential oils: A review. Food Res Int. 2012; 45(2): 722–34.
- [3]Tehseen M, Tahir M, Khan RU, Jabbar A, Ahmad B, Ahsan T, et al. The Philippine Agricultural Scientists. vol. 99. 2016.
- [4]Markowiak P, Ślizewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018; 10(1).
- [5]Faria DE, Borges Cam DM, Alfonso-To KA, Serpa Viei B, Rosa PS, Vaz AM, et al. Protein Levels for Heat-Exposed Broilers: Performance, Nutrients Digestibility, and Energy and Protein Metabolism. Int J Poult Sci. 2007; 6(3): 187–94.
- [6]Fuller AR. Probiotics in man and animals. J Appl Bacteriol. 1989; 66(5): 365–78.
- [7]Mohan B, Kadirvel R, Bhaskaran M, Natarajan A. Effect of probiotic supplementation on serum/yolk cholesterol and on egg shell thickness in layers. Br Poult Sci. 1995; 36(5): 799–803.
- [8]Duke GE. Alimentary Canal: Secretion and Digestion, Special Digestive Functions, and Absorption (4th ed.). Springer: New York, 1986.
- [9]Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000; 130(2): 396S-402S.
- [10]Ahmed ST, Islam MM, Mun HS, Sim HJ, Kim YJ, Yang CJ. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult Sci. 2014; 93(8): 1963–71.
- [11]Fallah R, Saghafi M, Rezaei H, Parvar R. Effect of bioplus 2B and protoxin probiotics supplementation on growth performance, small intestinal morphology and carcass characteristics of broiler chickens. Br J Poult Sci. 2013; 2(2): 11–5.
- [12]Ruttanavut J, Yamauchi K, Goto H, Erikawa T. Effects of Dietary Bamboo Charcoal Powder Including Vinegar Liquid on Growth Performance and Histological Intestinal Change in Aigamo Ducks. Int J Poult Sci. 2009; 8(3): 229–36.
- [13]De-Souza LFA, Araújo DN, Stefani LM, Giometti IC, Cruz-Polycarpo VC, Polycarpo G, et al. Probiotics on performance, intestinal morphology and carcass characteristics of broiler chickens raised with lower or higher environmental challenge. Austral J Vet Sci. 2018; 50(1): 35–41.
- [14]Razee A, Mahbub ASM, Miah MY, Hasnath MR, Hasan MK, Uddin MN, et al. Performance of Japanese Quails (Coturnix coturnix japonica) on Floor and Cage Rearing System in Sylhet, Bangladesh: Comparative Study. Iran J Appl Anim Sci. 2016; 6(4): 931–6.
- [15]Rezaei M, Karimi Torshizi MA, Wall H, Ivarsson E. Body growth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fibre. Br Poult Sci. 2018; 59(4): 422–9.
- [16]Afrin M, Amin T, Karim R, R IM. Effects of formaldehyde intoxication on liver of Swiss albino mice. IOSR J Agric Vet Sci. 2016; 9(9): 76–81.
- [17]Iji PA. The impact of cereal non-starch polysaccharides on intestinal development and function in broiler chickens. Worlds Poult Sci J. 1999; 55(4): 383–7.
- [18]Merchant IA, Packer RA. Veterinary Bacteriology and Virology (7th ed.). The Iowa State University Press: Ames, lowa, USA, 1967.
- [19]Inborr J. Swedish poultry production without ýn-feed antiobiotics – a testing ground or a model for the future? Australian Poultry Science Symposium (APSS). 2000; 12: 1–9.
- [20]Alagawany M, Abd El-Hack Me, Farag Mr, Sachan S, Karthik K, Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ Sci Poll Res. 2018; 25(11): 10611-10618.
- [21]Hetland H, Svihus B, Choct M. Role of Insoluble Fiber on Gizzard Activity in Layers. J Appl Poult Res. 2005; 14(1): 38–46.
- [22]Alam M, Ferdaushi Z. Use of probiotics instead of antibiotics in broiler production. Progress Agric. 2019; 29(4): 359–70.
- [23]Aristizabal-Gutierrez D, Narváez-Solarte W, Giraldo-Carmona J. Evaluation of a probiotic preparation in broilers under commercial breeding conditions. Vet y Zootec. 2016; 10(2): 53–61.
- [24]Awad WA, Böhm J, Razzazi-Fazeli E, Ghareeb K, Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. 2006; 85(6): 974–9.
- [25]Stęczny K, Kokoszyński D. Effect of probiotic preparations (EM) and sex on morphometric characteristics of the digestive system and leg bones, and caecal microflora in broiler chickens. J Appl Anim Res. 2020; 48(1): 45–50.
- [26]Munyaka PM, Echeverry H, Yitbarek A, Camelo-Jaimes G, Sharif S, Guenter W, et al. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult Sci. 2012; 91(9): 2164–72.
- [27]Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut, Science. 2001; 292(5519): 1115–1118.
- [28]Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 2009; 88(1): 49–56.
- [29]Hidayat MN, Malaka R, Agustina L, Pakiding W. Effect of Lactobacillus sp. probiotics on intestinal histology, Escherichia coli in excreta and broiler performance. J Indones Trop Anim Agric. 2018; 43(4): 445.
- [30]Samli HE, Senkoylu N, Koc F, Kanter M, Agma A. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and microbiota. Arch Anim Nutr. 2007; 61(1): 42–49.
- [31]Chichlowski M, Croom WJ, Edens FW, MacBride BW, Qiu R, Chiang CC, Daniel LR, Havenstein GB, and Koci MD. Microarchitecture and spatial relationship between bacteria and ileal, cecal and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult Sci. 2007; 86(6):1121–1132.
- [32]Kim JS, Ingale SL, Kim YW, Kim KH, Sen S, Ryu MH, et al. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J Anim Physiol Anim Nutr (Berl). 2012; 96(4): 618–26.
- [33]Samanya M, Yamauchi K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem Physiol. 2002; 133(1): 95–104.
- [34]Shamoto K, Yamauchi K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult Sci. 2000; 79(5): 718–723.
- [35]Jin LZ, Ho HW, Abdullah N, Jalaludin S. Digestive and bacteria enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult Sci. 2000; 79(6): 886–891.
- [36]Hamedi S, Rezaian M, Shomali T. Histological changes of small intestinal mucosa of cocks due to sunflower meal single feeding. Am J Anim Vet Sci. 2011; 6(4): 171–5.
- [37]Pelicano E, Souza P, Souza H, Figueiredo D, Boiago M, Carvalho S, et al. Intestinal mucosa development in broiler chickens fed natural growth promoters. Rev Bras Ciência Avícola. 2005; 7(4): 221–9.
- [38]Manafi M, Khalaji S, Hedayati M. Assessment of a probiotic containing bacillus subtilis on the performance and gut health of laying Japanese quails (coturnix coturnix japonica). Rev Bras Cienc Avic. 2016; 18(4): 599–606.
- [39]Siadati SA, Ebrahimnezhad Y, Salehi Jouzani G, Shayegh J. Evaluation of probiotic potential of some native lactobacillus strains on the growth performance and serum biochemical parameters of Japanese quails (Coturnix Coturnix Japonica) during rearing period. Rev Bras Cienc Avic. 2017; 19(3): 399–408.
- [40]Strompfova V, Marcinakova M, Gancarcikova S, Jonecova Z, Scirankova L, Guba P, et al. New probiotic strain Lactobacillus fermentum AD1 and its effect in Japanese quail. Vet Med (Praha). 2012; 50(9): 415–20.
- [41]Garcia-Mazcorro JF, Lanerie DJ, Dowd SE, Paddock CG, Grutzner N, et al. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol Ecol. 2011; 78(3): 542–554.
- [42]Vicente JL, Torres-Rodriguez A, Higgins SE, Pixley C, Tellez G, Donoghue AM, et al. Effect of a selected Lactobacillus spp.-based probiotic on Salmonella enterica serovar enteritidis-infected broiler chicks. Avian Dis. 2008; 52(1): 143–6.
- [43]Gibson GR, Roberfroid MB. Dietary modulation of human colonic microbiota: Introducing the concept of prebiotic. J Nutr. 1995; 125(6):1401–1412.
- [44]Abdelbasset M, Djamila K. Antimicrobial activity of autochthonous lactic acid bacteria isolated from Algerian traditional fermented milk Raib. African J Biotechnol. 2008; 7(16): 2908–14. doi: 10.5897/AJB07.753.
- [45]Parassol N, Freitas M, Thoreaux K, Dalmasso G, Bourdet-Sicard R, Rampal P. Lactobacillus casei DN-114001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol. 2005; 156(2): 256–262.
- [46]Ringø E, Salinas I, Olsen RE, Nyhaug A, Myklebust R, Mayhew TM. Histological changes in intestine of Atlantic salmon (Salmo salar L.) following in vitro exposure to pathogenic and probiotic bacterial strains. Cell Tissue Res. 2007; 328(1):109-16.
- [47]Hansen GF, Olafsen JA. Bacterial interactions in early life stages of marine cold water fish. Microb Ecol. 1999; 38(1): 1–26.
- [48]Fonseca BB, Beletti ME, da Silva MS, da Silva PL, Duarte IN, Rossi DA. Microbiota cecal, morfometria do íleo, pH do inglúvio e desempenho zootécnico de frangos de corte sob suplementação com probióticos. Rev Bras Zootec. 2010; 39(8): 1756–60.
- [49]Eizaguirre I, Urkia NG, Asensio A, Zubillaga I, Zubillaga P, Vidales C, et al. Probiotic supplementation reduces the risk of bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2002; 37 (5): 699-702.