Antiviral effect of honey extract Camelyn against SARS-CoV-2
Abstract
This study aimed to evaluate the potential antiviral effects of honey extract “Camelyn” against Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2). The baby hamster kidney cell line 21 (BHK‐21), bone marrow-derived hematopoietic stem cells (HSCs), and splenic cells were used for Camelyn cytotoxicity assay. After the isolation procedures, cell viability was assessed by trypan blue dye exclusion under a microscope using a hemocytometer. The in vitro cell growth rate was carried out using the cell counting Kit 8 (CCK-8) assay. The cells were seeded in growth media with various Camelyn concentrations (35 μg, 50 μg, 70 μg, 100 μg, 150 μg, and 200 μg). The absorbance at 450 nm was determined by the multiplate reader. The antiviral effect was assessed by plaque reduction assay for the determination of drug susceptibility against SARS-CoV-2. Serial dilution of the selected compounds was pre-incubated with 40 to 100 plaque-forming units (PFUs) of SARS-CoV-2. The pre-incubated mix of Camelyn and SARS-CoV-2 was then added to the confluent Vero E6 cells after incubation cells were fixed and stained and the number of PFUs was counted under an inverted microscope and plotted against the logarithm of antiviral concentrations. Our study showed that Camelyn is not cytotoxic, has a stimulatory effect on cell proliferation, and has an inhibitory effect against SARS-CoV-2 with EC50 (half-maximal effective concentration) from 85.7 μg/mL to 192.4 μg/mL depending on product concentration and viral plaque per cell.
INTRODUCTION
The current pandemic shows a great demand for every possible treatment and prevention approach against COVID-19 including existing natural products. Various organic compounds including bee honey, propolis, royal jelly, curcumin, resveratrol are extensively studied and utilized as potential treatment options for different infections [1]. Despite the critique of modern medicine in recent years, honey has got great attention due to its wide range of therapeutic properties including antimicrobial, anti-inflammatory, and antiviral activity [2, 3]. Researchers have described various phytochemical factors such as hydrogen peroxide, volatile organic acids, lysozyme, glucose oxidase, catalase as effective antibacterial factors [4]. The beeswax, pollen, and propolis are important chemical compounds that provide antimicrobial properties to honey [5, 6]. Honey also contains oligosaccharides in small quantities related to the growth inhibition of various microbes, such as intestinal bacteria [7]. Phenolic compounds, including flavonoids, of honey, propolis, and royal jelly are attributed to biologically active molecules that demonstrate antimicrobial effects [8, 9]. These physical and chemical factors give honey unique properties. It is determined that honey eliminates wound infections, provides minimization of scarring, suppresses inflammation, stimulates angiogenesis and epithelium growth [10]. The honey’s anti-inflammatory activity showed an inhibition of the expression of cytokines [11]. It is known that honey can improve the proliferation of T and B lymphocytes, stimulates phagocytosis, and regulates the production of cytokine.es from monocytes, such as tumor necrosis factor (TNF), interleukin 1 beta (IL-1β), and IL-6 [12]. It was determined that honey and its several components block the cell cycle of colon cancer cell lines in the G0/G1 phase [13, 14].
In vitro studies have shown the antiviral activity of honey against different types of viruses [15-17]. The antiviral effect of honey is attributed to its various ingredients, for instance, copper, which is a trace element part of honey inactivates viruses. The phenolic compounds, such as flavonoids, ascorbic acid, or hydrogen peroxide cause viral growth inhibition by interrupting viral transcription, translation, and replication [1], [18], [19]. To elucidate the possible action of honey, plaque inhibition assays were used in Watanabe et al. study [20]. It was reported that Manuka honey efficiently inhibited influenza virus replication (EC50 = 3.6 ± 1.2 mg/mL), which is related to its antiviral effects. In the presence of 3.13 mg/mL Manuka honey, the EC50 of zanamivir or oseltamivir was reduced to nearly 1/1000th of their single-use. The results showed that honey has a strong inhibitory activity against the influenza virus, and demonstrated a possible medicinal value it may have.
The different kinds of honey from eight floral sources were analyzed to evaluate their anti-HIV-1 activities as well as their effects on lymphocyte proliferation. The anti-HIV-1 activity of eight different kinds of honey was performed by quantitative polymerase chain reaction (PCR) assay. The study revealed that monofloral (the same plant species) kinds of honey had anti-HIV-1 activity depended on plant sources and the amount of methylglyoxal in these plants biomass [21].
The study of Abedi et al. [22] provided some evidence of the potential effects of honey and its compounds against the coronavirus due to their ability to regulate the attachment and entry of the virus into the host cell and RNA replication. Honey and its components may also regulate cellular signaling pathways including oxidative stress, inflammation, and apoptosis. One mechanism of the anti-viral action is inhibition of the viral proteins necessary for attachment and entry into host cells [23]. It has been pointed out that honey can affect the disulfide bonds in hemagglutinin protein HA receptors, which prevents the attachment of the influenza virus to the host cell surface. The coronavirus spike protein belongs to the same class of protein family [24]. It has been reported that [25,26] honey compounds such as quercetin, chrysin, kaempferol, galangin, and caffeic acid have anti-viral activity against COVID-19 through strong binding affinity to main protease and viral replication. The main compounds of honey, such as kaempferol, galangin, and caffeic acid can inhibit virus adsorption, invasion, and replication. Chrysin can prevent virus entry into the host cells and virus replication. Guercerin can inhibit virus coating, invasion, and replication [27–29]. The recent studies and the review article of the potential pharmacological effects of honey [30] indicate that honey and its main components have potential implications for the prevention and treatment of coronavirus infection, including COVID-19.
Although the antimicrobial activities of honey have been well studied against many bacteria and fungi [31] [32], its antiviral activities still need extensive investigations that it can be used as prevention and treatment of various viral infections. This study aimed to evaluate the antiviral effects of honey extract Camelyn against Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2).
MATERIALS AND METHODS
The commercial product Camelyn ampoules, obtained from JSC “Silicon Biotechnology”, is made from a selected honey extract. The contents of the ampoule consist of 35% of Camelyn, and 65% water for injection. Camelyn contains ketones, ethers, bioorganic acids, phenols, aldehydes, and furfural. For cytotoxicity and antiviral assays, Camelyn was diluted to a final concentration from 10 μg/mL to 2500 μg/mL.
Experimental animals
Six-week-old BALB/c mice (n=3) were bred and housed in a breeding facility at the State Research Institute Centre for Innovative Medicine (Lithuania). All procedures were carried out under the institutional guidelines of the European Union and were approved by the Lithuanian Ethics Committee on the Use of the Laboratory Animals under the State Veterinary Service No. G2–124 (2019.07.11). Animals were maintained in an environment of controlled temperature (23 ± 1 °C). Food and water were provided ad libitum.
Cells preparation
The baby hamster kidney cell line 21 (BHK‐21) was obtained from Vilnius University Life Sciences Center (Lithuania). Parental BHK-21 cells were seeded in high glucose Dulbecco’s Modified Eagle Medium (DMEM) (4.5 g/L) (Life Technologies, USA), supplemented with 10% FBS (Lonza, Switzerland) and 1% antibiotics (penicillin and streptomycin 10.000 U (Lonza, Switzerland). Cultures were maintained at 37 °C and 5% CO2 atmosphere. The cell monolayer was dispersed using a 0.25% trypsin – EDTA (Lonza, Switzerland) mixture.
Bone marrow-derived hematopoietic stem cells (HSCs) were isolated by flushing femur and tibiae of BALB/c mice as previously described by Juppperi et al. [33] with some modifications. Splenic cells were isolated by gentle pressure-dissociation of tissue using PBS and then passed through a 70 µm sterile cell strainer. Collected HSCs and splenic cell suspensions were washed with PBS and then fractionated in a density gradient using Lympholyte M (Cedarlane, USA) media according to the manufacturer’s recommendations. The isolated HSCs and splenic cells were washed three times in Roswell Park Memorial Institute RPMI-1640 media containing 10% FBS (Lonza, Switzerland), and 1% antibiotics (penicillin and streptomycin 10.000 U (Lonza, Switzerland), centrifuged for 10 minutes at 300 × g, resuspended, and counted. After the isolation procedures, cell viability was assessed by trypan blue dye (0.4%, w/v) exclusion under Nikon ECLIPSE 50i (Nikon, Japan) microscope using a hemocytometer.
Camelyn cytotoxicity assay
The in vitro cell growth rate was carried out using the cell counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Japan) according to the recommendation by the manufacturer. Two x 105 BHK-21, HSCs, and 5 x 105 splenic cells were seeded in growth media into 96-well plates and for 72 hours incubated with control and various Camelyn concentrations (35 μg, 50 μg, 70 μg, 100 μg,150 μg, and 200 μg) at 37 °C in a 5% CO2 atmosphere. The absorbance at 450 nm was determined by the multiplate reader Sunrise (Tecan, Austria). The viability of the Camelyn treated cells was compared to control cells (untreated) and treated with DMSO (positive control). All assays were performed in three independent experiments.
SARS-CoV-2 plaque reduction assay
The compound was assessed by plaque reduction assay for the determination of drug susceptibility against SARS-CoV-2/Quebec City/21697/2020. The selected compounds were assessed by plaque reduction assay, the gold standard phenotypic method for the determination of drug susceptibility against SARS-CoV-2. Briefly, confluent Vero E6 cells were seeded at 1 × 105 cells/well into 6 well plates. Serial dilution of the selected compounds was pre-incubated with 40 to 100 plaque-forming units (PFUs) of SARS-CoV-2 for 60 min at 37 °C in a 5% CO2 atmosphere. The pre-incubated mix of compound and SARS-CoV-2 was then added to the confluent Vero E6 cells and incubated for 60 minutes at 37 °C in a 5% CO2 atmosphere. Then inoculum was removed and the infected cells were incubated for three days (without the compound) in Minimum Essential Medium (MEM) (Merck, Germany) with 2% fetal bovine serum (Thermo Fisher Scientific, USA) containing 0.6% SeaPlaque agarose (Lonza, Switzerland). Cells were fixed and stained and the number of PFUs was counted under an inverted microscope and plotted against the logarithm of antiviral concentrations. The EC50 values then were calculated. In parallel, the antiviral drugs favipiravir and remdesivir were assessed by plaque reduction assay according to a standard protocol (no pre-incubation of a virus with drugs) for the determination of drug susceptibility against SARS-CoV-2.
Statistical analysis
Statistical analyses were done using Microsoft Excel and IBM SPSS Statistics software package 25. Spearman rank correlations were used to calculate relationships between variables. A probability level of p-value < 0.05 was taken as statistically significant.
RESULTS
Cytotoxicity assay
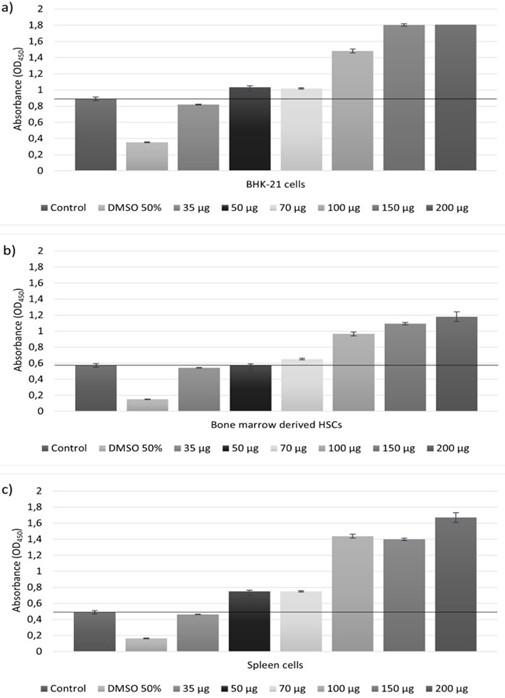
Cytotoxicity assay is a quantitative determination of the difference between cell death and cell growth and has been used in our experiments to evaluate the possible effect of the honey product on cell growth and proliferation. Each ampoule of honey extract Camelyn contained 2 ml of an amber-colored solution for injection. The content of active compounds was 0.035g/mL. None of the Camelyn concentrations (35 μg, 50 μg, 70 μg, 100 μg,150 μg, and 200 μg) tested had an adverse cytotoxic effect (Figure 1a, 1b, 1c). The higher Camelyn concentration significantly increased the hematopoietic stem cell amount. Compared with control, the number of HSCs ranged from 114%, when Camelyn concentration was 70 μg/mL, to 206% when concentration reached 200 μg/mL. Similar trends were observed in cell line BKH-21 assay (Figure 1a, 1b). The number of cells increased from 116% to 203% when the concentration increased from 70 μg/mL to 200 μg/mL. The stimulatory effect of Camelyn was even more notable for spleen cell growth. Starting from Camelyn concentration of 50 μg/mL the growth of spleen cells was increased, and at the end of the experiment, the average number of treated cells was more than 3 times higher compared with control (Figure 1c).
SARS-CoV-2 plaque reduction assay
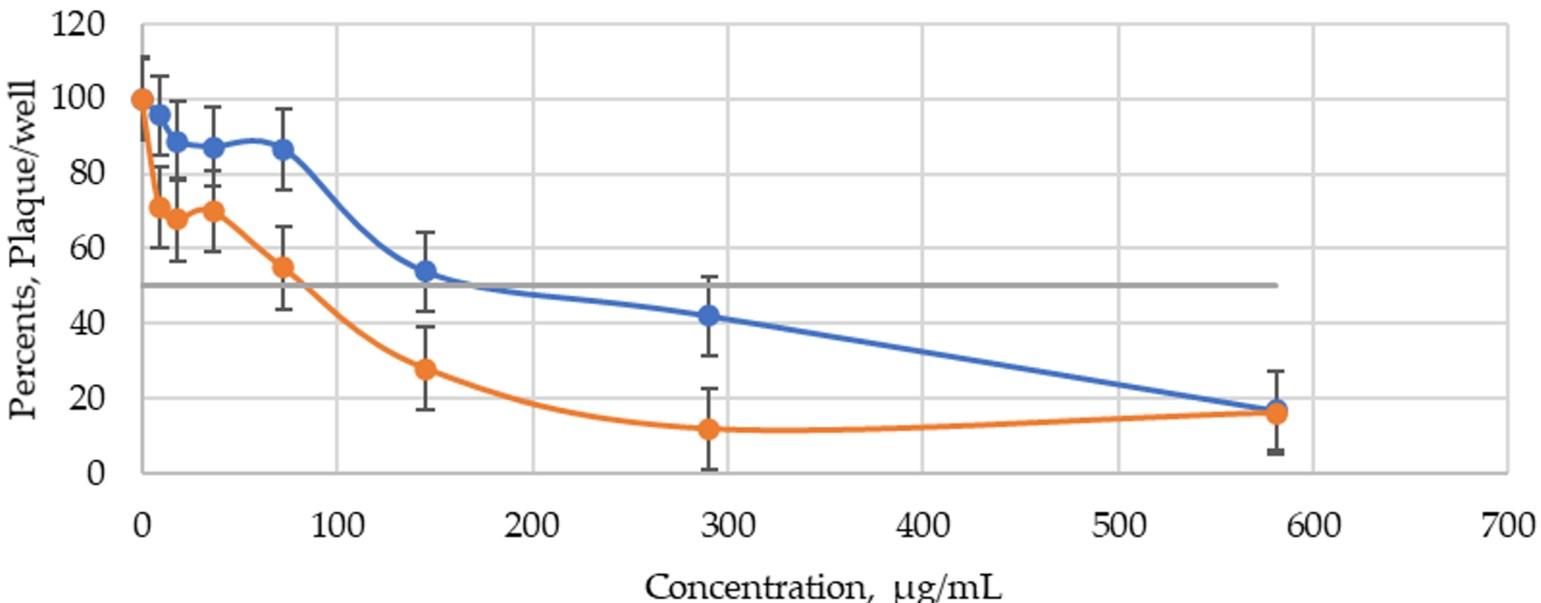
To assess the antiviral activity of the honey product Camelyn against SARS-CoV-2, its half-maximal effective concentration (EC50) was determined. Confluent Vero E6 cells were seeded in 6-well plates. Two-fold serial dilutions of Camelyn were pre-incubated with about 50–100 plaque-forming units (PFUs) of SARS-CoV-2/Quebec City/21697/2020 for 60 minutes and used to infect cells. After 3 days of incubation (without Camelyn) cells were fixed and stained with crystal violet. The number of PFUs was counted under an inverted microscope and plotted against the logarithm of antiviral concentrations to obtain the EC50.
Concentrations of honey extract Camelyn from 9.08 μg/mL to 72.6 μg/mL, had an insignificant effect on virus plaque reduction when cells were infected with 100 PFU. The number of virus plaques decreased by 4.5–13.5 %. The higher concentration of 145.3 μg/mL has reduced the number of virus plaques to 53.85% (Figure 2). The EC50 was 192.4 μg/mL.
When virus inoculum was reduced to 25–30 PFU, Camelyn concentrations from 9.08 μg/mL to 36.3 μg/mL decreased virus plaque number 30–33% (Figure 2). Starting from 72.6 μg/mL concentration, the number of virus plaques was reduced significantly compared to control. This assay revealed, the concentration of honey extract Camelyn has shown inhibitory activity with EC50 of 85.7 μg/mL. An additional test was done using the concentrate product, Camelyn tablets, to verify the similarity of the profile of inhibition. The number of virus plaque had a similar effect on Camelyn tablet inhibition. However, the dilutions of the concentrated Camelyn tablet’s solution have shown a stronger inhibitory effect with EC50 of 116.27 ± 73.39 μg/mL when infected with 100 PFU. Our results showed that Camelyn extract seems to have an inhibitory activity at the beginning of the replicative cycle of SARS-CoV-2.
In comparison, the antiviral drugs favipiravir and remdesivir activities were found to have EC50 of 15.71 μg/mL (100 μM) and 0.616 μg/mL (1.16μM), respectively. Wang et al. [34] identified favipiravir to have activity in vitro against SARS-CoV-2, albeit requiring a high concentration compared with remdesivir (EC50 = 61.80 μM). Notably, remdesivir potently blocked virus infection at low-micromolar concentration (EC50 = 0.77 μM) [35].
DISCUSSION
Honey is known for its medicinal benefits and receiving attention as natural medicine. The growing number of scientific and clinical reports suggest that honey could be used not only for home treatment but also for wound healing and tissue repair [36], [37], [38]. The beneficial effects of honey on wound healing mostly were attributed to antibacterial activity. High sugar content, which leads to high osmotic pressure, and low pH cause bacterial cell dehydration and cell wall disruption. Studies show that the antimicrobial activities of honey are related to increases in reactive oxygen species (ROS) hydrogen peroxide activity [39]. The antioxidant effect of honey is correlating with its anti-inflammatory and wound healing activity [40]. The honey samples may release hydrogen peroxide that is produced by the enzyme glucose oxidase and is responsible for the antimicrobial effect [39]. The harmful oxidizing effect of hydrogen peroxide is not observed on skin cells due to the honey polyphenolic component, which can antagonize the action of this ROS. Some researchers have noted the potential of honey to induce stem cell proliferation, stimulate hematopoietic stem cell migration, and also mediate the healing process by increasing tissue blood flow. On the other hand, the honey exhibited inhibitory effects on cellular growth by reducing the proliferation ability, inducing cell apoptosis, and inhibiting the cell cycle in a dose-dependent manner [41].
In many instances, honey should be used as it is. However, as needed, the active ingredients of honey, such as small peptides, amino acids, polyphenols, sugars, vitamins, can be extracted from honey. Camelyn is an original honey extract that was received from the special sort of honey by a patented extraction method and is a mixture of sugars, proteins, polyphenols, vitamins, minerals, and free amino acids. The data showed that Camelyn is not cytotoxic, and has a strong stimulatory effect on cell proliferation. Due to this property, Camelyn would be useful in wound healing as other honey products.
The data of Bakradze et al. clinical study of inflammatory diseases of parodentium revealed that Camelyn possesses immunostimulation, anti-inflammatory action, activates regeneration process has an analgesic effect. 56 patients with various forms of the disease (gingivitis, parodontitis) were under clinical observation. The study results have confirmed clinical appropriateness to use Camelyn for gingivitis, parodontitis, and periodontitis in combined treatment [42]. The research of Chumburidze et al. [43] showed an excellent regenerating and healing effect of Camelyn on damaged tissues. The features of Camelyn for treatment of different types of infections and tumors and pharmacokinetics of Camelyn in rat’s plasma were described in this study. The minimum inhibitory concentration (MIC) of Camelyn was determined against some of the bacterial and fungal strains in the study of Maglakelidze et al. [44]. Camelyn was seen to possess powerful inhibitory action (0,012-0,150 μg/mL) against most test bacteria in vitro studies. Camelyn exhibited potent in vitro activities against fluconazole-resistant strains of Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei, with MICs at which 90% of isolates were inhibited of 0.012 μg/ml, respectively.
As far as is known, no published scientific or clinical studies have observed the effects of honey on SARS-CoV-2. To date, four registered clinical trials estimate the efficacy of honey and its active compounds in patients with COVID-19 (NCT04323345, NCT04345549, NCT04347382, NCT04468139) [45]. Several studies that predicted the binding affinity of Manuka honey polyphenolic compounds to SARS-CoV-2 viral proteins have been conducted. Most of these studies investigated a possible antiviral effect of polyphenols based on the predicted binding to SARS-CoV-2 main protease (Mpro) [28] [46]. The study of Heba et al. [28] screened the biological activity of six compounds present in honeybee and propolis against the COVID-19. The study revealed that four compounds have strong binding affinity and may inhibit the COVID-19 virus replication. Polyphenols are among these bioactive compounds and are currently under phase 3 of clinical investigation as a treatment of COVID-19 patients [46]. Results from Watanabe et al. study [20] showed that honey, and particularly Manuka honey, has potent inhibitory activity against the influenza virus, demonstrating a potential medicinal value. Manuka honey efficiently inhibited influenza virus replication (EC50 = 3.6 ± 1.2 mg/mL). To compare with Camelyn inhibitory activity, this concentration is 10–20 times higher.
Based on scientific studies review, Hossain et al. [30] summarized that honey might be useful for COVID-19 patients by several major mechanisms; direct antiviral properties, regulating/boosting host immune signaling pathways, and curing and/or improving comorbid conditions. The use of drugs faces several problems such as bacterial multidrug resistance and possible side effects. This makes to think about new therapeutic alternatives such as honey and honey products.
CONCLUSIONS
The research has shown that honey extract Camelyn has no cytotoxic effect, is safe, and has demonstrated antiviral properties against SARS-CoV-2 as well. However, the molecular studies of the Camelyn effect on virus replication or immune system need to be done in detail in the future. There is no doubt that Camelyn doesn’t work in the same way as other investigational drugs currently used to treat COVID 19, however, given the current emergency caused by the COVID-19 pandemic and the limited therapeutic options, Camelyn is presented as a promising and relevant therapeutic option that is safe, easy to administrate orally, and is readily available as a natural supplement.
ACKNOWLEDGMENTS
Materials used for experiments provided by JSC “Silicon Biotechnology”. This research was funded by The Agency for Science, Innovation and Technology, Republic of Lithuania (MITA) (grant no. 01.2.1-MITA-T-851-02-0248 to P.J.) and from the Canadian Institutes of Health Research (grant no. 170629 to M.B.).
AUTHOR CONTRIBUTIONS
Conceptualization, LK; methodology, MB, LK, and GB; formal analysis, IG; investigation, MB and AL; writing – original draft preparation, LK; writing – review and editing, MB, AL, NJ; visualization, IG; supervision, RB; funding acquisition, PJ. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ali AM, Kunugi H. Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies. Molecules 2021;26:1231.
- [2]Molan P. Why honey is effective as a medicine: 2. The scientific explanation of its effects. Bee World 2001;82:22–40.
- [3]Olaitan PB, Adeleke OE, Ola IO. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. Afr Health Sci 2007;7:159–65.
- [4]Bansal V, Medhi B, Pandhi P. Honey – A remedy rediscovered and its therapeutic utility. Kathmandu Univ Med J (KUMJ) 2005;3:305–9.
- [5]Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 2008;46:3774–9.
- [6]Al-Hatamleh MAI, Boer JC, Wilson KL, Plebanski M, Mohamud R, Mustafa MZ. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020;10:1–28.
- [7]Shin HS, Ustunol Z. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria: An in vitro comparison. Food Research International 2005;38:721–8.
- [8]Cianciosi D, Forbes-Hernández TY, Afrin S, Gasparrini M, Reboredo-Rodriguez P, Manna PP, et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018;23:1–20.
- [9]Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci 2008;73:117–24.
- [10]Molan PC. The evidence supporting the use of honey as a wound dressing. Lower Extremity Wounds 2006;5:40–54.
- [11]Miguel MG, Antunes MD, Faleiro ML. Honey as a complementary medicine. Integr Med Insights 2017;12:1–15.
- [12]Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003;21:242–7.
- [13]Jaganathan SK. Honey constituents and their apoptotic effect in colon cancer cells. J ApiProduct and ApiMed Sci 2009;1:29–36.
- [14]Pichichero E, Cicconi R, Mattey M, Muzi MG, Canini A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int J Oncol 2010;37:973–81.
- [15]Shahzad A, Cohrs RJ. In vitro antiviral activity of honey against varicella zoster virus (VZV): A translational medicine study for potential remedy for shingles. Transl Biomed 2012;3:2.
- [16]Al-Waili NS. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit 2004;10:94–9.
- [17]Berretta AA, Silveira MAD, Cóndor Capcha JM, de Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother 2020;131.
- [18]Kwakman PHS, te Velde AA, de Boer L, Vandenbroucke-Grauls CMJE, Zaat SAJ. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 2011;6:3–9.
- [19]Yao L, Jiang Y, D’Arcy B, Singanusong R, Datta N, Caffin N, et al. Quantitative High-Performance Liquid Chromatography Analyses of Flavonoids in Australian Eucalyptus Honeys. J Agric Food Chem 2004;52:210–4.
- [20]Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N. Anti-influenza Viral Effects of Honey In Vitro: Potent High Activity of Manuka Honey. Arch Med Res 2014;45:359–65.
- [21]Behbahani M, Khan RH. Anti-HIV-1 Activity of Eight Monofloral Iranian Honey Types. PLoS ONE 2014;9:e108195.
- [22]Abedi F, Ghasemi S, Farkhondeh T, Azimi-Nezhad M, Shakibaei M, Samarghandian S. Possible Potential Effects of Honey and Its Main Components Against Covid-19 Infection. Dose-Response 2021;19:1–13.
- [23]Münstedt K. Bee products and the treatment of blister-like lesions around the mouth, skin and genitalia caused by herpes viruses—A systematic review. Complement Ther Med 2019;43:81–4.
- [24]Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein. Viruses 2012;4:1011–33.
- [25]Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003;300:1763–7.
- [26]Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 2014;281:4085–96.
- [27]Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res 2009;81:77–81.
- [28]Hashem HE. IN Silico Approach of Some Selected Honey Constituents as SARS-CoV-2 Main Protease (COVID-19) Inhibitors. EJMO 2020;4:196–200.
- [29]Mehla R, Bivalkar-Mehla S, Chauhan A. A flavonoid, luteolin, cripples HIV-1 by abrogation of Tat function. PLoS ONE 2011;6:e27915.
- [30]Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon 2020;6:e05798.
- [31]Israili Z h. Antimicrobial properties of honey. Am J Ther 2014;21:304–23.
- [32]Albaridi NA. Antibacterial potency of honey. Int J Microbiol 2019;1:1–10.
- [33]Juopperi TA, Schuler W, Yuan X, Collector MI, Dang C v., Sharkis SJ. Isolation of bone marrow-derived stem cells using density-gradient separation. Exp Hematol 2007;35:335–41.
- [34]Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71.
- [35]Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother 2020;75:2013–4.
- [36]Saikaly SK, Khachemoune A. Honey and Wound Healing: An Update. Am J Clin Dermatol 2017;18:237–51.
- [37]Martinotti S, Bucekova M, Juraj Majtan, Ranzato E. An Effective Regenerative Medicine Product in Wound Management. Curr Med Chem 2019;26:5230–40.
- [38]Martinotti S, Laforenza U, Patrone M, Moccia F, Ranzato E. Honey-mediated wound healing: H 2 O 2 entry through AQP3 determines extracellular Ca 2+ influx. Int J of Mol Sci 2019;20:764.
- [39]Nooh HZ, Nour-Eldien NM. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochem 2016;118:588–95.
- [40]Manjunatha HD, Chua LS. The anti-inflammatory and wound healing properties of honey. Eur Food Res Technol 2014;239:1003–14.
- [41]Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D, Reboredo-Rodriguez P, et al. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: the suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct 2018.
- [42]Bakradze MS, Chantladze VG, Shoniia NO. Using Camelyn in stomatology, results and vistas. Georgian Med News 2009;171:24–7.
- [43]Chumburidze TB, Murtazashvili TG, Kunchuliia LS, Nemsitsveridze NG. Analysis of pharmacokinetics of kamelin in rat’s blood plasma. Georgian Med News 2009;167:96–8.
- [44]Maglakelidze B, Abasshidze G, Dedashidze I, Mshvildadze V, Pichete A. Evaluation of in vitro and in vivo antibacterial and antifungal activity of “CAMELYN M”. Science end Technology Against Microbial Pathogens. Proceedings of the International Conference on Antimicrobial Research, 2011, p. 94–9.
- [45]Tantawy MA. The Efficacy of Natural Honey in Patients Infected with Novel Coronavirus (COVID-19): A Randomized, Controlled, Single Masked, Investigator Initiated, Multi-center Trial. 2020; NCT04323345.
- [46]Mustafa MZ, Shamsuddin SH, Sulaiman SA, Abdullah JM. Anti-inflammatory properties of stingless bee honey may reduce the severity of pulmonary manifestations in COVID-19 infections. Malays J Med Sci 2020;27:165–9.