Functional informativeness of lymphocytes’ cytomorphometric analysis of laboratory rats’ blood
Abstract
Immunological methods that objectively reflect the lymphogenetic processes in the examined organism are needed to assess the effectiveness of prophylactic or therapeutic agents that modulate regenerative processes in the immune system. Such requirements are met by the cytomorphometric method of analysis of circulating blood lymphocytes, the size (small, medium and large) populations of which reflect their proliferative processes in the lymphoid organs. However, the cytomorphometric characteristics of lymphocytes have species features that have to be determined experimentally. In the model of fecal peritonitis in white non-linear sexually mature male laboratory rats, the limits of statistical size classes of lymphocytes were determined, which were the same in control and experimental animals, namely: small – with a diameter of 8.5 μm and less, medium – with a diameter of more than 8.5 μm and less 11.0 μm, large – with a diameter of 11.0 μm and more. Acute infectious process significantly changed the levels of cytomorphometric classes of lymphocytes according to their functional activity in immunogenesis: small, medium and large size of lymphocytes as 42.5 to 7.0%, 45.0 to 54.0%, 10.5 to 30.0%, respectively, in the control and experimental groups of animals, which demonstrates the informativeness of the method. In this case, small and large lymphocytes belong to the activated lymphocytes, which determine the state of the immune system, while medium lymphocytes mainly make up the pool of memory cells. Based on the ratio of these size classes in the peripheral blood, a conclusion about the proliferative reaction of lymphocytes in the experiment is made, followed by the extrapolation of the results and in clinical practice.
INTRODUCTION
Objectification and personalization of the assessment of the state of immunity is a priority paradigm of modern laboratory diagnostics, and the development of computer technology will help automate and accelerate the results of the analysis. In this regard, modern advances in molecular cell biology have contributed to the progress of fundamental developments in the direction of modulation of regenerative processes in the immune system by natural and synthetic compounds. However, immunological methods that objectively reflect the lymphogenesis in the examined organism are needed to assess the effectiveness of the prophylactic or therapeutic agents application. The cytomorphometric method of the analysis of circulating blood lymphocytes meets such requirements. Cytomorphometry, as a method of computer image analysis includes analysis of nuclear and cellular morphometric parameters (radius, diameter, perimeter, area, volume, etc.), is widely used in laboratory diagnostics, medicine and veterinary medicine [1, 2]. Blood, as a part of the internal environment, reflects functional state of the organism and morphogenetic function of the immune system to ensure structural and humoral homeostasis during its physiological and pathological changes [3, 4, 5]. In this case, from all the variety of blood leukocytes, exactly lymphocytes as the main cells of the immune system clonospecifically respond to all changes in the organism and preserve cellular memory of them until subsequent immunological reactions. It is possible to study the processes of morphogenesis that occur in lymphoid organs at physiological and pathological (infectious, oncological, autoimmune) regenerative processes on circulating blood lymphocytes using a cytomorphometric method that takes into account the stages of activation, proliferation, differentiation and migration of lymphocytes which stereotypically reflect the formation of morphofunctional size classes of lymphocytes on small, medium and large [5, 6, 7]. However, the cytological characteristics of blood lymphocytes have species features. Thus, lymphocytes of human blood and laboratory rats differ in specific density, which is for the first 1.077 – 1.078 g/ml, for the second 1.087 – 1.088 g/ml [8, 9, 10]. The specific density of lymphocytes depends on the species characteristics of their nuclear-cytoplasmic ratio, which reflects their other cytomorphometric characteristics. Meanwhile, laboratory rats are one of the common objects of modeling physiological and pathological human conditions [11]. Therefore, without determining the cytomorphometric features of their lymphocytes, this immunological analysis cannot be extrapolated to the dynamics of human blood lymphocytes.
The aim of our study is to establish the cytomorphometric limits of small, medium and large blood lymphocytes of laboratory rats and their ratio in norm and pathology (acute fecal peritonitis) with definition of functional activity in immunogenesis of each size class of lymphocytes to further address fundamental and applied aspects of experimental and clinical immunology by assessment of modulation of regeneration processes under the influence of natural and synthetic factors.
MATERIALS AND METHODS
Animals and experimental conditions
The research was performed on 20 white non-linear sexually mature male laboratory rats aged 7 – 8 months and weighing 180 – 220 g. Animals were randomly divided into control/intact (n=10) and experimental groups (n=10). An acute diffused purulent fecal peritonitis was simulated in experimental animals by the method of Lazarenko et al. (2008) for the shift of immunological parameters due to activation of immunogenesis: injected into the abdominal cavity 10% filtered fecal mixture at the rate of 0.5 ml per 100 g of body weight [12, 13]. Animals were removed from the experiment on the 3rd day after injection. All manipulations were carried out in accordance with the rules and regulations for the handling of laboratory animals [14, 15]. All procedures performed were approved by the Bioethics committee of Zaporizhzhia National University Biology faculty (Protocol No. 1 dated August 28, 2019).
Collection of blood samples and laboratory analysis
Animals under ether anesthesia (Sorbpolimer-Analitic Ltd., Kyiv, Ukraine) were decapitated, arteriovenous blood was collected, stabilized by heparin (20 μg/ml, Spofa, Prague, Czech Republic) and immediately analyzed the number of leukocytes in Goryaevʼs chamber (MICROmed TM, Poltava, Ukraine), and in blood smears stained by Pappenheim using concentrated solution of May-Grünwald dye and 10% solution of Romanowsky-Giemza dye (Biomed Ltd., Shostka, Ukraine) – leukocyte formula and cytomorphometric studies of lymphocytes performed in accordance with the methodology [5, 6, 7, 16]. Measurements of lymphocytes performed using FemtoScan online software v. 2.3.219 (Advanced Technologies Center, Moscow, Russian Federation) in pixels, which were translated into micrometers based on calibration of the values obtained at a given magnification using an object micrometer (PZO, Warszawa, Poland; value of a point is 0.01 mm or 10 μm) using an oil immersion system (the objective lens 100×). Microphotographs were prepared using a PrimoStar iLED microscope and an Axio CamERc5s camera (Carl Zeiss, Goettingen, Germany). Since the gradation of cytomorphometric classes of lymphocytes is carried out in the range of 0.5 – 1.0 μm for greater accuracy formed a variation series of size classes of lymphocytes with an interval of 0.3 μm. Based on histograms comparison of lymphocytes cytomorphometric groups of control and experimental animals distributed the limits of small, medium and large cytomorphometric classes of lymphocytes and determined the percentage of each grouped class. The state of the immune system was evaluated by the ratio of small, medium and large lymphocytes [5, 6, 7].
Statistical analysis
Statistical processing and presentation of experimental results were performed using the MicrosoftXP Exel 2010 (Microsoft Corporation, Redmond, Washington, USA) and IBM SPSS Statistics 20.0 application (IBM corp., Armonk, NY, USA). The verification of quantitative indicators for the normality of distribution was performed using the one-sample Kolmogorov-Smirnov test, the Shapiro-Wilk test. At the nonparametric distribution at least by one indicator of data series, used the Mann-Whitney rank test to assess the significance of differences between independent samples. The values in the tables are represented as Me (Q1; Q3), where Me is median, Q1 and Q3 are the first (25%) and third (75%) quartiles. Differences were considered significant at P < 0.05 [17].
RESULTS
Disease symptoms, the total number of leukocytes and leukocyte blood count
On 3rd day after modeling of acute purulent peritonitis in laboratory rats a pronounced clinical picture of the disease was observed. Acute purulent peritonitis is one of the forms of inflammatory reaction of the whole organism (sepsis), therefore, corresponding changes were detected in peripheral blood (Table 1) increase in the number of leukocytes by 86.7%, the relative number of neutrophils by 2.5 times, including banded 4.5 times, segmented nuclei 2.3 times, two-fold decrease in the relative number of lymphocytes at almost equal their absolute values, compared with the control.
Table 1. The leukocyte blood formula of intact sexually mature laboratory rats (control) and in generalized infectious process (fecal peritonitis), Me (Q1; Q3).
Cytomorphometric parameters of lymphocytes
The results of cytomorphometric analysis of peripheral blood lymphocytes in intact laboratory rats and with systemic inflammation are presented on the histogram (Figure 1). Thus, cytomorphometric lymphocytes’ indices of healthy adult rats and with fecal peritonitis varied widely: in healthy animals – from 5.4 to 13.8 μm, and with fecal peritonitis – from 7.8 to 15.0 μm. Comparison of frequency distribution histograms of individual lymphocytes’ classes of intact and experimental animals in the presence of gaps in their dynamics (at the level of 8.4 and 10.8 μm) clearly separated the following cytomorphometric classes of lymphocytes (LC): small – with a diameter of 8.5 μm and less (LC ≤ 8.5 μm); medium – with a diameter of more than 8.5 μm and less than 11.0 μm (LC > 8.5 – < 11.0 μm) and large – with a diameter of 11.0 μm and more (LC ≥ 11.0 μm).
Using these cytomorphometric limits of lymphocytes classes in intact laboratory rats the following results were obtained (Table 2) the relative proportion of small lymphocytes is 42.50 (31.50; 59.75)%, medium – 45.00 (31.75; 56.50)%, large – 10.50 (7.50; 12.50)%, which corresponds to the physiological state of immune system in animals. Generalized infectious process (sepsis) in the experimental group of rats led to a redistribution of frequencies in cytomorphometric classes of lymphocytes in the peripheral blood: the number of small lymphocytes sharply decreased to 7.00 (6.00; 8.00)%, the number of medium lymphocytes increased to 54.00 (53.75; 56.50)% and large to 39.00 (36.50; 40.00)%. Wherein, a part of large lymphocytes was especially polymorphic (there were cells up to 15.0 μm), intermediate classes can be additionally distinguished in each cytomorphometric class of lymphocytes.
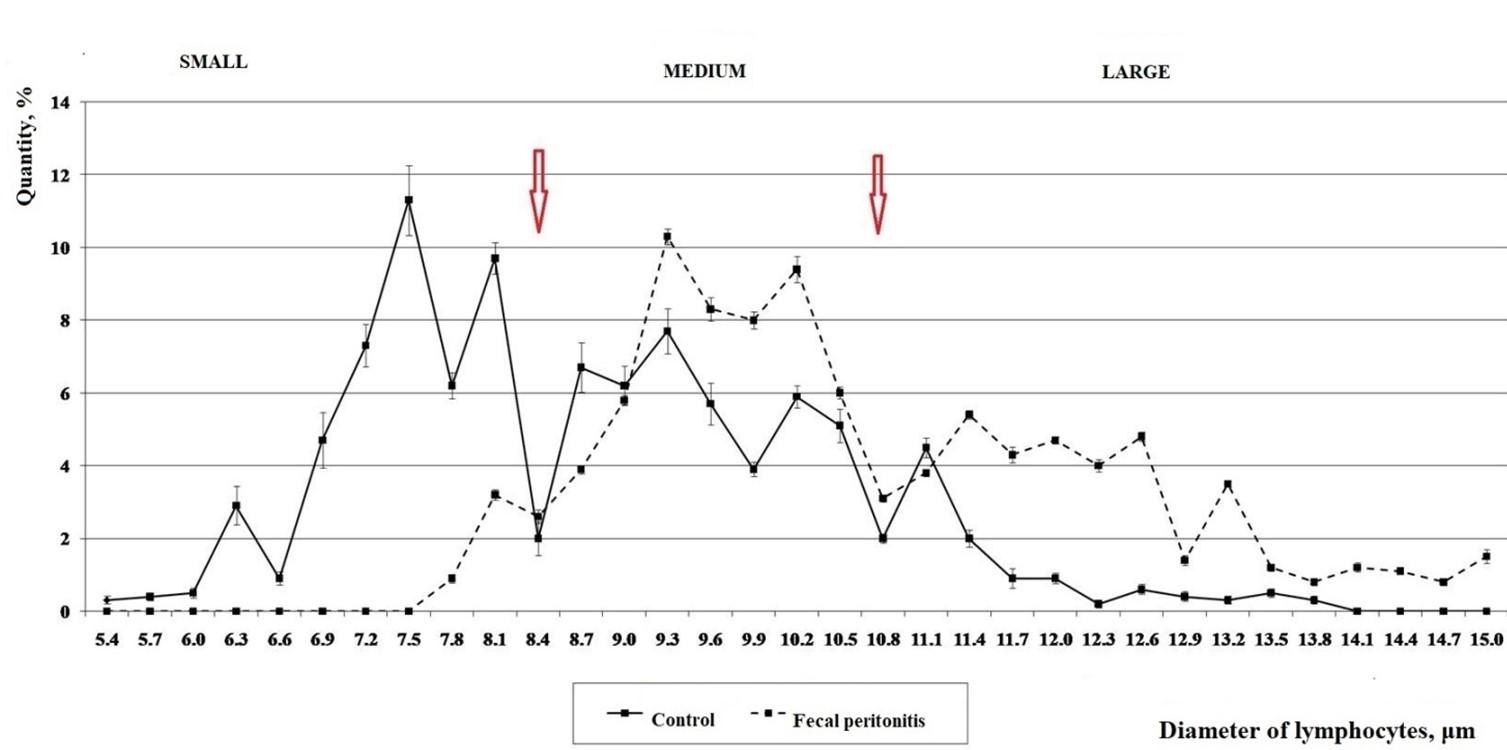
Table 2. Grouped cytomorphometric classes of lymphocytes in normal (control) and pathological (fecal peritonitis) states of sexually mature laboratory rats, Ме (Q1; Q3).
Morphology of lymphocytes
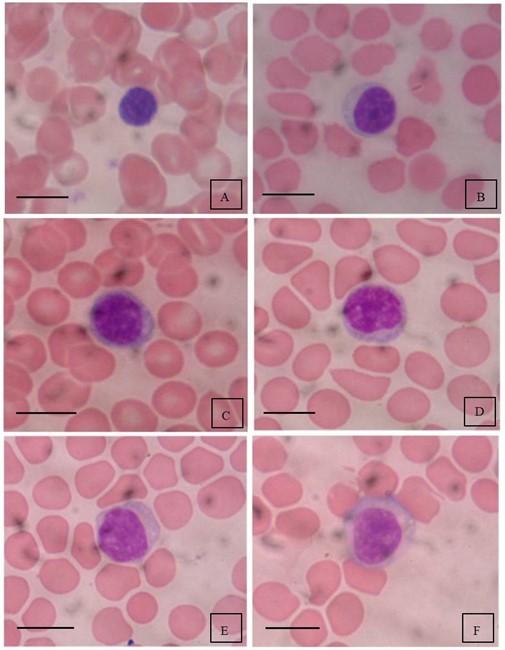
In addition to dimensional characteristics, small, medium and large lymphocytes also differ in morphology (Figure 2). Small lymphocytes are erythrocyte-sized, have round or oval compact nucleus, which occupies most of the cell, dense condensed granular or deep chromatin, almost without cytoplasm or with a narrow rim of cytoplasm with moderate basophilia (Figure 2, A). In medium lymphocytes, the nucleus is round, compact, dominates over cytoplasm, coarse-grained chromatin, no nucleoli, sometimes there are remnants of nucleoli, cytoplasm with reduced basophilia (Figure 2, B, C). Large lymphocytes (immunoblasts, Figure 2, D, E, F) have an active nucleus with fine-grained chromatin, 1 – 2 nucleoli can be distinguished in the nucleus, a broad basophilic cytoplasm due to the deployment of a protein-synthetic system, the main component of which are ribosomes.
DISCUSSION
In the study of leukocytes count and leukocyte blood formula in intact adult rats, the indicators corresponded to the reference values for this age [18, 19, 20] and had a lymphoid profile (predominance of lymphocytes over neutrophils) which coincides with the data of literature [21, 22, 23]. It is known that white blood in laboratory rats is quite labile, therefore, to shift the immune parameters due to activation of immunogenesis for infection animals simulated with fecal peritonitis, as one of the options for development of systemic inflammatory reaction, which in terms of force evens out individual physiological fluctuations. In the simulation of fecal peritonitis in all experimental animals, the development of a septic condition was observed. It sharply shifted all indicators of the leukocyte formula: significant leukocytosis and granulocytosis were detected, especially neutrophilia with shift of the blood formula to the left, quantitative and qualitative shifts of lymphocytes. Detailed morphological characteristics of blood cells and their ratio in normal laboratory rats and the ones with fecal peritonitis are presented in our previous research [24]. These changes in rats’ blood coincided with those obtained by other authors [25] and corresponded to our goals – to shift the homeostatic fluctuations of blood leukocytes to contrast them in control and experimental animals.
The proposed methodological approach to the analysis of morphofunctional features of circulating lymphocytes allowed us to address the following issues. Firstly, the boundaries of small, medium and large cytomorphometric classes of lymphocytes in outbred rats were clearly separated. Secondly, their species features in comparison with human lymphocytes are established. Thirdly, conceptually determined their formation at the stages of lymphogenesis. Fourthly, confirmed the justification of the functional activity in immunogenesis for each of cytomorphometric classes of lymphocytes previously formulated by Frolov et al. [5, 6, 7, 26].
Thus, according to the analysis of dips and peaks in histograms of control and experimental rats (Figure 1), the limits of small, medium and large cytomorphometric classes of lymphocytes coincided with each other. However, the development of peritonitis in experimental rats was noted by differences in the amplitude of these peaks and their uniformity (Figure 1), which depended on their functional significance. Thus, such histological feature as the specific density of lymphocytes and their size should be of a species character, which must be taken into account in this methodological direction, but other researchers, for the most part, do not observed this. The universality of grouping of circulating blood lymphocytes into cytomorphetric classes (small, medium and large) is due to the stereotype of their lymphogenesis (formation in the central and peripheral lymphoid organs and subsequent migration into tissues and organs of all vertebrates). Thus, according to the cytomorphogenetic concept of Frolov et al., lymphocyte, after antigenic or mitogenic stimulation stereotypically undergoes the following stages of lymphogenesis: recognition of the stimulus; cell activation; blast transformation; proliferation; differentiation; migration; immune response during which the nuclear-cytoplasmic ratio changes, which is reflected in cell size [5, 6, 7].
As we consider, in majority, lymphocytes of the medium cytomorphometric classes as their tissue characteristics for a specific type of organism, begin immunogenesis. Activation and blasttransformation are accompanied by the deployment of a protein-synthetic system, which leads to increasing cell size (3 – 5 times), which becomes maximum before a series of sequential (3 – 5 – 10 or more) mitoses depending on the specific immunological situation. Rapid sequential division of large blast-transformed cells leads to reduction of their offspring size to a functionally minimal value for lymphocytes of a particular species, which is one of the hallmarks of successful subsequent migration. During proliferation, lymphocytes undergo certain stages of differentiation inherent in each subpopulation of lymphocytes.
Migrating to the internal environment, these postproliferative lymphocytes form a pool of small activated lymphocytes with high migration capacity, which are deposited in organs, performing an inherent (committed) homeostatic function, after which they are mostly utilized by apoptosis. It is known that lymphocytes are special cells that have certain features of stem cells: they have the ability to proceed from the mitotic cycle of productive histogenesis to the final differentiation, returning to the G0 stage, replenishing the pool of source populations of T- and B-lymphocytes clonally receptor-committed to this antigen/structure (for example, СD2, CD3, CD4, CD8, CD19, CD20, CD22, CD25). Cellular memory before previous immunogenesis is based on this property. Thus, the part of the small lymphocytes comes from the subsequent committed immunogenesis, becoming memory cells, circulating in the organism for a long time (months, years, decades). Metabolic processes in this part of cells lead to the restoration of the average genetically determined nuclear-cytoplasmic ratio specific for medium cytomorphometric class of lymphocytes. A smaller part of them are activated as a transient cytomorphometric form of large immunoblast lymphocytes, which prematurely left the immune organs for migration. However, most medium lymphocytes are temporarily immunologically intact as memory cells. They have been activated long ago (during which their nuclear-cytoplasmic ratio has been restored) and retain potencies for future immunogenesis upon appropriate encounter with antigen/structure or under the influence of mitogenic signals through common activation markers (for example, CD25, CD28, CD69, CD71, CD95, CD127).
All stages of immunogenesis are influenced by positive and negative regulatory factors that keep the immunological response within physiological homeostatic limits. In the case of increased needs for immunocompetent cells, for example, in the case of infection or disruption of the structure of another genesis, leukocytes that have not passed all the relevant stages of differentiation enter the peripheral circulation. In hematology, this condition of leukopoiesis is called “shift of cells to the left”. Among granulocytes, this group of “immature” cells are young, banded, such as neutrophils [27, 28]. However, we determine that this condition is also characteristic of lymphogenesis. In the case of load on immune system, immunoblasts enter the circulation at different stages of blast transformation prior to the beginning of proliferation [29, 30]. As a rule, in such cases, medium, rarely large immunoblasts enter the circulation from the lymphoid organs. These activated lymphocytes with signs of blast transformation constitute the cytomorphometric class of large lymphocytes, which in the future, like small lymphocytes, migrate to tissues, organs and after performing the appropriate immunological function undergo apoptosis. Large lymphocytes also include a population of natural killers (CD16+) and a group of T-lymphocytes (T-helpers – CD4+, T-killers/suppressors – CD8+), which contain red-violet granules in the cytoplasm, which are also utilized in the relevant organs after immunological reactions.
After experimental and logical structural and functional determination of each cytomorphometric population of small, medium and large lymphocytes, it becomes timely to explain the species differences of these classes in rats and humans on one side and in intact and experimental rats on other. As might be expected, the average values of cytomorphometric classes of rats’ lymphocytes and their statistical fluctuations significantly exceeded those observed in humans. Thus, the higher specific density of lymphocytes in rats coincided with the larger size of these cells and an increase in the average size and their boundaries among the cytomorphometric classes of lymphocytes (small, medium and large) blood (Table 3). For example, the average size of small lymphocytes in the laboratory rats were higher by 30.8%, and large – by 15.8%, compared with those in humans (Table 3). These dimensional features of lymphocytes were not taken into account by other authors in similar cytomorphometric studies [31]. Therefore, the use of gradation of human blood lymphocyte size is not acceptable for similar cytomorphometric analysis of cells in other species.
In addition, species histograms differ in quantitative indicators of lymphocyte size classes. Thus, in a healthy middle-aged humans and intact rats 7 – 8 months of age (corresponding to the middle age category) they differ in the number of small lymphocytes, which include newly formed postproliferative highly migratory activated fractions of circulating lymphocytes, which mainly reflect the state of the immune system at the time of testing. According to the literature, the reference values of the proportion of small lymphocytes in middle-aged people are 15 – 20% [5, 6, 7], whereas according to our data in adult middle-aged rats it is 42.50 (31.50; 59.75)% (Table 3). We attribute these significant quantitative differences to the average individuals lifespan of these species and their body temperature. Thus, the average life expectancy in rats is 2 – 3.5 years (average 3 years) at a body temperature of + 38.5 – 39.5 ˚С, and humans – 80 years, at a body temperature of 37.0 ˚С [18, 32]. This assumption emphasizes the conditionality of the primacy of the morphogenetic function of immunity in the control and maintenance of structural histological homeostasis of the organism [4, 7, 33-37]. Thus, according to modern data, it is unequivocally proved that all clones of T- and B-lymphocytes during formation in the central lymphoid organs are sensitized (committed) to the autostructures of cells and their derivatives [3, 4, 33, 35], but without cytotoxic function, whereas allergic and autoallergic reactions develop with local or systemic dysregulation in the immune system. Homeostatic helpers/suppressors, autosensitized clones of T- and B-lymphocytes provide control and regulation of histogenetic reactions of all tissues at the stages of their morphogenesis from stem to completely differentiated cells.
This fact was convincingly proved by the example of blocking the regeneration of liver cells with chemical cytostatic in rats having surgery on this organ. During transfer of T- and B-lymphocytes from them to intact animals, the latest stimulated cell regeneration at that stage at which regeneration was stopped by a cytostatic in the experimental group of animals [34]. Other experiments have shown that it is the RNA extracted from lymphocytes from patients with polycythemia vera stimulate hematopoiesis in rats [33]. The regulatory function of autosensitized T- and B-lymphocytes explains the presence of autoantibodies without cytotoxic action to all without exception cell structures and their derivatives [4, 35, 38].
This has been confirmed by many authors since the early 20th century. From these positions it is possible to explain lymphoid type of leukocytes of blood in rats and mainly neutrophilic type in human. Thus, rats have accelerated structural and functional physiological processes that occur at +38,5 – 39,5 ˚С [18] according to various sources. Physiological and reparative processes are provided by proliferative reactions at histogenesis which level is regulated as it is specified above by lymphoid system. However, it has already been conclusively proven that the proliferative potencies of tissue differon stem cells are limited by the Hayflick-Olovnikov phenomenon, a reduction in the daughter chain in DNA reduplication from the 5′-end [39, 40]. Losing potency to cell proliferation occurs when the final telomeres of chromosomes are exhausted by replicative cycles: deletions, mutations are already exposed to structural or regulatory genes, as a result of which the cell activates (starts) the apoptosis program [39]. It has been experimentally shown that human somatic cells have potencies up to 40 – 60 mitoses [39, 41, 42], which provide the maximum biological age up to 100 – 110 years, while the ones of laboratory mice and rats have potencies for 10 – 15 divisions with a maximum lifespan of 3.5 years [43, 44, 45].
From the standpoint of homeostatic morphogenetic function of immunity, it is also possible to explain leukocyte and cytomorphometric shifts in the blood of experimental rats, which simulated acute fecal peritonitis. The development of a generalized infectious process (sepsis) was accompanied by an acute inflammatory reaction of the whole organism, in which all parts of the immune system of animals were activated, first of all, cells of innate immunity. Therefore, the burst of neutrophilia and its sharp shift to the left is fully explained as they perform the main clearance function from pathogens and cellular detritus, synthesize a network of various cytokines [46]. In parallel, innate immune cells activate homeostatic responses of acquired adaptive immunity, which through clonospecific and protective T- and B-lymphocytes regulate morphogenetic and protective cytotoxic responses to pathogens.
A significant contribution to understanding the rapid development of immunological responses to disorders of genetic-structural homeostasis was the discovery and subsequent study of the functional features of a new group of immunocompetent cells – innate lymphoid cells (ILC) [47-53]. They differ from well-studied T- and B-lymphocytes by the absence of clonospecific receptors for ligands, but have a wide variety of receptors that test the homeostatic state of the tissue microenvironment and react by synthesizing various inflammatory cytokines, rapidly involving innate and adaptive (T- and B-lymphocytes) cells in the inflammatory response. They are also relatively tissue-resident cells. As an analogue of T-helper lymphocytes, they are involved in a number of immunological reactions in the sequence ILC – Dendritic cell – Т-lymphocyte. At the last stage, ILC [47, 51] together with other auxiliary cells of the microenvironment [54] provide a dichotomy of activated committed T- and B-lymphocytes circulation: either in MALT (mucosa-associated lymphoid tissue) and/or in SALT (skin-associated lymphoid tissue).
If taking into account that the immune system of mucous membranes (MALT) is more than 70% of the mass of lymphoid organs, then in the case of acute inflammatory process, which occurs in a group of experimental animals, activated lymphocytes from the small cytomorphometric classes migrated and deposited mainly to these organs performing their immunological functions, after which they mostly undergo apoptotosis [55]. It becomes clear why the part of the small lymphocytes classes in the blood of these animals decreased to 7.00 (6.00; 8.00)%, with 42.50 (31.50; 59.75)% in control animals, i.e. by 83.5%. In addition, according to the literature [49, 51, 53], ILC provides rapid mobilization of metabolic morphogenetic responses, including in histogenesis of cells of myeloid and lymphoid tissues. These circumstances can explain the expressive and sharp shift to the left to differentiate granulocytes (the emergence of banded neutrophils) and lymphocytes (the presence of small and large blasts). Also with regard to lymphocytes, this phenomenon was manifested by the splitting (two-humped) of the medium cytomorphometric class of lymphocytes (intermediate immunoblast), and a sharp increase in the number of large lymphocytes (immunoblasts). The appearance of a significantly large proportion of immature forms among neutrophils and lymphocytes indicates an extreme degree of stress in the immune system, aimed at elimination of the acute inflammatory reaction to the infectious process.
Thus, thanks to modern discoveries in cellular immunology, the cytomorphometric method of analyzing the state of immune system acquires additional objectivity and informativeness. Considering the rapid dynamic processes of new formation and the relative dichotomy of the direction of predominant migration of activated lymphocytes in MALT (mucosa-associated lymphoid tissue) and/or SALT (skin-associated lymphoid tissue) [7, 47-50, 54], which puts this method in a number of objective methods of primary immunological level together with the general blood test: tests immunity intension on amplitude of activated lymphocytes (small and large) and localization of disturbance of structural homeostasis of tissues – by migratory dynamics of increase or decrease of the content of certain cytomorphometric classes of lymphocytes from their reference values.
Modern development of laboratory equipment (flow hemometers, cytometers) can make this method as operational as other methods of general analysis of the immune system at the time of blood collection for analysis. Assessment of the intensity of lymphogenic reactions in the organism of the subject gives the cytomorphometric method an advantage over other methods of cytological analysis, and its efficiency, objectivity and informativeness can be successfully used to address fundamental and applied aspects of experimental and clinical immunology to assess the modulation of physiological or reparative regeneration processes under the influence of natural and synthetic factors in preventive and curative measures.
The perspective of further research is to determine the density of CD structures on small, medium and large lymphocytes on the histogram of the lymphocyte suspension obtained using flow cytometers. Also the analysis of the percentage of small, medium and large cytomorphometric classes of blood lymphocytes of laboratory rats and other model animals (mice, guinea pigs) of different ages and their shift in various pathologies of infectious and non-infectious genesis with parallel analysis of humoral immunity factors including cytokines is of clinical interest.
Table 3. Comparative characteristics of cytomorphometric features of blood lymphocytes of laboratory rats and donors* of middle age.
CONCLUSION
The limits of variation of small, medium and large cytomorphometric classes of blood lymphocytes are species-specific and are due to the nuclear-cytoplasmic ratio of cells, which is tested for specific density in gradient solutions by centrifugation. Therefore, in each case it is necessary to make a cytomorphometric histogram of samples of whole blood lymphocytes, based on which separate the boundaries and average values of cytomorphometric classes of lymphocytes of a particular mammalian species.
Established division of circulating blood lymphocytes of laboratory rats into small (average cell size ≤ 8.5 μm), medium (average cell size > 8.5 – < 11.0 μm) and large (average cell size ≥ 11.0 μm) cytomorphometric classes is an objective reflection of their histogenetic events in the lymphoid organs of the body.
Normally, the proportion of small lymphocytes in laboratory rats is 42.50 (31.50; 59.75)%, medium – 45.00 (31.75; 56.50)%, large – 10.50 (7.50; 12.50)%, and in the case of generalized infectious process (sepsis) varies according to their functional value: the part of small lymphocytes, which include actively migrating postproliferative committed lymphocytes, decreases to 7.00 (6.00; 8.00)%; the part of medium lymphocytes includes memory cells, that are long-circulating, rarely transient cytomorphometric forms of large lymphocytes is 54.00 (53.75; 56.50)%, and the part of large lymphocytes that are immunoblasts, activated T-lymphocytes and NK increases to 39.00 (36.50; 40.00)%. Based on the ratio of these size classes in the peripheral blood, a conclusion about the proliferative reaction of lymphocytes in the experiment is made, followed by the extrapolation of the results and in clinical practice.
ACKNOWLEDGEMENT
The authors received no financial support for this research.
AUTHOR CONTRIBUTIONS
This work is a collaboration among all the authors. OKF, ROL and LVM were involved in conception and design of the experiments. ROL contributed to perform the experiments. OKF, ROL and LVM analyzed and interpreted data. ROL made the illustration. OKF, ROL and LVM contributed to drafting the article. OKF contributed to revising it critically for important intellectual content. LVM revised the manuscript for necessary changes in grammar and English standard. OKF, ROL and LVM made the final approval of the version to be published.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ruban GI, Berdnik VV, Marinitch DV, Goncharova NV, Loiko VA. Light scattering and morphology of the lymphocyte as applied to flow cytometry for distinguishing healthy and infected individuals. J Biomed Opt. 2010; 15(5): 057008. doi: 10.1117/1.3503404.
- [2]Shashni B, Ariyasu S, Takeda R, Suzuki T, Shiina S, Akimoto K, et al. Size-based differentiation of cancer and normal cells by a particle size analyzer assisted by a cell-recognition PC software. Biol Pharm Bull. 2018; 41(4): 487-503. doi: 10.1248/bpb.b17-00776.
- [3]Babaeva AG, Gevorkyan NM, Zotikov EA. Rol’ limfocitov v operativnom izmenenii programmy razvitiya tkanej [The role of lymphocytes in the operative change of the tissue development program]. Publishing house of the Russian Academy of Medical Sciences: Moscow, Russia, 2009. (in Russian).
- [4]Poletaev AB. Fiziologicheskaya immunologiya (estestvennye autoantitela i problemy nanomediciny) [Physiological immunology (natural autoantibodies and problems of nanomedicine)]. MIKLOSH: Moscow, Russia, 2010. (in Russian).
- [5]Frolov ОК, Kopiyka VY, Fedolov YеR, Frolova LO. Cytomorphometric method of finding out the activated lymphocytes in peripheral blood. Laboratory diagnostics. 2006; 4(38): 55-9. (in Russian, with English abstract).
- [6]Frolov AK. Patogeneticheskij analiz sostoyaniya immunnoj sistemy po dinamike novoobrazovaniya i migracii aktivirovannyh limfocitov vo vnutrennej srede organizma: metodicheskie rekomendacii [Pathogenetic analysis the state of immune system by the dynamics of new growth and migration of activated lymphocytes in the internal environment of the body: guidelines]. Zaporizhzhya State University: Zaporizhzhia, Ukraine, 1999. (in Russian).
- [7]Frolov OK, Fedotov YeR, Kopiika VV, Frolova LO. Pathogenetic analysis of immune system: basic principles. Experimental and Clinical Physiology and Biochemistry. 2004; 3(27): 14-21. (In Ukrainian, with English abstract).
- [8]Movsesyan HA, Alchujyan NKh, Movsisyan NH, Kevorkian GA. Nitrergic system activity and D-glucose uptake of leucocyte subpopulations and bone marrow of rats treated with half-conducted laser and red light diode in clostridial sepsis. National Academy of Sciences of Armenia Reports. 2009; 109(1): 68-77. (in Russian, with English abstract).
- [9]Fasanmade AA, Jusko WJ. Optimizing whole blood lymphocyte proliferation in the rat. Journal of Immunological Methods. 1995; 184(2): 163-7. https://doi.org/10.1016/0022-1759(95)00084-N.
- [10]Tompkins AB, Hutchinson P, de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor beta on lymphocyte subsets in vitro. Biol Reprod. 1998; 58(4): 943-51. doi: 10.1095/biolreprod58.4.943.
- [11]Modlinska K, Pisula W. The natural history of model organisms: the norway rat, from an obnoxious pest to a laboratory pet. eLife 2020; 9: e50651. doi: https://doi.org/10.7554/eLife.50651.
- [12]Lazarenko VA, Lipatov VA, Blinkov YuYu, Skorikov DV. Experimental model of diffuse fecal peritonitis. Kurskij Nauchno-Prakticheskij Vestnik “Chelovek i ego Zdorov’e” / Kursk Scientific and Practical Bulletin “Man and His Health”. 2008; 4: 128-32. https://cyberleninka.ru/article/n/eksperimentalnayamodel-rasprostranennogo-kalovogo-peritonita (in Russian, with English abstract).
- [13]Melo MC, Gadelha DN, Oliveira TK, Brandt CT. Alcohol extract of Schinu sterebinthifolius raddi (anacardiaceae) as a local antimicrobial agent in severe autogenously fecal peritonitis in rats. Acta Cir Bras. 2014; 29 (Suppl 1): 52-6. doi: 10.1590/s0102-86502014001300010.
- [14]European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, France). ETS No.123. 1986. https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/123.
- [15]The Law of Ukraine “Pro Zakhyst Tvaryn Vid Zhorstokoho Povodzhennia” [On protection of animals from cruel treatment]. No. 3447-IV. 2006. http://zakon4.rada.gov.ua/laws/show/3447-15.
- [16]Frolov OK, Litvinenko RO (2020). Sposib vyznachennia tsytomorfometrychnykh klasiv limfotsytiv krovi laboratornykh shchuriv [Method of determining cytomorphometric classes of blood lymphocytes of laboratory rats] (UA. Patent for a utility model No. 140246). Kyiv: State Enterprise “Ukrainian Intellectual Property Institute” (UKRPATENT). https://base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=265926. (in Ukrainian).
- [17]Dunn OJ, Clark VA. Basic statistics: a primer for the biomedical sciences (4th ed.). John Wiley & Sons: New York, USA, 2009.
- [18]Zapadnyuk IP, Zapadnyuk VI, Zakharia EA, Zapadnyuk BV. Laboratornye zhivotnye. Razvedenie, soderzhanie, ispol’zovanie v eksperimente [Laboratory animals. Breeding, maintenance, use in the experiment] (3rd ed.). Vyshcha shkola: Kiev, USSR, 1983. (in Russian).
- [19]Weiss DJ, Wardrop KJ. (eds). Schalm’s veterinary hematology (6th ed.). Wiley-Blackwell: Iowa, Ames, 2010.
- [20]Bailly Y, Duprat P. Normal Blood Cell Values, Rat. In: Jones TC, Ward JM, Mohr U, Hunt RD (eds). Hemopoietic System. Monographs on Pathology of Laboratory Animals. Springer: Berlin, Heidelberg, 1990, pp. 27-38. doi: https://doi.org/10.1007/978-3-642-84110-1_3.
- [21]Uzenbaeva LB, Vinogradova IA, Golubeva AG, Tchurov AV, Ilyukha VA. Age related changes in rat blood leucocytes count and morpho-metrical parameters of large granular lymphocytes under different light regims. Advances in Gerontology. 2006; 19: 79-84. (in Russian, with English abstract).
- [22]Khizhkin EA, Antonova EP, Ilyukha VA, Uzenbaeva LB, Ilyina TN, Baishnikova IV, et al. Age-related changes of physiological and biochemical parameters in rodents with different ecological specialization. Transactions of KarRC of RAS. 2015; 11: 15-25. doi: 10.17076/eb103. (in Russian, with English abstract).
- [23]Abrashova TV, Gushchin YaA, Kovaleva MA, Rybakova AV, Selezneva AI, Sokolova AP., et al. Spravochnik. Fiziologicheskie, biohimicheskie i biometricheskie pokazateli normy eksperimental’nyh zhivotnyh [Directory. Physiological, biochemical and biometric indicators of the norm of experimental animals]. Publishing house “LEMA”: St. Petersburg, Russia, 2013. (in Russian).
- [24]Lytvynenko RO, Makyeyeva LV. Hematological leukocytes ratio indices: predictors of acute purulent fecal peritonitis in nonlinear laboratory rats. J Adv Biotechnol Exp Ther. 2021; 4(2): 120-32. doi: https://dx.doi.org/10.5455/jabet.2021.d113.
- [25]Denisenko VL, Gain YM, Shakhrai SV, Veremey EI, Zhurba VA, Rukal VM, et al. Modeling of occlusive large-bowel obstruction in laboratory animals. Vestnik Vitebskogo gosudarstvennogo Meditsinskogo Universiteta / Vestnik of Vitebsk State Medical University. 2011; 10(1): 25-32. http://vestnik.vsmu.by/downloads/2011/vestnikVGMU-10-1-2011.pdf (in Russian, with English abstract).
- [26]Frolov AK, Artsimovich NG, Moskalets OV, Vedernikov AA, Kornev AV (1997). Sposob opredeleniya aktivirovannyh limfocitov v krovi [Method for determining activated lymphocytes in blood] (RU. Patent No. 2080597). Moscow: Russian Agency for Patents and Trademarks. https://rusneb.ru/catalog/000224_000128_0002080597_19970527_C1_RU/ (in Russian).
- [27]Honda T, Uehara T, Matsumoto G, Arai S, Sugano M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta. 2016; 457: 46-53. doi: 10.1016/j.cca.2016.03.017.
- [28]Farkas JD. The complete blood count to diagnose septic shock. J Thorac Dis. 2020; 12(Suppl 1): S16-S21. doi: 10.21037/jtd.2019.12.63.
- [29]El-Bolkainy MN, Nouh MA, El-Bolkainy TN, Farahat IG, Badawy OM. (eds). Pathology of Cancer. (5th ed.). The National Cancer Institute (NCI), Cairo University: Cairo, Egypt, 2016. http://www.elbolkainy.net/pathology-of-cancer-5th-ed.
- [30]Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood. 1997; 90(8): 2863-92. doi: https://doi.org/10.1182/blood.V90.8.2863.
- [31]Drogomyretska IZ, Mazepa MA. Effect of cadmium and nickel ions on morphometric parameters of leucocytes in Cyprinus carpio L. Immunology and allergology: science and practic. 2010; 1: 50-5. (in Ukrainian, with English abstract).
- [32]Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013; 4(6): 624-30.
- [33]Babaeva AG, Gevorkyan NM, Tishevskaya NV, Golovkina LL, Muratova YuO, Ragimov AA. About hematopoietic properties of peripheral blood lymphocytes RNA from patients with polycythemia vera and healthy donors. Oncohematology. 2015; 10(2): 58-62. doi: https://doi.org/10.17650/1818-8346-2015-10-2-58-62. (in Russian, with English abstract).
- [34]Gevorkyan NM, Babaeva AG. Variability of manifestations of lymphocyte morphogenetic function depending on a character and localization of organ damage. Bulletin of the Russian Academy of Natural Sciences. 2012; 1: 44-7. (in Russian, with English abstract).
- [35]Poletaev AB, Churilov LP. Immunophysiology, natural autoimmunity and human health. Herald of the International Academy of Sciences. Russian Section. 2009; 1: 11-6. https://readera.ru/14315162. (in Russian, with English abstract).
- [36]Tishevskaya NV, Babaeva AG, Gevorkyan NM. Effect of lymphocyte morphogenetic activity on organism reactivity and resistibility. Russ J Dev Biol. 2018; 49: 48-59. https://doi.org/10.1134/S106236041801006X. (in Russian, with English abstract).
- [37]Delgobo M, Paludo KS, Fernandes D, de Oliveira JG, Ortolan GL, Favero GM. Gut: key element on immune system regulation. Brazilian Archives of Biology and Technology. 2019; 62: e19180654. https://doi.org/10.1590/1678-4324-2019180654.
- [38]Frolova L, Frolov A, Kol`tsova І. Antiendotelial antibodies in development of essential hypertension during female climacteric period. Immunology and allergology: science and practic. 2012; 1: 83-7. http://nbuv.gov.ua/UJRN/Ita_2012_1_15. (in Ukrainian, with English abstract).
- [39]Hayflick L. The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research. 1965; 37(3): 614-36. doi: https://doi.org/10.1016/0014-4827(65)90211-9.
- [40]Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000; 28(22): 4474-8. doi: 10.1093/nar/28.22.4474.
- [41]Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000; 1(1): 72-6. doi:10.1038/35036093.
- [42]Sosińska P, Mikuła-Pietrasik J, Książek K. Molekularne podstawy komórkowego starzenia: fenomen Hayflicka 50 lat później [Molecular bases of cellular senescence: Hayflick phenomenon 50 years later]. Postepy Hig Med Dosw (Online). 2016; 70: 231-42. doi: 10.5604/17322693.1197485.
- [43]Steenstrup T, Kark JD, Verhulst S, Thinggaard M, Hjelmborg J, Dalgård C, et al. Telomeres and the natural lifespan limit in humans. Aging. 2017; 9(4): 1130-42. doi: 10.18632/aging.101216.
- [44]Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Semin Hematol. 2013; 50(2): 165-74. doi:10.1053/j.seminhematol.2013.03.030.
- [45]Drapkina ОМ, Shepel RN. Telomeres and telomerase complex. The main clinical manifestation of genetic malfunctioning. Cardiovascular Therapy and Prevention. 2015; 14(1): 70-7. http://dx.doi.org/10.15829/1728-8800-2015-1-70-77. (in Russian, with English abstract).
- [46]Dolgushin II, Mezentseva EA, Savochkina AYu, Kuznetsova EK. Neutrophil as a multifunctional relay in immune system. Russian Journal of Infection and Immunity. 2019; 9(1): 9-38. https://doi.org/10.15789/2220-7619-2019-1-9-38. (in Russian, with English abstract).
- [47]Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018; 174(5): 1054-66. doi: 10.1016/j.cell.2018.07.017.
- [48]Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol. 2018; 3(25): eaat1604. doi: 10.1126/sciimmunol.aat1604.
- [49]Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018; 19: 302-14. doi: https://doi.org/10.1038/s41590-018-0049-7.
- [50]Zhou S, Li Q, Wu H, Lu Q. The pathogenic role of innate lymphoid cells in autoimmune-related and inflammatory skin diseases. Cell Mol Immunol. 2020; 17: 335-46. doi: https://doi.org/10.1038/s41423-020-0399-6.
- [51]Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020; 20: 552-65. https://doi.org/10.1038/s41577-020-0282-9.
- [52]Rao A, Strauss O, Kokkinou E, Bruchard M, Tripathi KP, Schlums H, et al. Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs. Nat Commun. 2020; 11: 2049. https://doi.org/10.1038/s41467-020-15695-x.
- [53]Kansler ER, Li MO. Innate lymphocytes-lineage, localization and timing of differentiation. Cell Mol Immunol. 2019; 16: 627-33. https://doi.org/10.1038/s41423-019-0211-7.
- [54]Yarilin AA. Immunologiya [Immunology]. GEOTAR-Media: Moscow, Russia, 2010. (in Russian).
- [55]Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019; 10: 782. https://doi.org/10.1038/s41419-019-2015-1.