Molecular and immunological activity of Terminalia chebula extracts
Abstract
Several plant extracts and Ayurvedic formulations were used to treat ailments and such studies were well documented within the recent decades. Even a number of the plants were screened for immunomodulators to revive and rejuvenate the system. The present study is a screening trail for any possible healing activity of Terminalia chebula on IL-2 and IFN-γ levels. The raw and dried fruits of the sample were pulverized finely and extracted with methanol. Following which their aqueous solutions are re extracted with hexane, ester and chloroform to review the possible cytotoxic effects. Lipopolysaccharide (LPS)-stimulated macrophage cells were used throughout the study to assess the effect of extracts on nitric oxide (NO) production using Griess method. Expression of Cyclooxygenase-2 (COX2) and tumor necrosis factor (TNF)-α were studied by real time PCR quantification alongside estimation of IL-1β and IL-6 cytokine levels using the enzyme-linked immunosorbent assay (ELISA). The present study confirmed the positive effect of the chloroform extract in reducing the NO secretion and also by showing an inhibition within the expression of COX2, IL-1β, IL-6, and TNF-α. Thus, to conclude T. chebula might be used as anti-inflammatory candidate drug and also be used additionally to the various chemical compounds available within the medical markets.
INTRODUCTION
Immunomodulation is an adaptive terminology of the body to suppress its extra aggressive immune responses. During the immune reaction, several immune molecules get activated and this processes many free radicals like reactive oxygen and reactive nitrogen species are generated [1]. Gas nitric oxide (NO) which may be by product of reactive nitrogen species produced inducible nitric oxide synthase (iNOS) and superoxide (O2−) are released by pyrogen-activated macrophages and activated immune cells respectively increasing the cellular toxicity and oxidative stress within the body [2]. Such an imbalance between the radical molecules and therefore the anti-oxidative system results in accumulation of free radicals which eventually results in inflammation. Such an outsized number of free radicals inducing oxidative stress results in structural changes within the biomolecules causing mutagenesis [3]. This stress was also found to assist in developing chronic diseases like pulmonary and cardiovascular diseases including cancer [4]. Thus, studies are entirely focussed of the way to immunosuppress the system and to extend the activity of the anti-oxidative systems to scale back the inflammatory activities. Many natural compounds which are derived from traditional botanical plants and herbs are found to be rich in anti-oxidative substances which are studied and being studied to scavenge the free radicals.
Tithonia diversifolia, member of Asteraceae that able to grow commonly in several parts of Central and South America and Asian countries [5] was utilized in healing dermatitis, fever, arthritis and lots of pathogen infections [6]. Chronic inflammation is taken into account a serious threat which progresses into cancer because the inflammatory molecules generated during this process creates a hostile environment for the tumor and aids in metastasis. In many instances, such an environment is claimed to be the main predisposing factor for viral mediated tumorigenesis. On entry of the pathogen into the body, the innate system gets activated recruiting granulocytes to the injured site, producing inflammatory mediators like TNF-α, IL-1β and IL-6 and lots of lipid mediators like Prostaglandins (PGs) and leukotrienes (LTs) [7].
Mediators invoke an acute inflammatory process within hours to clear away the infection from the damaged tissues [8]. In due course, such an acute inflammation gets resolved when all of the pathogens and damaged tissue debris are cleared far away from the damaged site. But sometimes, these acute cases become chronic within weeks to months or sometimes years, resulting in autoimmune, neurodegenerative and vascular diseases [8]. Interleukin-2 (IL-2) was considered as a cytokine liable for the proliferation and differentiation of effector T cells and aids in cancer and a number of other infectious diseases. IL-2 is employed in passive therapy on the patients with melanoma or renal cell carcinoma which has been approved by the US Food and Drug Administration [9]. IL-2 is that the first line of defensive molecule which aids in treating autoimmunity and intrinsically considered as potent therapeutic agent [10]. The cytokines primarily produced by the induced T cells and dendritic cells (DCs) [11] but sometimes differential expression of IL-2 receptors also results in selective IL-2-driven expansion of Tregs which increases the affinity for IL-2 receptor alpha which pairs with the IL-2 receptor beta [12]. Current studies confirmed that under expression of IL-2 will resolve the inflammation by attracting and expanding the immune regulatory cells [13]. Cyclooxygenase 2 (COX-2) is one among such inflammatory mediators which is seen to be over expressed during an immune reaction against the pathogens. COX, also referred to as prostaglandin (PG) H synthase, catalyses the primary committed step within the synthesis of prostanoids, an outsized family of arachidonic acid metabolites comprising PGs, prostacyclin, and thromboxanes [14]. COX-2 is rapidly expressed in several cell types in response to growth factors, cytokines, and pro-inflammatory molecules and has emerged because the isoform primarily liable for prostanoid production in acute and chronic inflammatory conditions [15]. COX-2 is additionally a molecule which metabolizes the accumulated PGE2.
These results in up-regulation of several signalling pathways and down-regulation of apoptotic proteins which helps in physiological processes like proliferation, angiogenesis and metastasis [16]. When COX-2 is seen to urge over expressed, inflammation increases within the body resulting in reduction in apoptosis and metastasis which finally develops into cancer. COX-2 was said to make an immunosuppressive tumor environment where the pro-inflammatory eicosanoids, cytokines, chemokines and carcinoma cells all aids in forming an immunosuppressive environment. It also results in down-regulation of TH1 cytokines like TNF-α, IFNγ, IL-2, IL-12, and also said to upregulate the TH2 cytokines like IL-4, IL-10 which are proven immunosuppressive agents [17]. Many traditional herbs were screened for their anti-inflammatory nature. Terminalia chebula was found to exhibit a huge number of medicinal properties due to their phyto-constituents. Its fruit is claimed to possess vast number of health benefits and is employed since ages for several and human ailments. T. chebula was also quoted extensively in Ayurveda, Unani and Homoeopathic literatures [18]. Its aqueous extract was found to contain anti-inflammatory properties inhibiting the inducible gas synthesis [19]. Even studies done thus far, proved that the Chebulagic acid present within these fruits could suppress the onset and progression of arthritis within mice. T. chebula was also found to be a potent anti-inflammatory agent and is employed in Freund’s adjuvant [20].
Thus, this study was designed to review the effect of the fruit extracts as anti-inflammatory agents. T. chebula was studied for its anti-oxidative stress-related immunomodulation properties. But none of the studies were focused on the inflammatory mediators like TNF-α and IL-6. The study was planned to screen the expression of the inflammatory mediators like TNF-α, IL-6, COX2 by real time amplification and ELISA methods. LPS induced (J774.1A) macrophage cell lines were utilized in the study.
MATERIALS AND METHODS
Materials
ELISA kits for IL1β and IL6 were purchased from Everone Biosciences. All the reagents utilized in the study were of molecular grade and were purchased from HiMedia Deionized water was produced in-house employing a Milli-Q System from Millipore. All the primers were ordered from Sigma Aldrich.
Cell culture
Murine macrophage cell line (J774.1A) was donated from Stellixir Ltd, Bangalore, and was cultured in Dulbecco’s modified eagle medium (DMEM) containing 2mM L-glutamine, 100U penicillin/mL, 0.1mg streptomycin/mL, and 10% heat-inactivated FBS (HiMedia) and incubated during a humidified CO2 incubator. The cells were maintained until confluence and were passaged until further use.
Extract preparation
Raw fruits and dried ripened fruits of T. chebula were collected from the nearby farms, Bangalore. The samples each of 500gm were extracted with 1000mL methanol for 3 days using soxhlet extraction (45°C). The extract obtained was then concentrated during a rotary evaporator and lyophilized. About 5gm of this this extract was suspended in water then partitioned with n-hexane, ester and chloroform extracts. The extracts obtained were then concentrated during a rotary evaporator and therefore the residues obtained were stored at -20 °C until further use. The yields obtained were 16.89, 18.21 and 19.26% (w/w) for n-hexane, ester and chloroform extracts respectively. Each extract was then weighed and suspended in 5% sterile dimethyl sulfoxide (DMSO) to offer a 20mg/mL solution [21].
Cell viability assay
The effect of the obtained extracts on the viability of the cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. About 7500-9000 cells per well were incubated with 1-200μg/mL of the extracts for 24hr at 37°C during a humidified CO2 incubator. All the tests were wiped out triplicates. Wells containing DMSO because the solvent was considered as negative control. 10μL of MTT (5mg/mL) was added to all or any the wells and therefore the plate was incubated for 4hr at 37°C. Following incubation, the plates were removed and added with lysis buffer (0.1N HCl in isopropanol) to dissolve the formazan produced from the treated cells. Absorbance was measured with a microplate reader (Genetix, Germany) at 570nm. Absorbance of the negative control wells was considered as 100% viability [22]. The Results were expressed as percentages and was calculated using the subsequent equation: [%viability =(ODsample-ODBlank)/ (ODcontrol-ODBlank) *100].
Quantification of nitric oxide production
Nitric oxide production was assayed according to the protocol prescribed by [23]. In brief, the cell lines at a concentration of 1×106/100μl were seeded into 96-well plates. The seeded cells were then treated with 1μL lipopolysaccharide (LPS, HiMedia; 1μg/ml) and extracts at varying concentrations of 6.25-200μg/ml for 24hr. All the tests were done in triplicates and the cells treated with LPS and 0.1% DMSO were considered as positive control and negative control, respectively. NG-nitro-L-arginine-methyl ester (L-NAME, SIGMA; 1mM/mL) was used as an iNOS inhibitor in the study.
Following 24hr incubation, the supernatants were collected from the cultures and assayed for the production of NO with Griess reagent. The supernatant was incubated with the reagent for 15min at room temperature and the absorbance was read at 550nm in a plate reader (Genetix). The concentration of nitrate and the % of NO inhibition along with IC50 was calculated by sodium nitrite standard curve. Positive control was considered 100%.
Cytokine assay
The cytokines like IL1β and TNFα which would be released by the cell lines were measured using the ELISA kit (Everone Biosciences). The protocol was followed according to the manufacturer’s instructions. The supernatant of cells cultured (control and treatment) was spine down at 8000rpm, 10min and stored at −20°C until further use. The cells without stimulation with LPS was used as negative control. The day before the assay, the 96well plate was impregnated with the capture antibody against the specific molecules. The samples were added in their respective wells and done in triplicates.
The plate was incubated for about 2hours and following washing with secondary antibody conjugated with HRP. H2O2-tetramethylbenzidine was sued as the substrate and after the incubation, the reaction was stopped using 2N H2SO4 and the absorbance was read at 405nm in a plate reader (Genetix). The concentration of the study proteins released into the media was estimated using the standard graph.
RNA extraction
Since the chloroform extract showed maximum rate of reduction, it was considered for the expression studies. The cells (treated and control) with confluency were used for the RNA extraction [24] using RNeasy Mini Kit (Qiagen74104). The test was done according to the manual instructions. Following trypsinization, the cells were spin down at 6000rpm for 5min and resuspended in about 560μl of AVL buffer and 560μl chilled ethanol. After thorough mixing, the contents were centrifuged at 8000rpm for about 1min and the column was then washed with 700μl of wash buffer and then incubated with 60μl of AVE buffer. The RNA was then eluted and stored at −20°C. The RNA was analyzed qualitatively using UV spectrophotometer (260/280) and used in the cDNA synthesis.
cDNA synthesis
cDNA synthesis was carried according to the manual instructions from RT PCR kit using SuperScript TMII Reverse Transcriptase, 200U/μl (HiMedia). About 1.22μl (concentration obtained was 1.97μg/μl) of the RNA was used in the reaction. Random primers and 1μl of RT enzyme were added and incubated at 25°C for 10min. Following incubation at 70°C for 45 min, the cDNA was then stored and used in the real-time PCR assay.
Real-time PCR
Primers used in the real time assay (Table 1) were designed using primer3 software and were purchased from Sigma-Aldrich. The real-time amplification was carried according to [25] using the iQTM SYBR Green Supermix (HiMedia). The primers (600nM) and 1μl of the RT products were used and the reaction was carried in a total volume of 12.5μl. All the reactions were done in duplicates along with their respective negative controls.
Table 1. List of the primers used in the real time PCR.
Expression of COX-2 and TNF-α members
Quantitative PCR was done on the samples (Control and treatment) in the Corbett Research cycler (Bio-Rad). The COX-2 (forward: 5’ GTACTCCCGATTGAAGCCCC 3’ and reverse: 5’ TCGTGTAGCGGTGAAAGTGG 3’; 138bp) and TNF- α (forward: 5’ GGGTGTGAGAAGAGAGATGGG 3’ and reverse: 5’ GGCCAGAGGGCTGATTAGAG 3’; 342bp) of about 600nM was used in the amplification study. 1.22μl of the RNA products were used, and the program was run for about 40 cycles at 93°C for 60s, 62°C for 40s, and with an elongation at 72°C for 60s. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward: 5’ GCTGAGTACGTCGTGGAGTC 3’ and reverse: 5’ CCCATTCCCCAGCTCTCATA 3’; 455 bp) was also amplified along with the gene members for a comparative analysis of the mRNA expression. The comparative analysis was done using ΔCt method and the Ct values obtained for each sample were normalized to the housekeeping gene (GAPDH).
Statistical analysis
Throughout the study the data was analysed using a one-way ANOVA and using SPSS software (USA). All the values were expressed as means ± SD of at least three independent experiments and the significance level was maintained at P < 0.05.
RESULTS
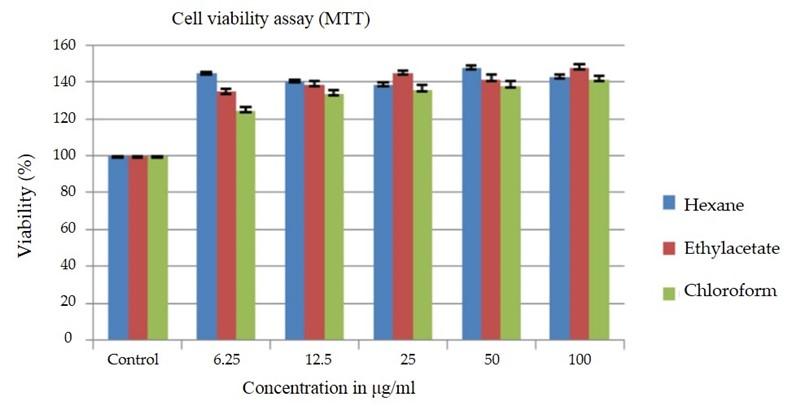
Effect of extract on cell viability assay
MTT colorimetric assay was done to assay the possible cytotoxic effect of varying concentrations (6.25to 100.00μg/ml) of the extracts on the growth of J774.1A cells. Extracts of n-hexane, ethyl acetate and chloroform showed no significant cytotoxicity on the cell lines even at a concentration of 100μg/ml. None of the extracts showed any reducing effect on the cell lines.
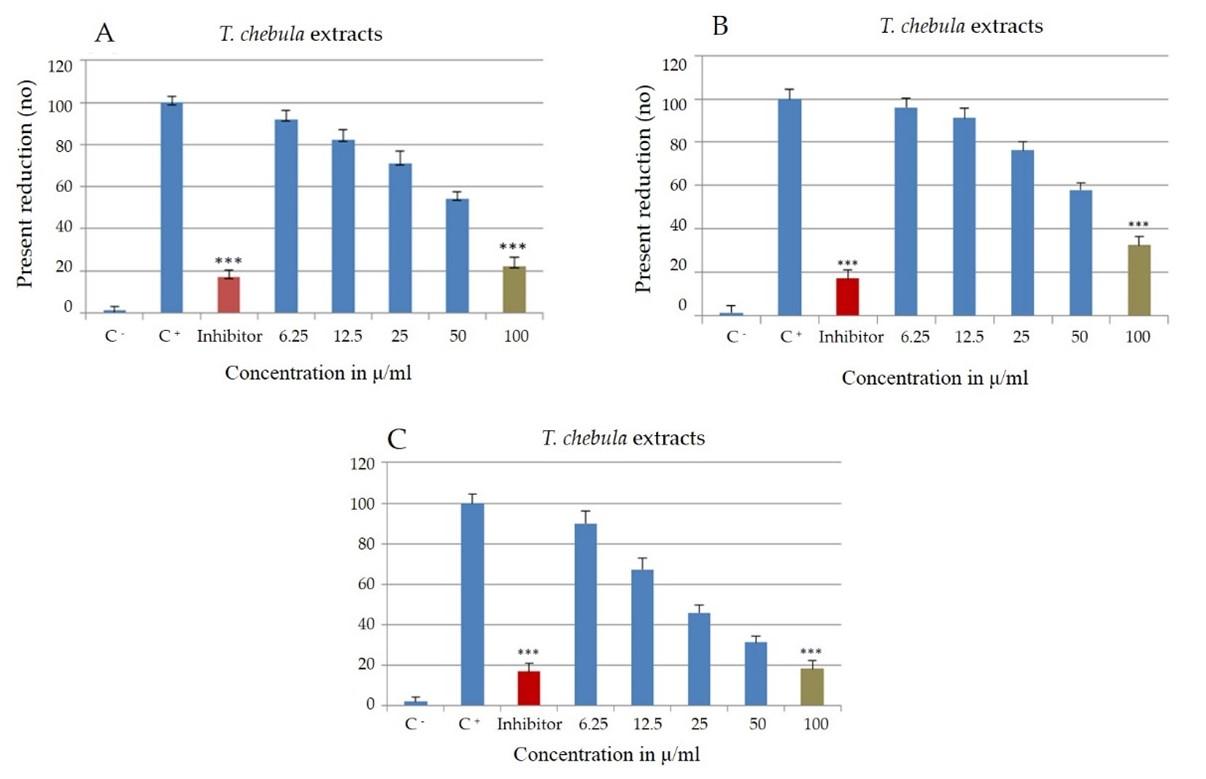
Reduction in NO production
The cell lines were treated with LPS, extracts and inhibitors to study the effect on NO production. Cells added with LPS stimulant showed a significant increase in the NO production to 87.23 ± 2.1μmol/L (p<0.005). Negative control without LPS treatment showed 15.67± 1.1μmol/L. The cells treated with inhibitor obviously showed a reduction in the NO production to 16.94± 2.9μmol/L (P < 0.005).
Chloroform extract showed significant reduction when compared to ethyl acetate and n-hexane. Chloroform extract showed significant reduction from 89.83± 1.6 μmol/L to 18.31± 1.6μmol/L. Hexane extract showed a reduction from 92.16 ± 3.5% at 6.25μg/ml to 22.3 ± 10.1% at 100μg/ml (Figure 2B). Ethyl acetate on the other hand also showed reduction but was significantly lower when compared to the other two extracts (32.4 ± 3.9% from 96.11± 2.3% at 100μg/ml, p<0.005).
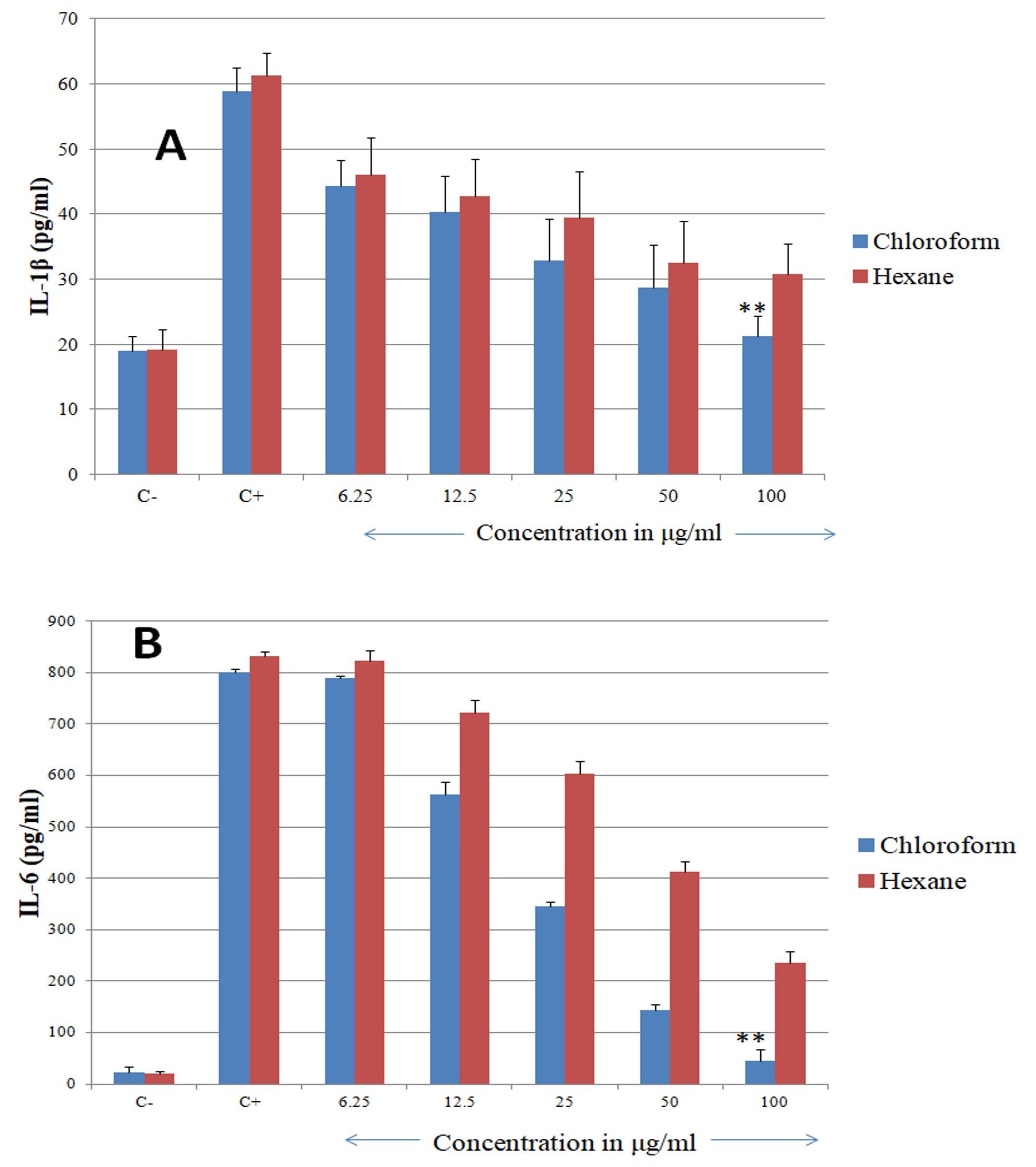
Effect of extract on cytokine assay
This ELISA method was used in estimating the expression of the IL-1β and IL-6 cytokines on treatment with the hexane and chloroform extracts only (ethyl acetate was excluded from the study). From the figure it was found that LPS stimulation has significantly increased the levels of IL-1β levels (47.84 ± 1.53pg/ml) when compared to the negative control (21.54 ± 2.11pg/ml), and even IL-6 levels were also increased to 894±17.01pg/ml from 11.3 ± 2.54pg/ml (negative control). Chloroform extract showed significantly higher reduction of the IL-1β and IL-6 levels when compared to the hexane extract.
Chloroform extract with varying concentrations (6.25-100μg/ml) significantly reduced IL-1β levels to 44.22 ± 0.31pg/ml (6.25μg/ml), 40.31 ± 1.48pg/ml (12.5μg/ml), 32.9 ± 0.21pg/m (25μg/mL), 28.75 ± 1.48pg/ml (50μg/mL), and 21.23 ± 0.21pg/ml (100μg/mL); P < 0.005. Hexane extract also showed reduction in the levels with varying concentrations (6.25-100μg/ml) to 46.12 ± 0.31pg/ml (6.25μg/ml), 42.82 ± 2.48pg/ml (12.5μg/ml), 39.45 ± 1.21pg/ml (25μg/mL), 32.45 ± 1.48pg/ml (50μg/mL), and 30.83 ± 2.21pg/ml (100μg/mL); P < 0.005. Similar results were seen in IL-6 secretion also and chloroform extract showed significantly lower levels of 45.67± 3.31pg/ml (100μg/ml) and 789.67± 1.39pg/ml (6.25μg/ml). Hexane showed reduction in IL-6 levels to 234.54± 1.77pg/ml (100μg/ml) and 821.91± 3.12pg/ml (6.25μg/ml).
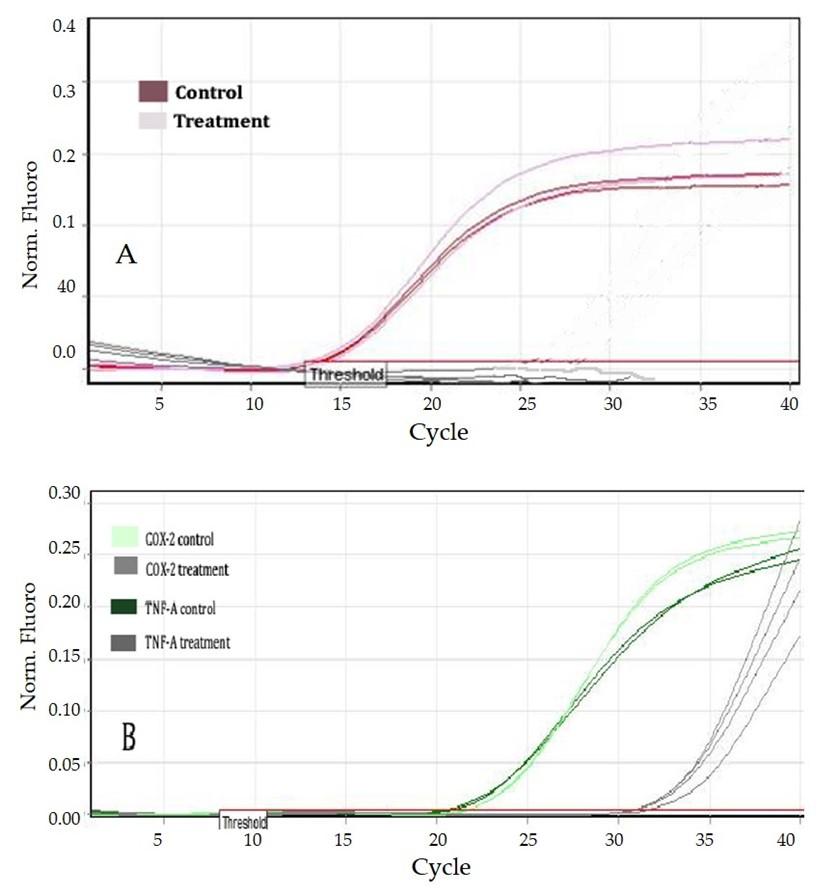
Expression of genes
The results obtained were normalized to GAPDH for comparative expression study. The mRNA expression was studied separately for each gene member (GAPDH, TNF- α and COX-2) was studied separately. The sample with the lowest ΔΔCt value was used as calibrator, and the remaining members were compared in relation to calibrator.
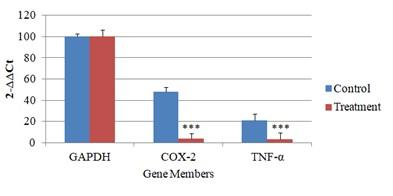
The Ct values of the GAPDH, TNF- α and COX-2 were found to be 11, 21, and 22 respectively for the control. And the Ct values for the GAPDH, TNF- α and COX-2 after treatment was found to be 11, 30 and 33, respectively.
From the Ct values, (Figure 4) after normalizing with the GAPDH, it was observed that the relative expression of both the gene members were downregulated in treatment samples when compared to the control. The GAPDH gene expression was considered as 100%.
From the graph, (Figure 5) and the calculated 2−ΔΔCt values, it was found that the relative expression of the COX-2 and TNF- α was downregulated by 12 and 7 times, respectively when compared to their respective controls.
DISCUSSION
Macrophages are considered very critical in case of inflammation due to their characteristic antigen presenting nature. Moreover, they are good at phagocytosis and other immunomodulation properties. The main role of these macrophages is to enhance the production of inflammatory molecules and mediators like cytokines [26]. During this crucial time, host tries to suppress the activity of these cytokines to minimise the damage caused to the tissues. Many previous studies reported that several medicinal plants are employed to heal the inflammatory process [27]. E. amoenumis studied for its anti-inflammatory and analgesic properties and as such commonly used as folk medicine in Iran to cure the common cold [28]. In the present study we studied the effects of T. chebula as anti-inflammatory on stimulation with LPS.
We first studied the extracts for their healing effects on J774.1A macrophage cell lines viability to nullify the cytotoxic effects, followed by estimating the inhibition of NO production. NO is said to be crucial mediator in regulating the inflammation process [29] as such its effects were studied. In the current study, the percent of cytotoxicity as shown by MTT assay determines the safety of using the sample material. None of the extracts (hexane, ethyl acetate and chloroform) showed any cytotoxicity on the cell lines. The cytotoxicity was significantly less even at the highest concentration of 100μg/ml.
In this study, T. chebula extract showed significant inhibition on the NO production in a dose dependent manner. Most of the studies reported so far suggest that the extracts which are significantly good at lowering the No production are good anti-inflammatory agents [30]. From the results, it was proved that chloroform extract (18.31± 1.6μmol/L) was significantly good at reducing maximum NO produced followed by hexane (22.3 ± 10.1 μmol/L) at highest concentration of 100μg/ml. The inhibition activity was quite promising when compared to the inhibitor reduction activity (16.94± 2.9μmol/L). This reduction might be either due to the inhibition of iNOS activity or at the level of locking the transcription signalling methods. As such the study was planned in the method specified by [31].
The present study so far concluded the possible evaluation on the inhibition of NO by the extracts. Additionally, there are several studies reporting the same NO reducing activities. Eucalyptus globules and Thymus vulgaris [26] were studied for their capability in reducing the NO production among the LPS induced J774.1A cells. Even studies done on Andrograp hispaniculata [32] and Echinacea [29] also confirmed the possible inhibitory role of the extracts in NO production and iNOS activity among the LPS-stimulated macrophages. Mentha longifolia also was concluded that it is a good anti-inflammatory agent on regulating the production of NO among the LPS-stimulated J774.1A cells [27].
COX2 is also said to play a crucial role in inflammation [33] which converts arachidonic acid to prostaglandins. Moreover, NSAIDs are used to suppress the inflammatory process by inhibiting the COX enzyme [34]. Studies done on the hexane extract of E. amoenum proved that they are potent anti-inflammatory by inhibiting the COX2 gene expression among the J774.1A macrophage cell lines [3].
Our study confirmed that the extracts strongly showed a reduction in the COX2 and TNF-α gene expressions. TNF-α and IL-1β were said to cytokines mostly involved in inflammation, where TNF-α produced by the macrophages takes part in initiating the acute phase of inflammation. This mode of action is done by attracting the neutrophils to the target site.
IL-1β also aids in bringing an inflammatory response on infection with a pathogen. Many chemical moieties were used as inhibitors to reduce the effect of IL-1β so as to relieve the inflammatory arthritis among the experimental organisms [11]. IL-6 is also said to be proinflammatory in nature and takes part in the generation of inflammation [35]. Our study confirmed that, chloroform extract showed a significantly downregulation of these two gene members (COX2 and TNF-α).
The real time expression studies done on the cell lines on induction with LPS confirmed the possible role of the extracts (chloroform) in downregulating the expression of inflammatory chemokines. COX2 and TNF-α were downregulated by 12 and 7 times when compared to the control. Even studies done on several medicinal plants Mentha longifolia [27] and Echinacea [29] showed that they could reduce the expression of TNF-α, IL-6, and IL-1β proteins at the mRNA level among the LPS induced macrophages. As major reduction effects were observed with chloroform extracts on the cell lines, further we need to plan to isolate and purify the phytochemicals from the extract and study the same at pinpoint level.
CONCLUSION
The plant material T. chebula and its extracts were studied for the possible effects on the macrophages as anti-inflammatory agents. Among them chloroform extract showed significant reduction of NO production followed by the hexane extract. Chloroform extract was found to be more effective in reducing the levels of IL-1B and Il-6 gene members. Real time PCR analysis also confirmed that the chloroform extract was significantly down regulating the expression of COX2 and TNF-α levels when compared to control. The findings in the current study reveal that the plant material can act as strong anti-inflammatory agent as confirmed on the LPS induced macrophages. Further if the study could isolate and purify the extract into its constituent photochemical, then the mode of action could be traced to the pinpoint level. Our study confirms the possible potent role of T. chebula as strong anti-inflammatory agent.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONS
HA, select the scope of the study, and AB and AM, supervised the lab work. All authors wrote and read the manuscript and approved it.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiation research, 2012; 178(6), 505-523.
- [2]Lundberg JO, Carlström M, Larsen F J, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovascular research, 2011; 89(3), 525-532. 187.
- [3]Jones D P, Sies H. The redox code. Antioxidants & redox signaling, 2015; 23(9), 734-746.
- [4]Penticuff JC, Kyprianou N. Therapeutic challenges in renal cell carcinoma. American journal of clinical and experimental urology, 2015; 3(2), 77.
- [5]Sharrock, R. A., Sinclair, F. L., Gliddon, C., Rao, I. M., Barrios, E., Mustonen, P. J., Godbold, D. L. A global assessment using PCR techniques of mycorrhizal fungal populations colonizing Tithoniadiversifolia. Mycorrhiza, 2004; 14(2), 103-109.
- [6]Maregesi, S. M., Ngassapa, O. D., Pieters, L., &Vlietinck, A. J. Ethnopharmacological survey of the Bunda district, Tanzania: Plants used to treat infectious diseases. Journal of ethnopharmacology, 2007; 113(3), 457-470.
- [7]AL-Saad, N.F., Nadhim, M.H. and Nawar, HY. Serological, Culture and Urea Breath Test for Detection of H. Pylori in Gastric Ulcers Patients. Indian Journal of Forensic Medicine & Toxicology, 2020; 14(4), p.1317.
- [8]Yao, C., &Narumiya, S. Prostaglandin‐cytokine crosstalk in chronic inflammation. British journal of pharmacology, 2019; 176(3), 337-354.
- [9]Zhu, J., Paul, W. E. CD4 T cells: fates, functions, and faults. Blood, The Journal of the American Society of Hematology, 2008; 112(5), 1557-1569.
- [10]Lan, R. Y., Selmi, C., & Gershwin, M. E. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2). Journal of autoimmunity, 2008; 31(1), 7-12.
- [11]Dayer, J. M. Interleukin 1 or tumor necrosis factor-alpha: which is the real target in rheumatoid arthritis. The Journal of rheumatology Supplement, 2002; 65, 10-15.
- [12]Khan, S. H., Martin, M. D., Starbeck-Miller, G. R., Xue, H. H., Harty, J. T., &Badovinac, V. P. The timing of stimulation and IL-2 signaling regulate secondary CD8 T cell responses. PLoS pathogens, 2015; 11(10), e1005199.
- [13]Granucci, F., Feau, S., Angeli, V., Trottein, F., &Ricciardi-Castagnoli, P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. The Journal of Immunology, 2003; 170(10), 5075-5081.
- [14]Hinz, B., & Brune, K. Cyclooxygenase-2—10 years later. Journal of pharmacology and experimental therapeutics, 2002; 300(2), 367-375.
- [15]Vane, J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature new biology, 1971; 231(25), 232-235.
- [16]Satoh, H., Amagase, K., Ebara, S., Akiba, Y., & Takeuchi, K. Cyclooxygenase (COX)-1 and COX-2 both play an important role in the protection of the duodenal mucosa in cats. Journal of Pharmacology and Experimental Therapeutics, 2013; 344(1), 189-195.
- [17]Stolina, M., Sharma, S., Lin, Y., Dohadwala, M., Gardner, B., Luo, J., .Dubinett, S. M. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. The Journal of Immunology, 2000; 164(1), 361-370.
- [18]Bag, A., Bhattacharyya, S. K., & Chattopadhyay, R. R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pacific journal of tropical biomedicine, 2013; 3(3), 244-252.
- [19]Moeslinger, T., Friedl, R., Volf, I., Brunner, M., Koller, E., Spieckermann, P. G. Inhibition of inducible nitric oxide synthesis by the herbal preparation Padma 28 in macrophage cell line. Canadian journal of physiology and pharmacology, 2000; 78(11), 861-866.
- [20]Pratibha, N., Saxena, V. S., Amit, A., D’souza, P., Bagchi, M., &Bagchi, D. Anti-inflammatory activities of Aller-7, a novel polyherbal formulation for allergic rhinitis. International journal of tissue reactions, 2004; 26(1-2), 43-51.
- [21]Saha, S., & Verma, R. J. Antioxidant activity of polyphenolic extract of Terminalia chebulaRetzius fruits. Journal of taibah university for science, 2016; 10(6), 805-812.
- [22]Wang, M., Yang, L., Ji, M., Zhao, P., Sun, P., Bai, R. & Li, C. Aqueous extract of Terminalia chebula induces apoptosis in lung cancer cells via a mechanism involving mitochondria-mediated pathways. Brazilian Archives of Biology and Technology, 2015; 58, 208-215.
- [23]Naseri, N., Kalantar, K., & Amirghofran, Z. Anti-inflammatory activity of Echiumamoenum extract on macrophages mediated by inhibition of inflammatory mediators and cytokines expression. Research in pharmaceutical sciences, 2018; 13(1), 73.
- [24]Abbas, S., & Malla, S. Cytotoxicity and expression studies of angiogenesis-promoting genes in cancer cell lines under the treatment of cancer candidate drugs. Asian J Pharm Clin Res, 2019; 12(5), 130-134.
- [25]Doersch, K. M., DelloStritto, D. J., & Newell-Rogers, M. K. The contribution of interleukin-2 to effective wound healing. Experimental Biology and Medicine, 2017; 242(4), 384-396.
- [26]Vigo, E., Cepeda, A., Perez‐Fernandez, R., & Gualillo, O. In‐vitro anti‐inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A. 1 murine macrophages. Journal of Pharmacy and Pharmacology, 2004; 56(2), 257-263.
- [27]Karimian, P., Kavoosi, G., &Amirghofran, Z. Anti-inflammatory effect of Menthalongifolia in lipopolysaccharide-stimulated macrophages: reduction of nitric oxide production through inhibition of inducible nitric oxide synthase. Journal of immunotoxicology, 2013; 10(4), 393-400.
- [28]Heidari, M. R., Azad, E. M., & Mehrabani, M. Evaluation of the analgesic effect of EchiumamoenumFisch& CA Mey. extract in mice: possible mechanism involved. Journal of ethnopharmacology, 2006; 103(3), 345-349.
- [29]Zhai, Z., Solco, A., Wu, L., Wurtele, E. S., Kohut, M. L., Murphy, P. A., & Cunnick, J. E. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. Journal of ethnopharmacology, 2009; 122(1), 76-85.
- [30]Stangeland, T., Alele, P. E., Katuura, E., & Lye, K. A. Plants used to treat malaria in Nyakayojo sub-county, western Uganda. Journal of ethnopharmacology, 2011; 137(1), 154-166.
- [31]Booke, M., Hinder, F., McGuire, R., Traber, L. D., &Traber, D. L. Selective inhibition of inducible nitric oxide synthase: effects on hemodynamics and regional blood flow in healthy and septic sheep. Critical care medicine, 1999; 27(1), 162-167.
- [32]Minaiyan, M., Asghari, G., Sadraei, H., & Feili, E. Anti-inflammatory effect of Pycnocyclaspinosa extract and its component isoacetovanillone on acetic acid induced colitis in rats. Research in pharmaceutical sciences, 2015; 10(4), 345.
- [33]Hugo, H. J., Saunders, C., Ramsay, R. G., & Thompson, E. W. New insights on COX-2 in chronic inflammation driving breast cancer growth and metastasis. Journal of mammary gland biology and neoplasia, 2015; 20(3), 109-119.
- [34]Buffum, M., Buffum, J. C. Nonsteroidal anti-inflammatory drugs in the elderly. Pain Management Nursing, 2000; 1(2), 40-50.
- [35]Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology, 2014; 6(10), a016295.