COVID-19 drugs: A necessary strategy for living with COVID-19 in the new normal and the mutations of the SARS-CoV-2 such as omicron variant
Abstract
The COVID-19 pandemic has spread rapidly and caused significant damage to global public health as well as the economy. While the race of vaccine development witnessed a spectacular breakthrough with the introduction of highly effective vaccines such as BNT162b2, mRNA-1273, or AZD1222, no specific drug for COVID-19 treatment has been discovered yet. Recently, repurposing drugs classified into three main groups of mechanisms, including antivirus, anti-SARS-CoV-2 antibodies, and immunomodulators, are investigated. As a result, Remdesivir and six other drugs are authorized by the Food and Drug Administration (FDA) to treat patients infected by the SARS-CoV-2. This work aims to highlight that, besides vaccines, COVID-19 drugs should get more attention and be considered as one of the most promising strategies to safely coexist with the SARS-CoV-2 virus in the new normal status and for the mutations of the virus, such as the omicron variant.
INTRODUCTION
The global pandemic, the COVID-19, has caused 279,114, 972 confirmed cases and 5,397,580 deaths worldwide (data taken on December 27, 2021) [1]. Since then, the race of vaccine development has never observed a more spectacular breakthrough in the COVID-19 pandemic. A variety of COVID-19 vaccines with their high efficacy, including BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), Sputnik V, and AZD1222, were introduced after only one year of investigation [2]. In contrast, no specific treatment for COVID-19 has been announced yet, although being applied to cutting-edge technologies, including machine learning or artificial intelligence. Therefore, this review will summarize the status of COVID-19 drug development to propose the new strategies for COVID-19 treatment using newly developed drugs for the normal and the mutated SARS-CoV-2 virus, such as the omicron variant.
THE DEVELOPMENT AND STATUS OF DRUGS FOR COVID-19
De novo drug discovery is a high-cost and risky procedure, and usually takes at least 10 years to reach patients. Due to globally urgent demand for an effective COVID-19 treatment, drug repurposing is widely evaluated as a reasonable alternative for traditional drug development. This strategy recognizes a new indication related to COVID-19 treatment for existing drugs. Drug repurposing significantly saves time and cost thanks to the available pharmacokinetic and safety profiles as well as dosage forms of drug candidates.
Much research for drug repurposing has been published with several approaches [3] including computational studies, in silico experiments, and clinical trials [4, 5]. In computational research, disease-based and target-based processes are the main approaches to indicate a new application for the approved drug. Disease-based process means comparing the similarities and characteristics of diseases, while in target-based process, researchers establish the interaction between drug and a list of targets. Computational and in silico studies are valuable for initially screening out drug candidates that effectively suppress COVID-19 progression for further clinical trials [6]. Besides, related to clinical trials, there are 7219 studies about COVID-19 therapeutic treatment registered on ClinicalTrials.gov by the time this review is edited (December, 2021). Of 3573 studies about drugs, the vast majority are in Phase 2 and Phase 3, with nearly 1857 drug interventions.
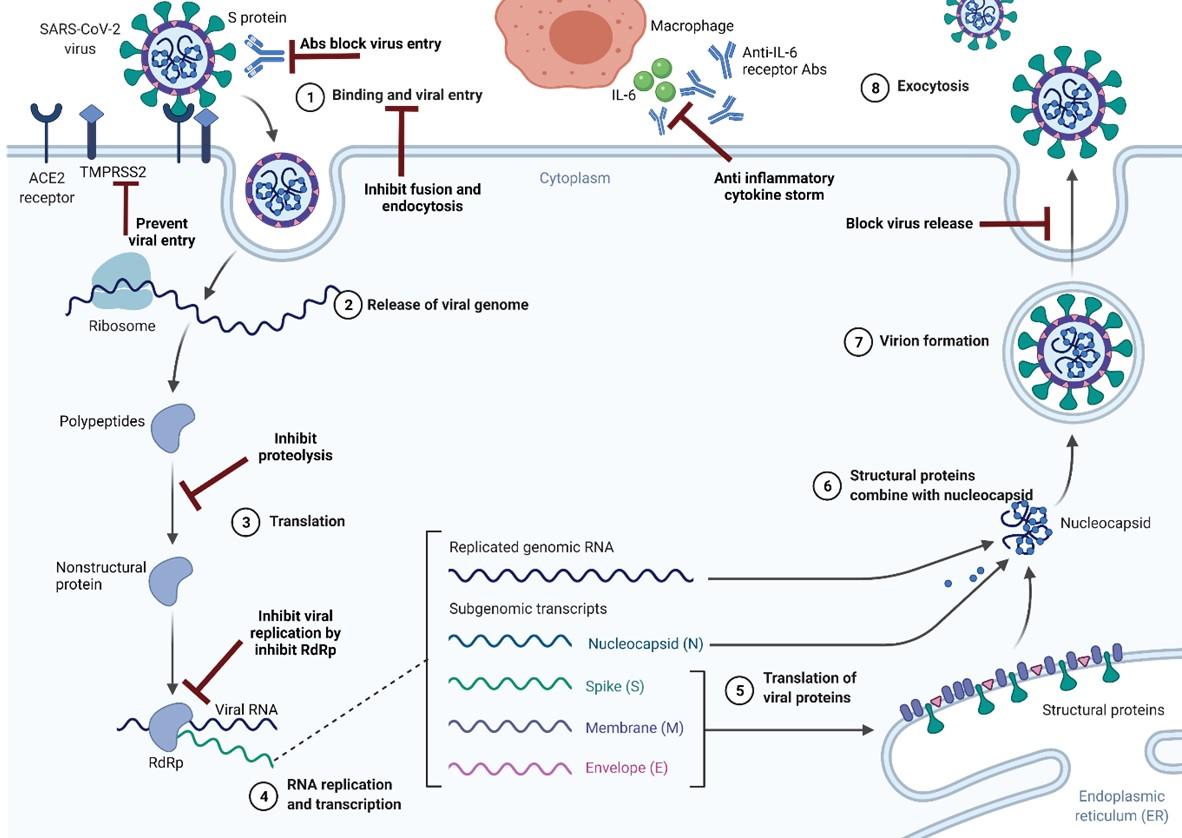
Summarizing from the three above approaches, currently proposing drugs for COVID-19 could be classified into three main groups based on their mechanisms, including antiviral drugs, human antibody products, and immunomodulators (Table 1). SARS-CoV-2 is a single RNA-strand virus targeting human cells by binding to angiotensin-converting enzyme 2 (ACE2) receptors by spike protein. After fusion, released viral RNA is translated into polypeptides, including structural and nonstructural proteins. A new RNA strand is synthesized through viral RNA-dependent RNA polymerase (RdRp), and the virus is then assembled and successfully replicated. During the life cycle of the SARS-CoV-2 virus, researchers focus on several specific therapeutic targets (Figure 1) [7]. For example, Lopinavir and Darunavir suppress proteolysis via inhibiting 3-chymotrypsin-like protease, while Remdesivir and Favipiravir target viral RdRp to reduce viral replication. Other antiviral drugs like Chloroquine and Hydroxychloroquine inhibit virus fusion into human cell membranes by increasing the pH of endocytosis [7, 8]. Several monoclonal antibodies, such as Sotrovimab, Casirivimab, or Bamlanivimab targeting viral spike protein, are suggested to block viral invasion and show clinical benefits in COVID-19 treatment. Notably, Bamlanivimab was reported to significantly reduce its activity on beta and gamma variants [8]. This finding raises concerns about resistant viruses that some variants can adopt mutations to reduce their susceptibility to monoclonal antibodies.
Systemic inflammation and cytokine storm are major factors leading to multiple organ failures and death. While corticosteroids, especially dexamethasone, are shown to be effective in suppressing inflammatory responses, several immunomodulators are utilized in combination with dexamethasone to inhibit cytokine storm and give beneficial clinical outcomes, like Tocilizumab, Sarilumab, or Baricitinib [8]. Of many existing drugs belonging to the 3 groups mentioned above, only Remdesivir was approved by the Food and Drug Administration (FDA) to treat COVID-19 disease. Drugs declared in the Emergency Use Authorization (EUA) list should be cautiously used despite being recommended by the FDA and National Institutes of Health (NIH) Panel. The remaining drug candidates are advised against using or still need more randomized trials to conclude [8, 9].
Table 1. Summary of FDA-authorized COVID-19 drugs (up to Sep, 2021)
COVID-19 DRUGS AS A NECESSARY STRATEGY FOR THE NEW NORMAL AND THE MUTATION OF THE SAR-COV–2 VIRUS SUCH AS OMICRON VARIANT
Since its first outbreak in Wuhan in December 2019, the COVID-19 pandemic has rapidly spread to 223 countries and territories globally, with more than 225 million confirmed cases and nearly 5 million deaths [1]. Effective strategies are urgently required to minimize the great pressure COVID-19 puts on global public health. New case detection, general prevention methods, and quarantine are proven effective. However, they can only be utilized as contemporary plans due to their devastating impacts on the global economy and society. Therefore, vaccines and drugs for COVID-19 are always of paramount importance to battle against the SAR-CoV-2 virus for long-term.
Due to the rapid mutation and spread of the SARS-CoV-2 virus, governments and scientists should accept that humans cannot get rid of this coronavirus with zero confirmed cases. It is time to reset our goal into safe coexistence with the SARS-CoV-2 virus and prepare for the new normal life. The first step of preparation is to accelerate the implementation of vaccination for citizens. Vaccine enhances human immunity and reduce the risk of severe illness and other consequences like death. mRNA vaccines such as BNT162b2 and mRNA-1273 were reported to have more than 90% protective efficacy after 2 doses. On the contrary, the effectiveness of Sputnik V and AZD1222, which are viral vector vaccines, is 91.6% and 76.0%, respectively [2]. Regarding concerns about the reduction in the effectiveness of current vaccines against new variants, a study by Bernal et al. (2021) stated that after 2 doses of BNT162b2 or AZD1222 vaccines, only subtle differences in protective efficacy were observed with the delta variant, which is the most common variant in India since April 2021 and recently the omicron variant, compared to alpha variant [10]. Therefore, current vaccines are expected to retain their vigorous activity against SARS-CoV-2 variants and partly resolve the fast mutation issue.
On the other hand, repurposing drugs play crucial roles in treating patients with SARS-CoV-2 infection. Casirivimab in combination with Imdevimab, as well as Sotrovimab, which were issued as EUA by the FDA, were also proven to decrease the risk of both COVID19-associated hospital admission and deaths in mild to moderate COVID-19 outpatients. Remdesivir, the only FDA-approved COVID-19 drug, was reported to reduce recovery time from 15 days to 10 days and gave beneficial clinical outcomes to hospitalized patients requiring oxygen support. Their effectiveness remains unchanged against 6 key variants of the SARS-CoV-2 virus [8]. Belonging to the same group with Remdesivir, Nirmatrelvir + Ritonavir (Paxlovid), and Molnupiravir, which recently attracted much attention due to their promising results in clinical trials, have been expected to be the game changers in this long-term battle [11].
While waiting for a breakthrough in drug development, other alternative options have been proposed for the treatment of COVID-19. These findings usually focused on naturally originated medications since they are safer and more compatible than therapeutic drugs. Mahamud et al. suggested the food-originated bioactive peptides drugs, which have been proven to have a better health impact than therapeutic drugs and inhibit chronic disease without side effects [3]. Hossain et al. showed that honey could be a possible component against SARS-CoV-2 infection [12]. Another study by Farjana et al. used vitamin C against the pathology of COVID-19 due to its crucial role in the immune system [13]. It is also said that Nigella savita seed showed promising results in treating and preventing COVID-19 [14]. Other than physical drugs, mental health also played an initial role in preventing this disease, as stated in Hannan et al. [15]. However, the effectiveness of alternatives is still controversial, and need more reports.
While promoting the development of specific COVID-19 drugs, another emerging challenge for treatment also needs overcoming. The increasing demand for medicine, high cost, and the scarcity of drug resources prevent developing countries from accessing qualified drugs and vaccines. This significantly decelerates the new normal status while COVID-19 causes more damage. Transparent and reasonable criteria for COVID-19 drug allocation need to be rapidly established to support more and more countries in the battle against the SARS-CoV-2 virus [16].
CONCLUSION
Besides developing new vaccines against this disease during this public health crisis, it is vital to make progress in the development of specific drugs for COVID-19 treatment to co-exist with the SARS-CoV-2 in the new normal. The current strategy is mostly focused on reproposing drugs with three groups, including antiviral drugs, human antibody products, and immunomodulators. Meanwhile, alternative therapies should be considered.
ACKNOWLEDGEMENT
We also thank Dr. Le Bui Minh (NTT Hi-tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam) for the license to use BioRender to create the figures in this work. No external funding supported this work
AUTHOR CONTRIBUTIONS
DTC conceptualized this manuscript. DTC, NLB, YVNT, and SMVN wrote the manuscript. NLB, YVNT, and SMVN prepared the figures and tables. All authors contributed to the revision and approved the final manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]WHO. WHO Coronavirus (COVID-19) Dashboard. 2021 Dec 27, 2021 [cited 2021 Sept 23, 2021]; Available from: https://covid19.who.int/.
- [2]Carl Zimmer, JaS-LW. Coronavirus Vaccine Tracker. 2021 Sept. 22, 2021 [cited 2021 Sept. 23, 2021]; Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- [3]Mahamud, AGMSU, Kabir ME, Sohag AAM, Chen C, Hannan MA, Sikder MH et al., Food-derived bioactive peptides: a promising substitute to chemosynthetic drugs against the dysregulated renin-angiotensin system in covid-19 patients. Journal of Biologically Active Products from Nature, 2021. 11(4): p. 325-355.
- [4]Wang, X. and Y. Guan, COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays. Medicinal Research Reviews, 2021. 41(1): p. 5-28.
- [5]Sohag, AAM, Hannan MA, Rahman S, Hossain M, Hasan M, Khan MK, et al., Revisiting potential druggable targets against SARS-CoV-2 and repurposing therapeutics under preclinical study and clinical trials: A comprehensive review. Drug Development Research, 2020. 81(8): p. 919-941.
- [6]Mohamed K,Yazdanpanah N, Saghazadeh A, Rezaei N. Computational drug discovery and repurposing for the treatment of COVID-19: A systematic review. Bioorganic chemistry, 2021. 106: p. 104490-104490.
- [7]Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA, 2020. 323(18): p. 1824-1836.
- [8]NIH. COVID-29 Treatment Guidelines. 2021 Sept 15, 2021 [cited 2021 Sept 23, 2021]; Available from: https://www.covid19treatmentguidelines.nih.gov/.
- [9]FDA, Coronavirus Disease 2019 (COVID-19) EUA Information. 2021, FDA.
- [10]Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, et al., Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New England Journal of Medicine, 2021. 385(7): p. 585-594.
- [11]Katherine J, Wu, CZaJC. Coronavirus Drug and Treatment Tracker. 2021 Sept. 13, 2021 [cited 2021 Sept. 23, 2021]; Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-drugs-treatments.html.
- [12]Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M, et al., Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon, 2020. 6(12): p. e05798-e05798.
- [13]Farjana M, Moni A, Sohag AAM, Hasan A, Hannan MA, Hossain MG, et al., Repositioning Vitamin C as a Promising Option to Alleviate Complications associated with COVID-19. Infection & chemotherapy, 2020. 52(4): p. 461-477.
- [14]Islam MN, Hossain KS, Sarker PP, Ferdous J, Hannan MA, Rahman MM, et al., Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytotherapy Research, 2021. 35(3): p. 1329-1344.
- [15]Hannan MA, Islam MN, Uddin MJ, Self-confidence as an immune-modifying psychotherapeutic intervention for COVID-19 patients and understanding of its connection to CNS-endocrine-immune axis. 2020. 3: p. 14-17.
- [16]Henn W. Allocation criteria for an initial shortage of a future SARS-CoV-2 vaccine and necessary measures for global immunity. Vaccine, 2020. 38(34): p. 5396-5397.