HPTLC fingerprinting analysis of phytoconstituents from Bixa orellana and Beta vulgaris plant pigment
Abstract
Pigments are a type of coloring component which are utilized by humans to enhance colors in their lives. Using synthetic pigments for the purpose of coloring food, clothes, fruit juices, paints are accepted worldwide previously, but due to hazardous impact of synthetic colors on environment, and on human health made to go for alternative sources of the pigments which are safe to use. Isolation of natural pigment is another preference that will increase the supply of pigment from natural sources while minimizing environmental and health risks. Thus, there is a growing necessity for biocolor derived from natural sources that can substitute synthetic colors. Natural colorants are commonly found from plants, animals, and microorganisms. Plant pigments have several benefits, so it seems much of prominence for pigment production. Bixa orellana and Beta vulgaris were isolated for yellow, orange, and red color pigments from natural ecological source. Pigment extraction from plants requires extract preparation and then isolation of pigments using different solvents. Extracted pigments were analyzed by preliminary screening techniques such as phytochemical assay and various confirmation tests. We found positive results for flavonoid, tannin, carbohydrate, protein, saponin and alkaloid using phytochemical assays. HPTLC fingerprinting was done for each extract and found positive result for alkaloid and phenolic compounds. Thus, it was aimed to develop the extraction analytical methods for determination of Bixa Orellana and Beta Vulgaris spp. by HPTLC fingerprint approach.
INTRODUCTION
Plants play a significant part in the survival of life on Earth. Natural colorant production is increasing all over the world. Plant pigments are unique chemicals found in plants that absorb different wavelengths of light and give them a colorful appearance. It play an essential role in photosynthesis, plant growth and development [1]. Plant pigments are metabolic byproducts that give plants their characteristic colors referred to as biochromes [2]. Each pigment category is made up of a group of compounds with their own name, chemical structure, chemical properties, and color.
Bixa orellana L. (family Bixaceae) is a neotropical plant that is known in Mexico as achiote [3]. The predominant orange-red colored component of natural achiote pigments is bixin, which is known as annatto [4]. The aim of this research was to determine the bioactive elements of Bixa orellana through qualitative pharmacognostic and phytochemical analyses of the seeds, as well as to determine the plant part/organ with the highest concentration of phytoconstituents [5].
Plant-derived pigments such as betalains have become popular for use as natural colorants in the food industry [6]. Beta vulgaris contains a number of bioactive compounds that can reveal health-promoting effects, including betalains, ascorbic acid, flavonoids, polyphenols, saponins and nitrate [7]. Several in vitro investigations have revealed that betalain pigments protect cellular components from oxidative damage [8]. Extraction is required for the preparation of specific pigments as well as for gaining natural colorant extracts. Phytochemical screening revealed the presence of tannins, saponins, flavonoid, alkaloid, and phenolic component in plant. A comprehensive assortment of phytoconstituents in different extracts through HPTLC fingerprinting profiles displayed the existence of alkaloids, flavonoids and phenolics compounds.
MATERIALS AND METHODS
The plant material for the proposed study such as Bixa orellana was collected from serenity botanical garden and Beta vulgaris was collected from local market. Dr. Hitesh solanki, Professor at Department of Botany, Bioinformatics and climate change impact management, University school of sciences, Gujarat University, Ahmedabad, Gujarat has authenticated the plants gathered. The plant authentication number of Bixa orellana and Beta vulgaris were found GU/BOT/B/O4 and GU/BOT/A/V12 respectively.
Processing of the plant
Washing
To remove the clinging undesirable particles, the gathered healthy leaves were rinsed with water.
Drying of plant material
Because it lowers the moisture content of fresh materials, drying is a crucial step of dried material preparation for subsequent processing. However, drying conditions have been proven to have a considerable impact on sensory quality, bioactive component stability and activity. The plant material was dried in the shade for 7-15 days.
Grinding
Grinding to get a homogeneous sample and to increase the surface contact of the sample with the solvent solution.
Storage
Plant powders are stored at lower temperatures.
Physicochemical parameters
The numerous physicochemical characteristics are established in accordance with The Unani Pharmacopoeia of India. Odor, taste, color, moisture content, total ash value and extraction yield were all included.
Determination of moisture content
1.5 gm powdered leaves were measured into a weighted plane & slim Porcelain dish. It was dehydrated in the oven at temperatures ranging from 100o to 105oC. Cooling in desiccators and observing weight loss is commonly measured as ‘moisture’ [9].
Determination of total ash content
Weigh about 2 grams of the air-dried material in a formerly burned and tarred silica crucible. Spread the material out evenly and progressively raise the temperature to 500 – 600°C until it is white, showing the carbon absence.
Let the remains to cool for 30 minutes in a desiccator before weighing with no time interval. Using air-dried material standards, percentages of ‘total ash’ were calculated [10].
Total ash value of the sample =100(Z-x)/y %
X= ‘weight of empty dish’
Y= ‘weight of the drug taken’
Z= ‘weight of the dish + ash (after complete incineration)’
Extraction process
The dehydrated plant material of both plant species was grind using mortar and pestle to obtain fine powder and then it was passed through 1 mm sieve. To obtain crude extracts, 10 gram fine powders of both plant materials were soaked in 100 ml of various solvents such as methanol, acetone, chloroform, dichloromethane, ethanol and water separately for 5 to 7 days [11]. The plant material was filtered and the remaining solid was extracted to remove all the remaining liquid. The obtained liquid was purified by filtration. The solvent was extracted using rotatory vacuum evaporator under reduced pressure. The dried extracts were placed in an airtight container and kept at 4°C until further examination [12]. The yields of weighted extracts were kept in small bottles at refrigerator (4oC) (Figure 1). Yield percentages were calculated using the following formula:
Extract yield % = weight of dried extract /weight of dried leave × 100

Preliminary screening for extracted pigment by using chemical method
To examine the numerous chemical groups found in extracts, qualitative preliminary phytochemical experiments were performed (Table 1). Using recognized techniques, the presence of primary metabolites such as proteins, carbohydrates, fixed oils and fats was determined [13]. Secondary metabolites in Bixa orellana and Beta vulgaris leaf extracts included alkaloids, flavonoids, saponins, polyphenols, tannins, terpenoids and glycosides [14].
HPTLC fingerprinting analysis
HPTLC studies were carried out using the standard method described by Wagner et al. 10ul of sample were loaded in Silica gel TLC plate [15]. The samples loaded plate was kept in TLC twin trough developing chamber (after being saturated with solvent vapor) with respective mobile phases, namely toluene- acetone-formic acid (4.5 : 4.5 : 1) for flavonoids and Ethyl acetate-methanol-water(10:1.35:1) for alkaloid [16]. The plate was developed up to 90mm. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was kept in photo documentation chamber and the images were captured under visible light, UV 254 nm and UV 366 nm. The peak table, peak display and peak densitogram were noted [17]
Table 1. Qualitative phytochemical screening of selected plant extract.
RESULTS
Collection of plant material
The first phase in this research was to gather and treat plants such as Bixa orellana and Beta vulgaris. following that, several physicochemical features of plant powder should be noted. The following Table 2 shows the observed outcome:
Table 2. Moisture content and Ash value of Bixa orellana and Beta vulgaris.
Extraction of natural colorant
Table 3 displays the extraction yields as well as the physical properties of plant extracts. Bixa Orellana extraction yields ranged from 12.34 % to 17.8 % and Beta vulgaris extraction yields range from 22.78 % to 30.47 % in various solvent systems. The yields of extracts varied greatly depending on the extraction solvent and plant material utilized. Extraction yields achieved in Methanol, Acetone, Chloroform, Dichloromethane, Aqueous methanol, Aqueous ethanol, Aqueous acetone, and Methanol solvent systems of 17.81 %, 13.71 %, 12.34 %, 13.47 %, 30.47 %, 28.69 %, 27.71 % and 22.78 %, respectively.
The color of extract from the solvents Methanol, Acetone, Chloroform, Dichloromethane were discovered to be Orange and the sense of touch was found to be sticky. While the extract from Aqueous methanol, Aqueous ethanol, Aqueous acetone, and Methanol were intense red in color and sticky to the sense of touch.
Table 3. Physical characteristics and % yield of extract: Bixa orellana and Beta vulgaris.
Preliminary screening for extracted pigment by using chemical method
The phytochemicals found in plant samples are the focus of natural product biological activity. A small amount of the dry extract was used for qualitative phytochemical screening. The presence of significant phenolic compounds, saponins, tannin, Flavonoid and other substances has been identified in the plants. (Table 4).
Table 4. Preliminary tests for Bixa orellana and Beta vulgaris extract.
HPTLC fingerprinting of extracted pigments
Alkaloid profile
HPTLC fingerprint profile, chromatogram and densitogram for Alkaloid is presented in figure 2, 3, and 4, respectively. A variety of extracts like methanol extract, acetone extract, chloroform extract, dichloromethane extract, aqueous methanol extract, aqueous ethanol extract, aqueous acetone extract and methanol extract of Bixa orellana and Beta vulgaris were used for HPTLC Alkaloids profile that represented the presence of bands with Rf values ranged from 0.03 to 0.93, 0.09 to 0.92, 0.04 to 0.94, 0.03 to 0.75, 0.03 to 0.92, 0.09 to 0.91, 0.04 to 0.83, 0.09 to 0.90, respectively.
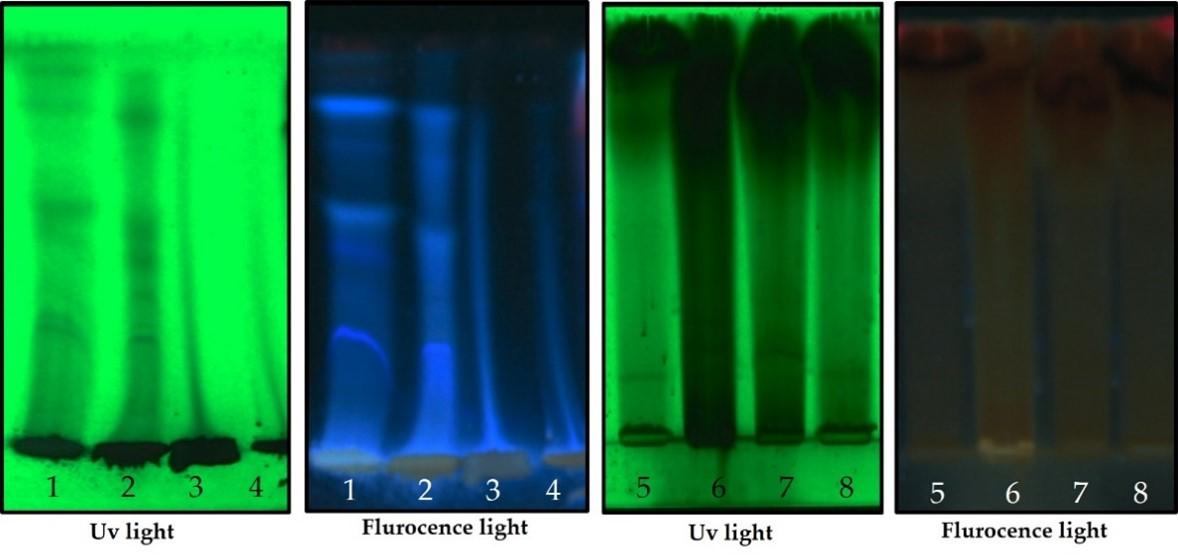
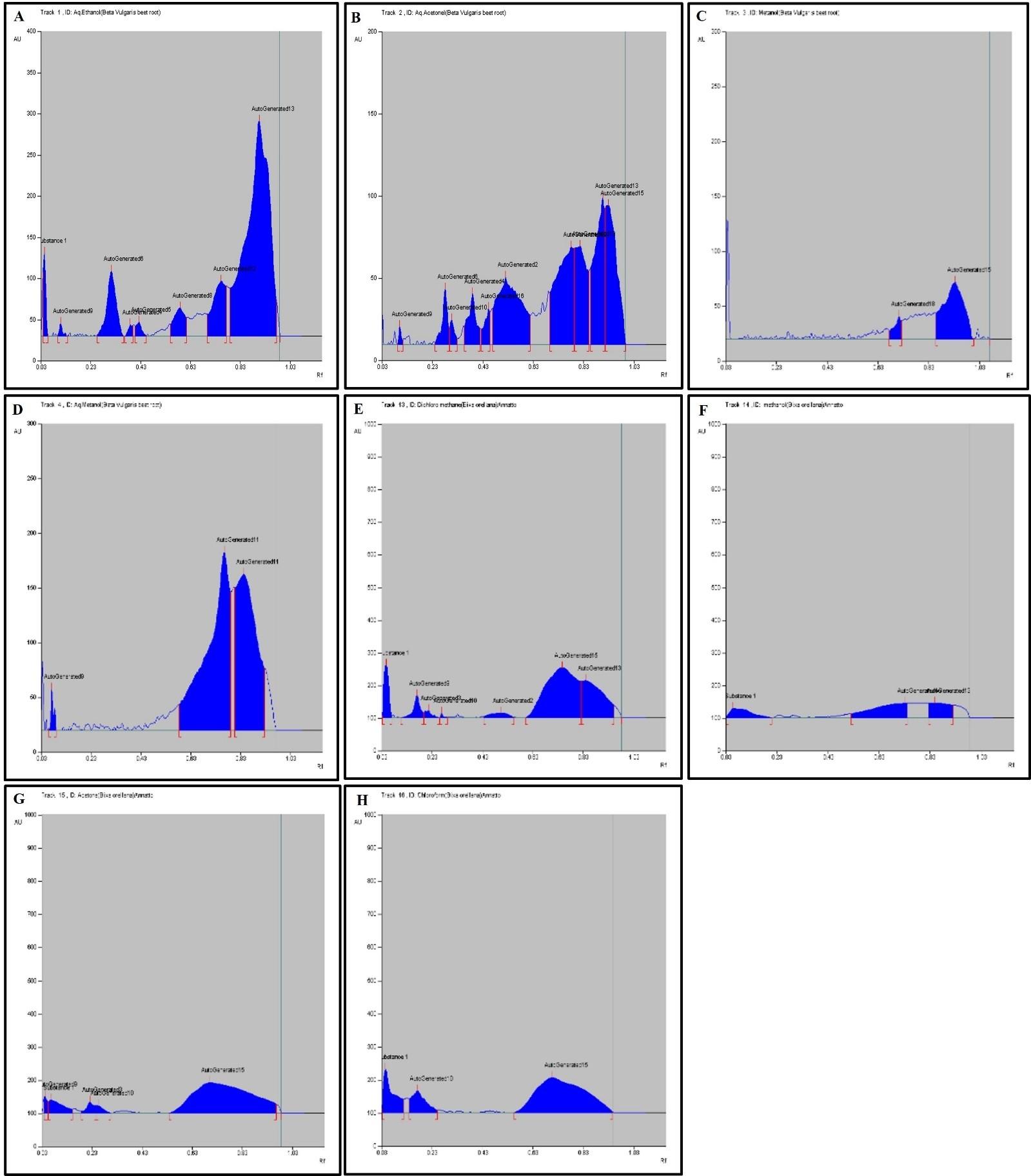
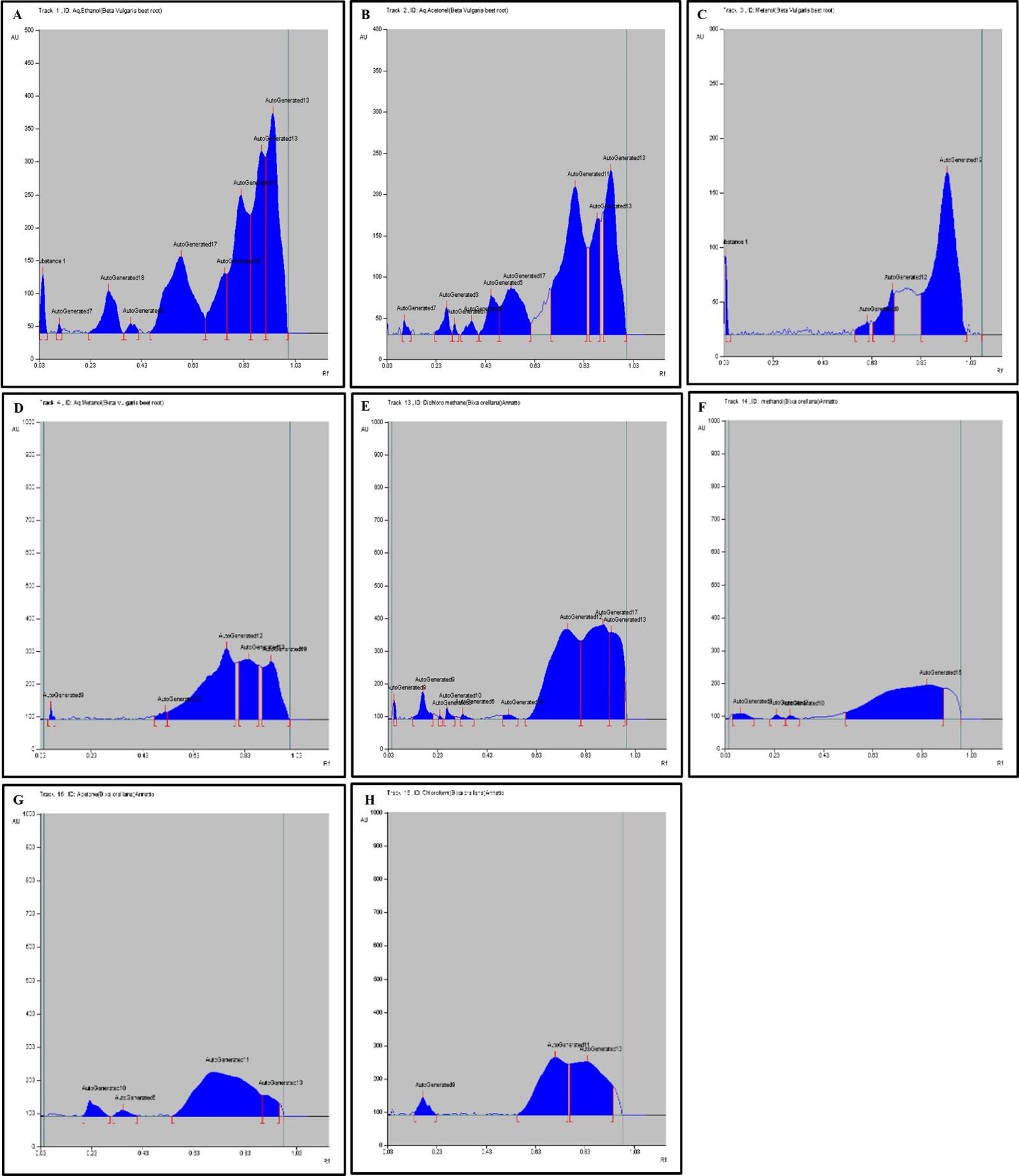
Table 5. Peak table with retention factor (Rf) values of alkaloid compounds of Bixa orellana extract.
Table 6. Peak table with retention factor (Rf) values of alkaloid compounds of Beta vulgaris extract.
Phenolic profile
HPTLC fingerprint profile, chromatogram and densitogram for Phenolic compounds is presented in figure 5, 6 and 7, respectively. A variety of extracts like Methanol extract, Acetone extract, Chloroform extract, Dichloromethane extract, Aqueous methanol extract, Aqueous ethanol extract, Aqueous acetone extract and Methanol extract of Bixa orellana and Beta vulgaris were used for HPTLC Phenolics profile that represented the presence of bands with Rf values ranged from 0.04 to 0.83, 0.05 to 0.43, 0.05 to 0.76, 0.05 to 0.72, 0.16 to 1.01, 0.13 to 1.04, 0.05 to 1.01 and 0.13 to 1.04, respectively.
Chromatograms of plant extract for phenolic profile (Beta vulgaris and Bixa orellana) in HPTLC analysis
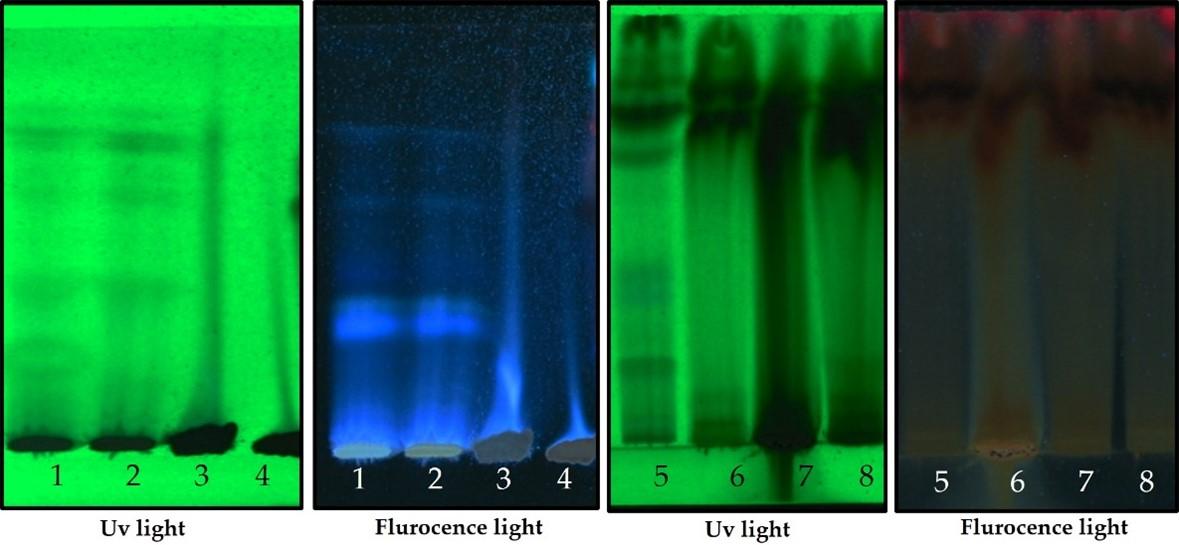
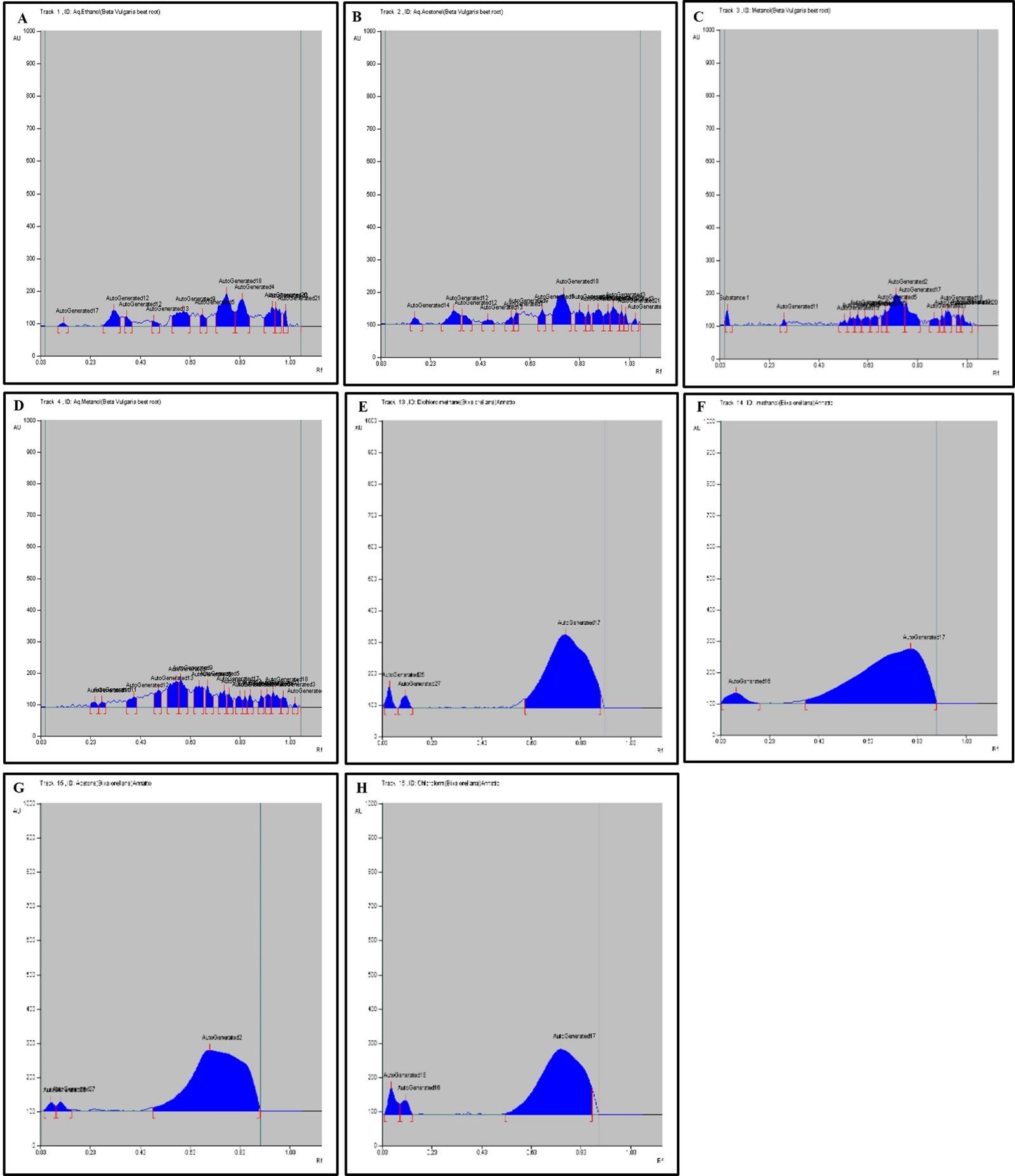
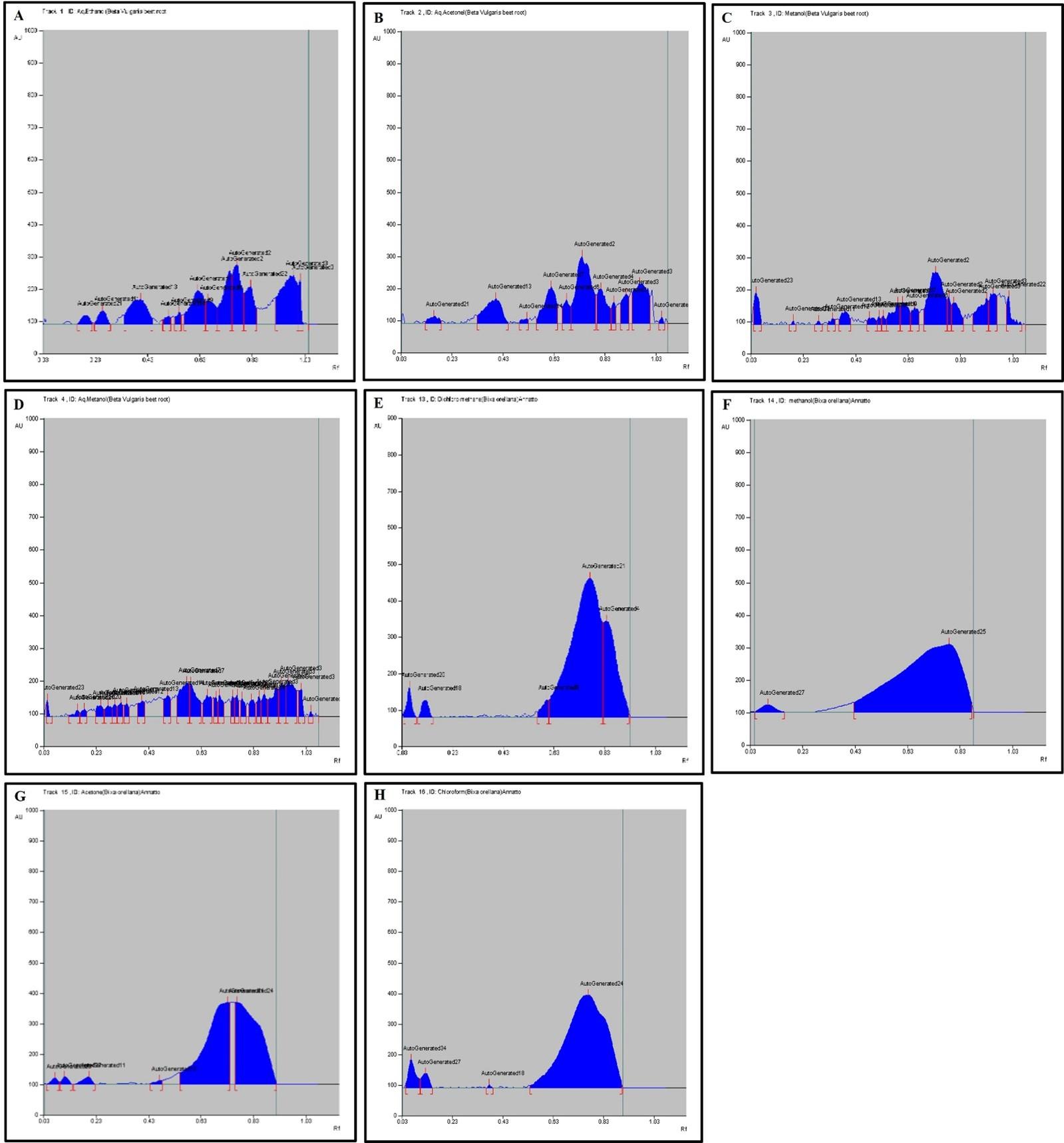
Table 7. Peak table with retention factor (Rf) values of phenolic compounds of Bixa orellana extract.
Table 8. Peak table with retention factor (Rf) values of phenolic compounds of Beta vulgaris extract.
DISCUSSION
The purpose of this study was to evaluate the HPTLC fingerprinting analysis of extracted pigments from plants for usage in a variety of industries. The extraction yield of chloroform extract of Bixa orellana was the lowest at 12.34 %. Methanol extract had the highest extraction yield of 17.81 %. The extraction yield of chloroform extract of Beta vulgaris was the lowest at 22.78 %. Aqueous methanol extract had the highest extraction yield of 30.47 %. Physicochemical properties of Plant extract such as colour and feeling of touch were observed.
The phytochemical screening of different extracts of plant samples of Bixa orellana and beta vulgaris revealed the presence of some secondary metabolites such as alkaloids, phenolics, flavonoids, steroids, and terpenoids.
HPTLC can be used as a phytochemical marker and is more effective in the field of plant taxonomy for secondary metabolite identification. Alkaloids are amino acids like lysine, ornithine, phenyl alanine, tyrosine, tryptophan, and histidine that are present in plants and responsible for detection and analytical analysis of phytoconstituents or pigments. The HPTLC results determined the presence of 16 different types of alkaloids bands and validated 11 different Rf values ranged from 0.03 to 0.93 (Table 5 and 6). The alkaloid band with 𝑅𝑓 value 0.22 confirmed the presence of Colchicine, 0.31 confirmed the Strychnine, 0.09 confirmed the nicotine and 0.41 confirmed the Chelidonine in the BVAE, BOD, BOM, BVAA extract, respectively.
The HPTLC results determined the presence of 16 different types of phenolics bands and validated 21 different Rf values ranged from 0.05 to 1.04 (Table 7 and 8). The phenolic band with 𝑅𝑓 value 0.75 confirmed the presence of quercetin in the BVAA, BVAM and BVAE extract.
CONCLUSION
Considering the need for alternative synthetic pigment in food products, pharmaceutical science, and cosmeceutical application, it was thought interesting to analyze the natural plant extracts. This survey suggests that all the extracts derived from the Beta vulgaris and Bixa orellana plants include numerous phytochemicals such as phenol, tannin, flavonoid, saponin and others. The more phytochemicals were identified in the methanolic extract due to higher polarity of the solvent. Natural pigments have a lower stability than synthetic colorants, which poses certain issues in terms of color loss during food processing, storage, and marketing. HPTLC fingerprinting confirmed the presence of some flavonoid and alkaloid compound in different extract of selected plants which might be responsible for stability properties for plant pigment.
ACKNOWLEDGEMENT
Authors are thankful for the contribution of colleagues and institution. Authors would like to acknowledge Ms. Kruti Dave for her continuous support.
AUTHOR CONTRIBUTIONS
PP; Conceived and designed the experiments. PP and MP; analyzed the data and drafted the manuscripts. PP and MP; reviewed and corrected the manuscripts. All authors read and approved the final version of the article.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Carvalho RF, Takaki M, Azevedo RA. Plant pigments: the many faces of light perception. Acta Physiologiae Plantarum. 2011; 33(2): 241-248.
- [2]Tanaka Y. Plant pigments for coloration: anthocyanins, betalains and carotenoids. Plant J. 2008; 54:733-749.
- [3]Carvalho JL, Moreira PA, Dequigiovanni G, Clement CR, Veasey EA. The domestication of Annatto (Bixa orellana) from Bixa urucurana in Amazonia. 2015; doi: 10.1007/s12231-015-9304-0.
- [4]De-Oliveira AC, Silva IB, Manhaes-Rocha DA, Paumgartten FJ. Induction of liver monooxygenases by annatto and bixin in female rats. Brazilian Journal of Medical and Biological Research. 2003 Jan;36(1):113-118.
- [5]Conrad OA, Dike IP, Agbara U. In vivo antioxidant assessment of two antimalarial plants–Allamamda cathartica and Bixa orellana. Asian Pacific journal of tropical biomedicine. 2013; 1;3(5):388-94.
- [6]Gengatharan A, Dykes GA, Choo WS. Betalains: Natural plant pigments with potential application in functional foods. LWT-Food Science and Technology. 2015 Dec 1;64(2):645-9.
- [7]Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015 Apr;7(4):2801-22.
- [8]Kanner J, Harel S, Granit R. Betalains a new class of dietary cationized antioxidants. Journal of Agricultural and Food chemistry. 2001 Nov 19;49(11):5178-5185.
- [9]Thiex N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. Journal of AOAC international. 2009 Jan 1;92(1):61-73.
- [10]Motegaonkar Manorama B, Salunke Shridar D. The ash and iron content of common vegetable grown in Latur District, India. Research Journal of Recent Sciences. 2012; 2277:2502.
- [11]Patel M, Dave K. and Patel P. “A review on different extraction method of plants : innovation from ancient to modern technology. International journal of biology, pharmacy and allied sciences. 2021; vol. 10(12): 511-527.
- [12]Wang L, Curtis L. Weller, Recent advances in extraction of nutraceuticals from plants. Trends in Food Science & Technology. 2006;17:300-312.
- [13]Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Ancient science of life. 2009; 29(2):12.
- [14]Poongothai A. Qualitative and quantitative phytochemical analysis of lantana camara leaf extract. 2019; 6(12): 603–607.
- [15]Dave K, Patel P, and Patel R. HPTLC Fingerprint Profile and Antifungal activity of selected botanical extract against Fusarium oxysporum for management of cumin wilt disease. 2021; 10: 54–68.
- [16]Cortés N, Mora C, Muñoz K, Díaz J, Serna R, Castro D, Osorio E. Microscopical descriptions and chemical analysis by HPTLC of Taraxacum officinale in comparison to Hypochaeris radicata: a solution for mis-identification. Revista Brasileira de Farmacognosia. 2014; 24:381-388.
- [17]Senguttuvan J, Subramaniam P. HPTLC Fingerprints of Various Secondary Metabolites in the Traditional Medicinal Herb Hypochaeris radicata L. Journal of Botany. 2016; 14.