Zoo-chemical profiling, in vivo toxicity and in vitro anti-inflammatory properties of Luffariella herdmani marine sponge extract
Abstract
The present study investigates the zoo-chemical profiling, anti-inflammatory, and radical scavenging activities of Luffariella herdmani marine sponge extract. The sponge crude extract (SCE) was prepared by methanol/dichloromethane extraction, followed by rotary evaporation. The percentage yield was calculated, and the zoo-chemicals were investigated by standard methods, while anti-inflammatory activity was tested by protein denaturation assay. Radical scavenging activity of the SCE was tested against 2, 2-diphenyl-1-picrylhydrazy (DPPH), nitric oxide (NO) and peroxide radicals, while in vivo toxicity was evaluated by the Artemia salina lethality assay. The results indicated the presence of terpenoids, alkaloids, anthraquinones, unsaturated sterols, sterols, and saponins in the SCE while flavanoids, quinones, tannins, and phenols were absent. In vitro anti-inflammatory activity against protein denaturation with IC50 of 58.54 µg/ml was evidenced while radical scavenging activity was not reported. The SCE was toxic to A. salina larvae with LC50 of 14.34 µg/ml. In conclusion, L. herdmani sponge extract possesses in vitro anti-inflammatory, in vivo toxic properties, yet radical scavenging activity was absent. The presence of terpenoids, alkaloids, anthraquinones, unsaturated sterols, sterols, and saponins with reported anti-inflammatory properties is suggestive of the use of L. herdmani sponge extract as an anti-inflammatory drug lead.
Keywords
INTRODUCTION
Marine drug discovery has gained much attention in the past few decades, resulting a vast array of bioactive compounds with therapeutic potential [1]. Sponges (Phylum Porifera) are particularly amongst the most abundant and ancient donors of novel natural products with unique biological properties, which can be used as drug candidates [2]. As a result, novel drug leads with antibacterial, antiviral, antifungal, antimalarial, antitumor, immunosuppressive properties, as well as cardiovascular and anticancer activities are reported from marine sponges [3].
The production of bioactive compounds by marine sponges is quite fascinating. Being the oldest metazoan, sponges are exclusively aquatic animals that dominate many benthic habitats [4]. Each individual contributes on average 3–5 chemical compounds, regardless of their order [5], as a result of their evolutionary adaptations to repel predators, parasites, microbial pathogens, biofouling, and overgrowth by other sessile organisms [5]. These compounds are toxic at very high concentrations, yet display numerous biological activities at low concentrations, enabling scientists to utilize them as potential drugs.
Sponges of the genus Luffariella are rich sources of bioactive metabolites [5], most commonly cytotoxic compounds. For example, luffariolides A-E and neomanoalides with potent cytotoxicity against murine lymphoma L1210 cells were reported from Luffariella sp. [6]. Further, L. variabilis has shown cytotoxicity against murine and human cell lines in various instances, as they contained manoalide, variabines A and furanosesterterpenoides [7, 8, 9, 10]. Some Luffariella sp. with luffariolides H and J are reported with cytotoxicity against murine lymphoma L1210 and Epidermoid carcinoma KB cells while indicating antimicrobial activity against Staphylococcus aureus, Bacillus subtilis and Mycrococcus luteus [11]. However, a large number of Luffariella sp. still remain understudied, creating a knowledge gap.
L. herdmani was originally reported from Sri Lankan waters in 1905 by Dendy [12], yet its distribution around the Sri Lankan coast was not clearly documented elsewhere. As a result of the long slumber of 115 years since its discovery, this species was not investigated for its taxonomy or related bioactive properties. However, this species was reported in two regions of the South Arabian coast during the 1933-1934 John Murray expedition [13] and recently from Kadmat Island, Agatti Island and Kavaratti Island in west coast of India [14].
Despite the availability of plethora of synthetic as well as natural drugs, inflammation still remains as a global health dilemma, resulting social and economic burden to many nations [15]. The inflammatory response is a defense mechanism that allows infections, damaged cells, toxic substances, irradiation, and other harmful conditions to be removed from the body while allowing injured cells to repair [16]. A large number of humoral and cellular mediators known as anti-inflammatory agents, in which if disrupted, leads to inflammatory diseases [17]. Non -steroidal anti-inflammatory drugs (NSAIDs), suppress the symptoms associated with the inflammation. However, most of these drugs are associated with adverse side effects such as gastric ulceration [15]. Other than NSAIDs, glucocorticoids too, are used in the treatment of inflammation [18] to inhibit leukocyte function. The importance of novel therapeutic entities which can successfully constrain inflammation by both these mechanisms is equally important. As such, the drug discovery with novel mechanisms for the successful management of inflammation is emphasized.
Despite the large number of sponges derived-bioactive compounds discovered to-date, only handful have been successfully developed into commercially viable drugs. One of the major reasons for this delay may be the ethical issues of using live animals in the early stages of drug discovery pipeline [19]. The use of higher animals such as rodents, as a model regardless of their innate tendencies, is now being highly criticized [19]. Most of the in vivo experiment procedures to evaluate anti-inflammatory activities of extracts; carrageenan induced paw edema, cotton pellet induced granuloma and leukocyte migration cause pain and discomfort to model organisms, thus affect the end result of the experiment. These are extremely time consuming and require technical skills such as anesthetics and surgical procedures [20]. Further, the repetition of the experiments is not possible in most of the circumstances. Therefore, alternative methods in drug screening are highly recommended. Alternative in vitro methods/models which are simple, low cost, repeatable, and easy to perform are being developed to be employed in the early phases of the drug development process.
Marine sponge extracts are usually endorsed with inherent cytotoxic properties. The evaluation of the toxicity prior assessing any other bioactivity is thus highly recommended. The lethality assay on Artemia salina is rapid and requires minimal resources, thus widely accepted as a convenient animal model to test toxicity [46].
Being one of the world’s richest biological hotspots, Sri Lanka abundantly harbours a plethora of diverse marine sponge fauna, yet scarcely explored with respect to their biodiversity, bioactivities and zoo-chemical constituents. A few bioactivities of Sri Lankan marine sponge extracts were reported such as immunomodulatory activity [1, 21] but a vast number is still remained overlooked creating research gap.
The order Dictyoceratida is considered as the highest producer of bioactive compounds among all Poriferan orders, which is responsible for more than 20% of all discovered bioactive compounds [5]. To date, no reports are available in Sri Lanka on Dictyoceratida sponges and their bioactive properties. We anticipate that the present study will serve as a model in the discovery for new anti-inflammatory drug lead from L. herdmani marine sponge species. The study will further provide some baseline data with respect to the L. herdmani marine sponge extract with radical scavenging activity and toxicity while identifying it zoo-chemical profile, which may be responsible for these bioactivities.
MATERIALS AND METHODS
Chemicals
Ferric chloride, sodium dihydrogen orthophosphate, potassium hydroxide, sodium chloride, sodium hydroxide, sodium monohydrogen orthophosphate, and sodium nitroprusside were obtained from Research-lab fine chem industries (Mumbai, India); sulfuric acid, absolute ethanol, hydrochloric acid, mercuric chloride, and glacial acetic acid were obtained from Breckland scientific suppliers (Norfolk, UK); and dichloromethane 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and Griess from Sigma Chemical Company Ltd. (Aldrich, USA). The standard anti-inflammatory drug, dichlofenac sodium (Voltaren® 50, Switzerland) and the standard drug, ascorbic acid for radical scavenging activities were obtained from Glorchem Enterprise, Colombo, Sri Lanka. The sponge crude extract (SCE) was obtained by vacuum rotary evaporation (BUCHI TYPE, India), and the absorbances were measured using a UV visible spectrophotometer (Jenway 6305, UK) and a micro plate reader (s/n MR05405, USA).
Collection, identification and preparation of the sponge crude extract (SCE)
The sponge material (500 g approximately) was collected from Unawatuna, Sri Lanka (6° 00ʹ 13.3ʺ N, 80° 14ʹ 46.9ʺ E), 1-1.5 km offshore via commercial scuba diving at a depth of 9-20 m (Department of Wildlife, Sri Lanka, permission number: WL/3/2/64/17). The specimen was carefully observed for external morphology, ectosome and choanosme structure, internal fiber arrangement and identified as L. herdmani [22, 23]. The identification was further confirmed by Dr. Marco Bertolino of Department of Earth, Environment and Life (DISTAV), University of Genova, Italy. A type specimen (Specimen No. 23/12/19/001) was deposited at the museum of the Department of Zoology, Faculty of Applied Sciences, and University of Sri Jayewardenepura. To prepare the SCE, sponge material was weighed, diced and incubated in a mixture of methanol/dichloromethane (1:1 v/v) for 72 hours. The resulted extract was filtered through Grade 1 filter paper, followed by rotary evaporation (BUCHI TYPE, India) at 40 °C [21]. Once the solvents are completely evaporated, the percentage yield was calculated, by dividing the weight of the SCE by the wet weight.
Qualitative zoo-chemical analysis
Exactly, 20 mg of SCE was dissolved in 10 ml of 5% (v/v) ethanol to obtain ethanolic SCE, which was used for zoo-chemical analysis [24].
Test for alkaloids
Approximately 1 ml of ethanolic SCE was mixed with a few drops of 50% HCl and a few milliliters of Mayer’s reagent and observed for the formation of a yellow-coloured precipitate.
Test for anthraquinones
Approximately 1 ml of ethanolic SCE was mixed with a few drops of 10% KOH (v/v). After agitation, the solution was observed for a red colour appearance.
Test for flavonoids
Approximately 2 ml of ethanolic SCE was mixed with 2 ml of Conc. HCl and a few small, cleaned magnesium stripes were added. The solution was observed for an orange to red colour appearance.
Test for quinones
For approximately 1 ml of ethanolic SCE, 10% (v/v) NaOH was added and observed for a yellow, red or purple colour appearance.
Test for saponins
Approximately 1 ml of ethanolic extract SCE was placed in a glass vial and shaken vigorously. The solution was observed for the formation and persistence of the froth for more than 10 minutes.
Test for sterols
Approximately 1 ml of acetic anhydride and 1 ml of Conc. H2SO4 were added into 1 ml of ethanolic SCE successively and observed for a colour change from red brown to purplish-brown.
Test for tannins
Approximately 1 ml of ethanolic SCE was mixed with a few drops of 1% (v/v) FeCl3 and observed for a blue black colour appearance which characterizes the presence of gallic tannins and a greenish brown colour appearance, which characterizes the presence of tannins.
Test for terpenoids
Approximately 1 ml of ethanolic SCE was mixed with 2 ml of Conc. H2SO4 and heated in a water bath at 40 0C for 2 minutes. The solution was observed for a brown to red colour appearance.
Test for unsaturated sterols
A few drops of Conc. H2SO4 were added drop wise along the wall into 1 ml of ethanolic SCE and observed for the formation of a red colour ring at the interface.
Test for phenols
Approximately 3 drops of 10% (v/v) FeCl3 were added to 3 ml of ethanolic SCE and observed for the appearance of a blue-violet or greenish colouration.
Evaluation of in vitro anti-inflammatory activity
Effect of SCE on inhibition of protein denaturation
The anti-inflammatory activity of SCE was evaluated by egg albumin denaturation assay, followed the previously described method with minor modifications [25]. Briefly, 5 ml reaction mixture containing 2 ml of different concentrations of SCE (100, 50, 25, 12.5, and 6.25 µg/ml) or standard drug diclofenac sodium (2500, 1250, 625, 312.5, and 156.25 µg/ml), 2.8 ml of phosphate buffered saline (PBS) (pH 6.4) and 0.2 ml of egg albumin (from fresh hen’s egg) was incubated at 27+2 °C for 15 minutes. Followed by incubation, the reaction mixture was further incubated at 70 °C in a water bath for 5 minutes to induce the protein denaturation.
After cooling the reaction mixtures to room temperature, the absorbance was measured at 660 nm, by UV visible spectrophotometer (Jenway 6305, UK) using PBS as a blank and 5% ethanol as the negative control. The test was triplicated and the percentage of inhibition of protein denaturation was calculated as follows.
Percentage inhibition of protein = [(Ac – As)/ Ac] ×100
Where Ac = absorbance of control, and As = absorbance of sample.
The IC50 values were calculated using the graph generated by Excel 2013.
Evaluation of radical scavenging activity
Effect of SCE on 2, 2-diphenyl-1-picryl-hydrazil (DPPH) scavenging activity
A reaction mixture of 100 µl of DPPH solution (125 µM in ethanol) with 100 µl of different concentrations of SCE (400, 200, 100, 50, 25, 12.5 and 6.25 µg/ml) or standard ascorbic acid (500, 250, 125, 62.5 and 31.25 µg/ml) was incubated for 30 minutes at room temperature and the absorbance was measured at 517 nm using a microplate reader (s/n MR05405, USA). The test was triplicated, and the percentage inhibition was calculated as below [26].
Percentage inhibition of DPPH = [(Ac – As)/ Ac] ×100
Where Ac = absorbance of control, and As = absorbance of sample.
The IC50 values were calculated using the graph generated by Excel 2013.
Effect of SCE on Nitric oxide (NO) scavenging activity
Accurately, 30 µl of a serial diluted SCE (400, 200, 100, 50, 25, 12.5 and 6.25 µg/ml) or Ascorbic acid (500, 250, 125, 62.5 and 31.25 µg/ml) and 30 µl of 10 mM sodium nitroprusside in PBS (pH 7.4) were added into a 96 well plate. The plate was incubated at room temperature for 150 minutes. After incubation, an equal volume of Griess reagent was added to each well in order to measure nitrite content.
After chromophore was formed at room temperature in 10 minutes, the absorbance at 546 nm was measured in a microplate reader. The test was triplicated, and the percentage inhibition was calculated as below [27].
Percentage inhibition of NO = [(Ac – As)/ Ac] ×100
Where Ac = absorbance of control, and As = absorbance of sample.
The IC50 values were calculated using the graph generated by Excel 2013.
Effect of SCE on hydrogen peroxide scavenging activity
Approximately, 150 µl of 4 mM hydrogen peroxide in PBS (pH 7.4) and 850 µl of various concentrations of SCE (400, 200, 100, 50, 25, 12.5 and 6.25 µg/ml) or ascorbic acid (500, 250, 125, 62.5 and 31.25 µg/ml) were mixed, followed by 10 minutes incubation period. The absorbance was measured at 230 nm. 5% ethanol was taken as the negative control. The percentage inhibition was calculated as below [28].
Percentage inhibition of hydrogen peroxide = [(Ac – As)/ Ac] ×100
Where Ac = absorbance of control, and As = absorbance of sample.
The IC50 values were calculated using the graph generated by Excel 2013. The test was triplicated.
Assessment of in vivo toxicity
Effect of SCE on Artemia salina nauplii lethality
A. salina cysts were allowed to hatch in sterile artificial seawater (after incubation for 24 hours under illumination) at 27±2°C temperature. Ten nauplii were delivered to each 5 ml crude extract at different concentrations (25, 20, 15, 12.5, 6.25 µg/ml). The test was done in triplicates. The positive control was 10% (v/v) Dimethyl sulfoxide (DMSO) while the negative control was 5% (v/v) ethanol.
The number of the dead and alive nauplii was observed under a compound microscope after 24 hours. The percent mortality of the brine shrimp’s larvae in each SCE concentration and the LC50 value of SCE were calculated using the graph generated by Excel 2013 [29].
Percentage mortality of A. salina nauplii = (N0-N1/N0) ×100
Where, N0= the total number of nauplii taken; N1= the number of nauplii alive.
Statistical analysis
Results represented as the mean ± SEM (standard error mean). The intergroup comparison was performed using the Minitab (version 17.1.0) through one sample t-test. Pearson’s product – moment correlation was run to assess the nature and magnitude of the relationship between given variables. P-value (<0.05) was considered statistically significant. The LC50 and IC50 values of the crude extract and drug control were calculated from the dose response curves using Excel 2013.
RESULTS
Percentage yield and qualitative zoo-chemical analysis of SCE
The extraction resulted a yield of 4.10% of SCE. The qualitative zoo-chemical analysis revealed the presence of secondary metabolites; terpenoids, saponins, anthraquinones, sterols, unsaturated sterols, and alkaloids. However, flavonoids, quinones, tannins, and phenols were absent (Table 1).
Table 1. Results of qualitative zoo-chemical analysis of the SCE.
Effect of SCE on in vitro protein denaturation
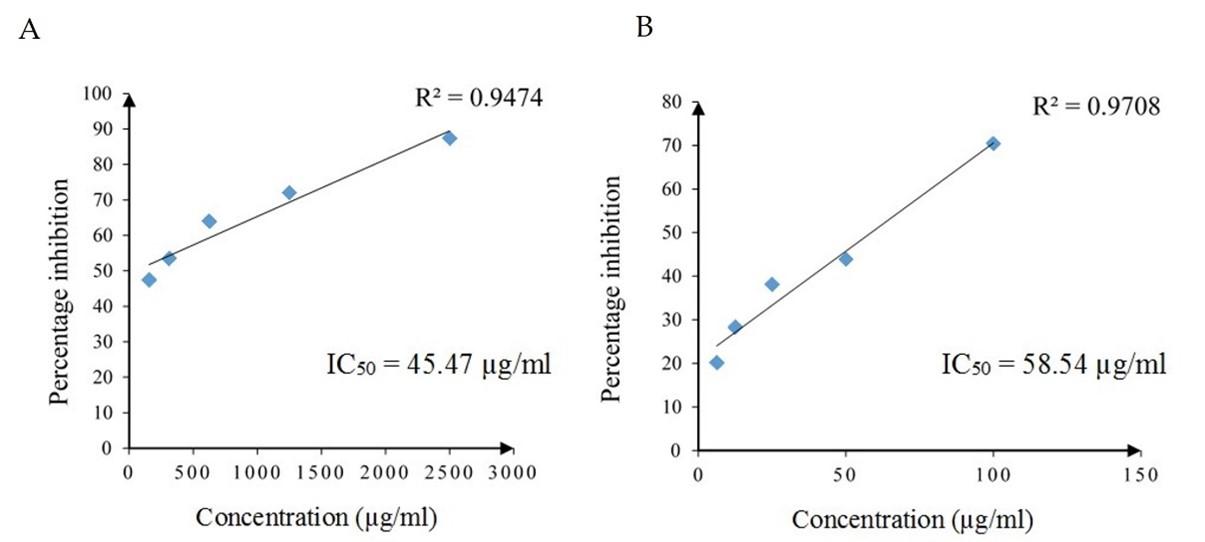
The inhibitory effect on thermally induced egg albumin denaturation by different concentrations of the standard drug diclofenac sodium and SCE are given in Figure 1. The inhibitory effect increased with increasing concentration in both diclofenac sodium and SCE and had strong positive linear relationships with concentration (Pearson, r = 0.973, p = 0.005 and r = 0.985, p = 0.002, respectively). The IC50 values of diclofenac sodium and SCE for inhibiting 50% of egg albumin denaturation were 45.47 µg/ml and 58.54 µg/ml, respectively. According to IC50 values, diclofenac sodium required lower concentration to inhibit 50% of egg albumin denaturation than SCE. Further, the SCE reported a strong percentage inhibition at 100 µg/ml (70.42%), which was lower than the percentage inhibition of the standard drug at 1250 µg/ml (71.98%).
Effect of SCE on DPPH radical scavenging activity
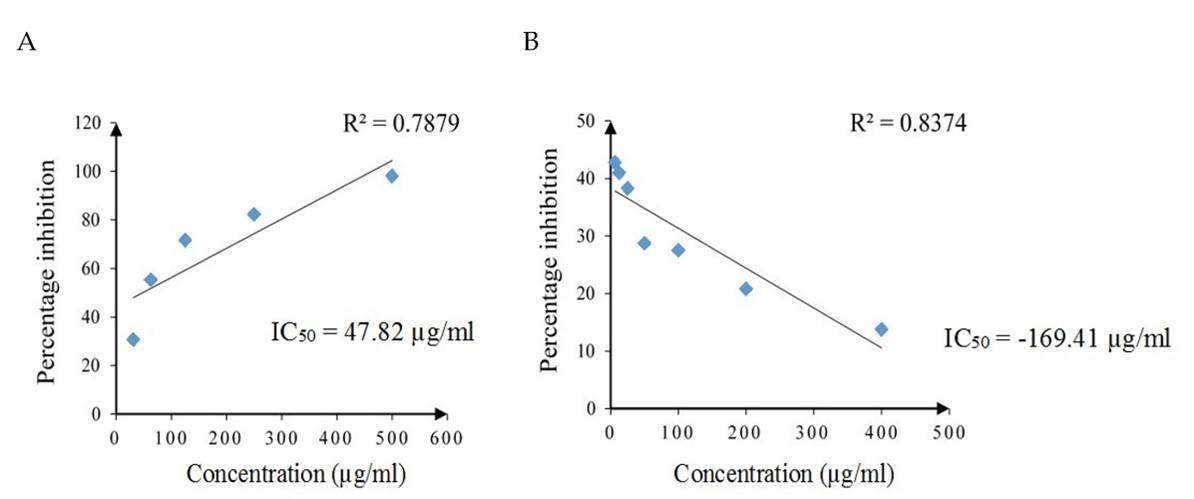
The radical scavenging activity of standard drug ascorbic acid and SCE against DPPH free radicals is given in Figure 2. The DPPH scavenging activity was increased with increasing concentration of ascorbic acid with a strong positive linear relationship (Pearson, r = 0.888, p = 0.044), yet decreased with increasing concentration of SCE which had a strong negative linear relationship (Pearson, r = -0.915, p = 0.004). The IC50 values of ascorbic acid and SCE were 47.82 µg/ml and -169.41 µg/ml, respectively.
Effect of SCE on NO radical scavenging activity
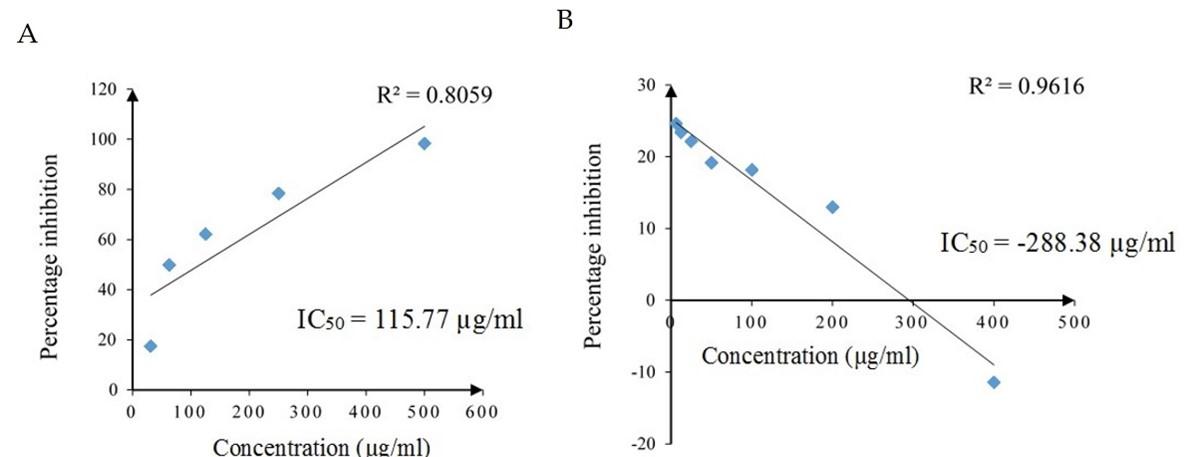
The NO scavenging activity of different concentrations of standard drug ascorbic acid and the SCE is shown in Figure 3. The NO scavenging activity of ascorbic acid was dose dependently increased with increasing concentration and had a strong positive linear relationship (Pearson, r = 0.898, p = 0.039). The NO scavenging activity of SCE decreased with increasing concentration and had a strong negative linear relationship (Pearson, r = -0.981, p = 0.000). The IC50 values of ascorbic acid and SCE were 115.77 µg/ml and -288.38 µg/ml, respectively.
Effect of SCE on Peroxide scavenging activity
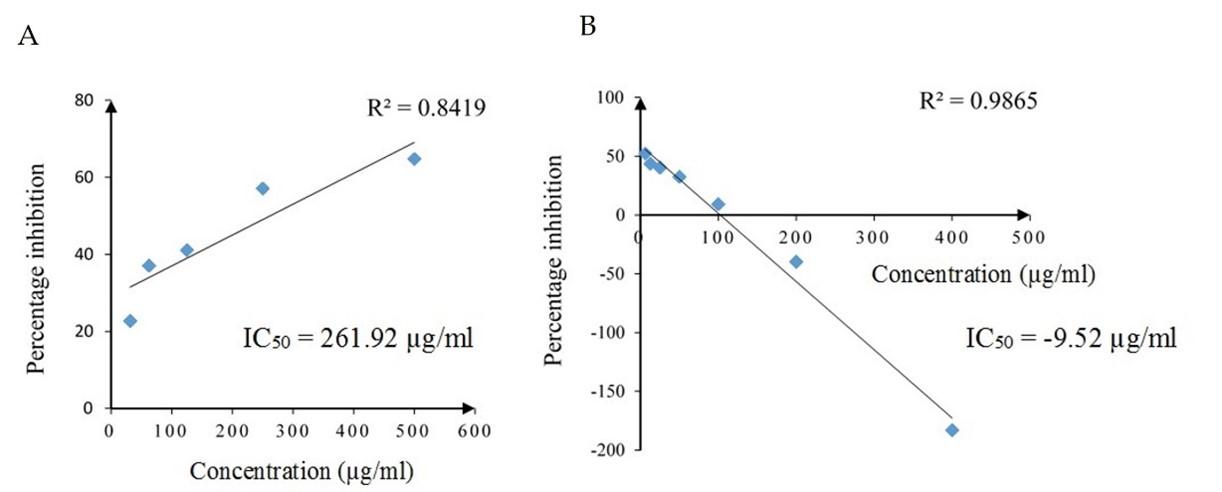
The hydrogen peroxide scavenging activity at various concentrations of standard drug ascorbic acid and SCE is given in Figure 4. The standard drug ascorbic acid showed increasing peroxide scavenging activity in a concentration-dependent manner and had a strong positive linear relationship (Pearson, r = 0.918, p = 0.028). The peroxide scavenging activity decreased with increasing concentration in SCE and had a strong negative linear relationship (Pearson, r = -0.993, p = 0.000). IC50 values of ascorbic acid and SCE were 261.92 µg/ml and -9.52 µg/ml, respectively.
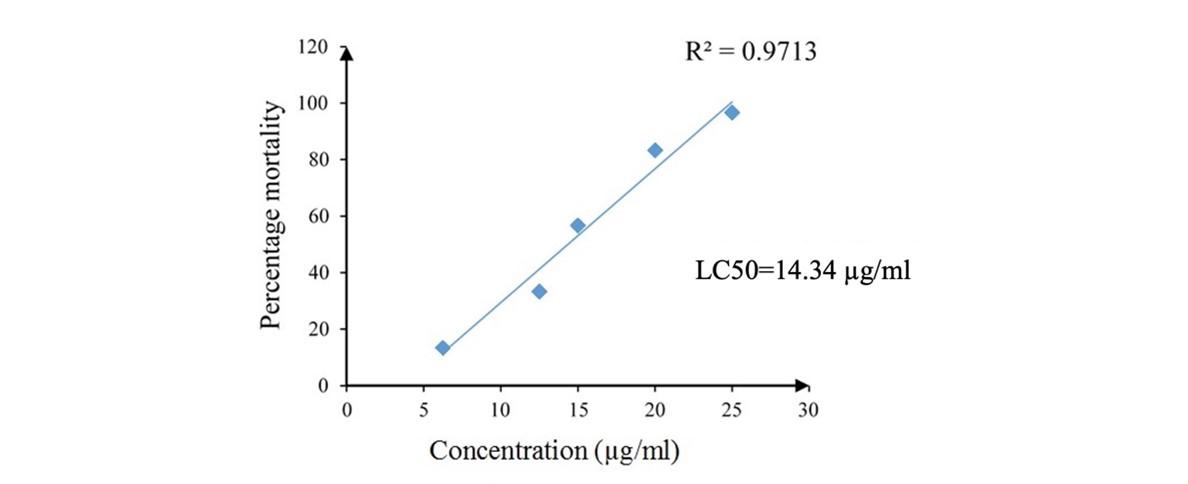
Effect of SCE on the mortality of Artemia salina larvae
Strong larvicidal activity was reported by the SCE with LC50 of 14.34 µg/ml (Figure 5). Hundred percent mortality was observed after 1 hour exposure of A. salina larvae to 25 µg/ml concentration of SCE while no mortalities in positive control. The number of mortalities was significantly higher compared to negative control except in 6.25 µg/ml concentration (p = 0.423) of SCE. The number of mortalities was significantly low in lower concentrations (20, 15, 12.5 and 6.25 µg/ml) in SCE compared to positive control (1-sample t test, p < 0.05). The percentage mortalities of A. salina larvae in SCE increased with increasing concentration and had a strong positive linear relationship (Pearson, r = 0.986, p = 0.002).
DISCUSSION
The attention to marine pharmacognosy has reached its spire during the past few decades, resulting in several hundred novel marine compounds in the clinical pipeline. Marine natural products have proven to be a rich source of bio-active compounds with therapeutic potential. In particular, marine sponges are considered the most important source of biologically active marine natural products that can be used for pharmacotherapeutic purposes [1]. Following the first compounds, Ara-C and Ara-A, isolated from the marine sponge Tethya crypta [30], various compounds have been isolated from marine sponges. As a result, wide array of therapeutic properties ranging from anti-infective activities such as antibacterial, antiviral, antifungal, antimalarial, anthelminthic to antitumor, anti-inflammatory, immunosuppressive activities are recorded from marine sponges worldwide. Due to the unique steriochemical structures of the carbon skeleton, these compounds are either toxic to pathogens or have the ability to interfere with the disease mechanism in the human body [31].
The genus Luffariella of order Dictyoceratida is well renowned for the abundance of bioactive terpenoids and terpenes, particularly sestertepenes [32], which are responsible for wide range of bioactivities including cytotoxicity (luffariellolides [33, 6], (4E, 6E)-dehydro-25-O-methylmanoalide [9]), antibacterial activities (manoalide, secomanoalide, (E)-neomanoalide, (Z)-neomanoalide, luffarins [33]) and anti-inflammatory activities such as PLA2 irreversible (manoalide [6, 34], luffariellin A [33]) and reversible (luffariellolides [33, 35], luffariellin B [33]) inhibitory activities. The dominancy of the terpenoids over other secondary metabolites is proven in Luffariella herdmani extract as well, highlighting that these metabolites may be responsible for the resulted bioactivities.
The odyssey of sponge biochemistry is strongly coiled with their long evolutionary history. The emission of mucus containing toxins is used as a defense mechanism against predators, pathogens and epibiomes [5, 36]. Thus, investigating for potential toxicity is considered as a first-line assessment to be performed on any natural extract, prior screening for its bioactivities. Artemia salina toxicity bioassay is a simple, inexpensive, reproducible, short-term method of screening large number of extracts such as plant crude extracts [37, 38] and is an indicator of potential antitumor, insecticidal, and fungicidal activity [39, 40, 41]. It has also been used to guide the isolation of bioactive compounds, testing of water quality, and detection of fungal toxins [42].
The mode of action causing toxicity is unknown, but the results typically correlate with more specific bioactivity tests. In the present study the Artemia salina toxicity assay was successfully applied to investigate the toxicity of the L. herdmani crude extract. Further, the reported toxicity (LC50 = 14. 34 µg/ml) is relatively high compared to the toxicities reported within the same Poriferan genus, L. cf. variabilis lipid extract (LC50 = 50 µg/ml) [43] and other genera, Dactylospongia elegans hexane extract, Haliclona sp. dichloromethane extract (20-30 µg/ml) [44], Xestospongia testudinaria chloroform extract (39.81 µg/ml) [45] etc.
The toxicity of natural extracts, especially plant extracts expressed as LC50 values is commonly validated either by comparison to Meyer’s or to Clarkson’s toxicity index [46]. According to Meyer’s toxicity index, extracts with LC50 < 1000 µg/ml are considered as toxic while Clarkson’s toxicity index classifies extracts with LC50 of 0 – 100 µg/ml as highly toxic [46]. According to both toxicity indices, the L. herdmani crude extract has shown potent toxicity. Further, the cell cytotoxicity indicates the ability of certain compounds or mediator cells to damage or destroy replicating live cells. Thus, the assessing of cell cytotoxicity is critical for the development of therapeutic anti-cancer drugs [47]. Further, the toxic compounds with IC50 value of ≤10 µM (or 4–5 µg/ml) is considered as possible anti-cancer drug candidates suggestive of developing SCE as an anticancer drug lead in future [47].
Inflammation is mainly resulted by tissue damage and leads to atherosclerosis, fever and heart problems [48]. Protein denaturation is occurred in the inflamed tissue, resulting pain and swelling. This is observed in most of the inflammatory diseases such as rheumatoid arthritis. During protein denaturation, the secondary and tertiary structures of proteins are broken down causing the loss of function [25]. During inflammation, the heat increment and the production of auto antigens cause the denaturation of tissue proteins [25]. The series of physiological events takes place in an inflamed tissue results adverse effects such as excessive accumulation of fluid within the cells, cellular rupture, plasma leakage and free radical damage [49]. The excess amount of free radicals that accumulated within the cells stimulate NF-κB pathways to release pro inflammatory cytokines, which considered as a major reason for inflammatory damage.
The NF-ҡB pathway is the major signaling pathway involved in regulation of genes that encode pro-inflammatory cytokines, chemokines, and inducible enzymes (e.g., COX2, iNOS). It also regulates cellular apoptosis [50]. The NF-κB family has five related transcription factors including p50, p52, RelA (p65), RelB, and c-Rel [51]. Upon stimulation by TNF, IL-1 etc., cytoplasmic NK-ҡB RelA–p50 heterodimers are released from IҡB and are transported to the nucleus, where they trigger selective gene expression [52]. This activation process is regulated by IκB kinase (IKK) [51].
Although an IC50 of 58.54 µg/ml was reported for protein denaturation assay during the present study, free radical scavenging effect was not reported by L. herdmani extract. Thus, the mechanism lying behind the anti-inflammatory activity, may be resulted by some other mechanisms, which has to be investigated by further inflammatory models. Anti-inflammatory activities of the genus Luffariella was well documented in previous studies as well. Both manoalide and seco-manoalide isolated from L. variabilis are irreversible inhibitors of the enzyme PLA2, which is a modulator of inflammatory process and manoalide is by far the best characterized PLA2 inhibitor from natural sources [36].
Free radical mediated cell damage can be seen in many different diseases and currently, a number of sponge crude extracts have shown radical scavenging properties [53]. Various types of radicals have been used in previous radical scavenging experiments, where DPPH, NO and peroxide radicals being the most common.
Many extracts which proven for potent scavenging activities is due to the presence of phenolic compounds such as phenolic acids, flavonoids, tannins etc. [54, 55]. The antioxidant activities of phenolic compounds is mainly attributed to their redox properties, which allow them to act as reducing agents, hydrogen or electron donors. The absence of phenolic compounds in the SCE may be the reason the negative results reported for of antioxidant properties.
Although the synergistic effect of the zoo-chemical constituents in the SCE cannot be disregarded, bioactivity guided fractionation of the SCE too, is equally important in drug discovery. Therefore, a comprehensive chemical characterization aimed at structure elucidation, followed in vivo and ex vivo models are highly recommended.
CONCLUSION
The potential anti-inflammatory activity of L. herdmani were investigated for the first time in the present study. The presence of a considerable amount of terpenoids along with a moderate amount of saponins and trace amounts of anthraquinones, sterols and unsaturated sterols may be responsible for the resulted activity. These metabolites may individually or synergistically be attributed to the resulted anti-inflammatory activity. The potent cytotoxicity resulted by the SCE is suggestive to develop this important drug lead as an anticancer drug, followed by comprehensive experiments. Future studies are underway to investigate the anti-inflammatory activity using different in vitro /ex vivo models and bioactive guided fractionation, isolation, and characterization of active ingredients in the SCE.
ACKNOWLEDGEMENT
Authors would like to thank Dr. Marco Bertollino of the Department of Earth Environment and Life Sciences at the University of Genova in Italy, for the assistance provided in sponge identification.
AUTHOR CONTRIBUTIONS
VG and SK equally responsible for the research. VG formulated and guided the research while participated in the manuscript writing. SK conducted the laboratory work, interpretation of the results and statistical analysis. The final draft was thoroughly checked and revised by both authors. Authors read and agreed on the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Gunathilake V, Bertolino M, Bavestrello G, Udagama P. Immunomodulatory Activity of the Marine Sponge, Haliclona (Soestella) sp. (Haplosclerida: Chalinidae), from Sri Lanka in Wistar Albino Rats: Immunosuppression and Th1-Skewed Cytokine Response. J Immunol Res. 2020; 2020: 7281295. doi: 10.1155/2020/7281295.
- [2]Abdelmohsen UR, Cheng C, Viegelmann C, Zhang T, Grkovic T, Ahmed S et al. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar Drugs. 2014; 12(3): 1220-44. doi: 10.3390/md12031220.
- [3]Anjum K, Abbas SQ, Shah SA, Akhter N, Batool S, Hassan SS. Marine Sponges as a Drug Treasure. Biomol Ther (Seoul). 2016; 24(4): 347-362. doi: 10.4062/biomolther.2016.067.
- [4]Van Soest RW, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ et al. Global diversity of sponges (Porifera). PLoS One. 2012; 7(4): e35105. doi: 10.1371/journal.pone.0035105.
- [5]Mehbub MF, Lei J, Franco C, Zhang W. Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar Drugs. 2014; 12(8): 4539-4577. doi: 10.3390/md12084539.
- [6]suda M, Shigemori H, Ishibashi M, Sasaki T, Kobayashi J. Luffariolides AE, new cytotoxic sesterterpenes from the Okinawan marine sponge Luffariella sp. J. Org. Chem.. 1992; 57(12): 3503-7.
- [7]Skindersoe ME, Ettinger-Epstein P, Rasmussen TB, Bjarnsholt T, de Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol (NY). 2008; 10(1): 56-63. doi: 10.1007/s10126-007-9036-y.
- [8]Sakai E, Kato H, Rotinsulu H, Losung F, Mangindaan RE, de Voogd NJ, Yokosawa H, Tsukamoto S. Variabines A and B: new β-carboline alkaloids from the marine sponge Luffariella variabilis. J Nat Med. 2014; 68(1):215-9. doi: 10.1007/s11418-013-0778-8.
- [9]Hamada T, Harada D, Hirata M, Yamashita K, Palaniveloo K, Okamura H, Iwagawa T, Arima N, Iriguchi T, de Voogd NJ, Vairappan CS. Manoalide-related Sesterterpene from the Marine Sponge Luffariella variabilis. Nat Prod Commun. 2015; 10(6): 863-4.
- [10]Ahmadi P, Higashi M, Voogd NJ, Tanaka J. Two Furanosesterterpenoids from the Sponge Luffariella variabilis. Mar Drugs. 2017; 15(8): 249. doi: 10.3390/md15080249.
- [11]Tsuda M, Endo T, Mikami Y, Fromont J, Kobayashi J. Luffariolides H and J, new sesterterpenes from a marine sponge Luffariella species. J Nat Prod. 2002; 65(10):1507-8. doi: 10.1021/np0202071.
- [12]Dendy A, Herdman SW. Report on the sponges collected by Professor Herdman, at Ceylon, in 1902.London: Royal Society; 1905. 57–246. Report No.: XVIII.
- [13]Burton, M., Murray J. Scientific Reports. John Murray Expedition, 1933- 34. London: British Museum (Natural History); 1959. 151-281.
- [14]George AM, VAN Soest RWM, Sluka RD, Lazarus S. A checklist of marine sponges (Porifera) of peninsula India. Zootaxa. 2020; 4885(2): 277–300. doi: 10.11646/zootaxa.4885.2.10.
- [15]Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018; 9(1): 143-150. doi: 10.14336/AD.2017.0306.
- [16]Delves PJ, Martin SJ, Burton DR, Roitt, IM. Roitt’s essential immunology. John Wiley & Sons: UK, 2017.
- [17]Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010; 140(6): 935-950. doi: 10.1016/j.cell.2010.02.043.
- [18]Punchard NA, Whelan CJ, Adcock I. The Journal of Inflammation. J Inflamm (Lond). 2004; 1(1): 1. doi: 10.1186/1476-9255-1-1.
- [19]Sarveswaran R, Jayasuriya WJ, Suresh TS. In vitro assays to investigate the anti-inflammatory activity of herbal extracts a review. World J. Pharm. Res. 2017; 6(17): 131-141. doi: 10.20959/wjpr201717-10058.
- [20]Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ et al. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int J Mol Sci. 2019; 20(18): 4367. doi: 10.3390/ijms20184367.
- [21]De Silva DP, Bertollino M, Gunathilaka KV. Evaluation of in vitro anti-inflammatory activity of five selected marine sponges against denaturation of protein-a pilot study. Int. J. Curr. Res. 2018; 10(4): 68349-68353.
- [22]Hooper JN, Van Soest RW. Systema Porifera: A guide to the classification of sponges. Springer: Boston, MA, USA, 2002.
- [23]de Voogd, NJ, Alvarez B, Boury-Esnault N, Carballo JL, Cárdenas P, Díaz MC et al.World Porifera Database. Luffariella herdmani (Dendy, 1905) [Internet]. Ostend, Belgium: Flanders Marine Institute (VLIZ); 2005 [updated 2010 July 10; cited 2022 Jan 14] . Available from: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=165364.
- [24]Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1966; 55(3): 225-276. doi: 10.1002/jps.2600550302.
- [25]Alhakmani F, Khan SA, Ahmad A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac J Trop Biomed. 2014; 4(2): S656-S660. doi:10.12980/APJTB.4.2014APJTB-2014-0273.
- [26]Prastya ME, Astuti RI, Batubara I, Wahyudi AT. Antioxidant, antiglycation and in vivo antiaging effects of metabolite extracts from marine sponge-associated bacteria. Indian J Pharm Sci. 2019; 81(2): 344-53.
- [27]Kumar AN, Bevara GB, Laxmikoteswramma K, Malla RR. Antioxidant, cytoprotective and anti-inflammatory activities of stem bark extract of Semecarpus anacardium. Asian J Pharm Clin Res. 2013; 6(1): 213-219.
- [28]Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010; 3(1): 43-53. doi:10.1016/j.arabjc.2009.12.008.
- [29]arah QS, Anny FC, Mir M. Brine shrimp lethality assay. Bangladesh J Pharmacol. 2017; 12(2): 186-9. doi: 10.3329/bjp.v12i2.32796.
- [30]Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010; 8(10): 2619-2638. doi:10.3390/md8102619.
- [31]Sipkema D, Franssen MC, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Mar Biotechnol (NY). 2005;7(3):142-162. doi:10.1007/s10126-004-0405-5.
- [32]Ettinger-Epstein P, Motti CA, Nys Rd, Wright AD, Battershill CN, Tapiolas DM. Acetylated sesterterpenes from the Great Barrier Reef sponge Luffariella variabilis. J Nat Prod. 2007; 70(4): 648-51. doi: 10.1021/np060240d.
- [33]Ebada SS, Lin W, Proksch P. Bioactive sesterterpenes and triterpenes from marine sponges: occurrence and pharmacological significance. Mar Drugs. 2010 Feb 23; 8(2): 313-46. doi: 10.3390/md8020313.
- [34]Zhou GX, Molinski TF. Manoalide derivatives from a sponge, Luffariella sp. J Asian Nat Prod Res. 2006; 8(1-2): 15-20. doi: 10.1080/10286020500246022.
- [35]Albizati KF, Holman T, Faulkner DJ, Glaser KB, Jacobs RS. Luffariellolide, an anti-inflammatory sesterterpene from the marine sponge Luffariella sp. Experientia. 1987; 43(8):949-50.
- [36]Perdicaris S, Vlachogianni T, Valavanidis A. Bioactive natural substances from marine sponges: new developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013; 1(3): 3-8. doi: 10.4172/2329-6836.1000115.
- [37]Waghulde S, Kale MK, Patil VR. Brine shrimp lethality assay of the aqueous and ethanolic extracts of the selected species of medicinal plants. Proceedings. 2019; 41 (1): 47. doi:10.3390/ecsoc-23-06703.
- [38]Viol DI, Chagonda LS, Moyo SR, Mericli AH. Toxicity and antiviral activities of some medicinal plants used by traditional medical practitioners in Zimbabwe. American Journal of Plant Sciences. 2016; 7(11):1538-1544. doi: 10.4236/ajps.2016.711145.
- [39]Michael AS, Thompson CG, Abramovitz M. Artemia salina as a Test Organism for Bioassay. Science. 1956 Mar 16; 123(3194): 464. doi: 10.1126/science.123.3194.464.
- [40]Harwig J, Scott PM. Brine shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl Microbiol. 1971; 21(6):1011-6. doi: 10.1128/am.21.6.1011-1016.1971.
- [41]McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998; 32(2): 513-24.
- [42]Reddy S, Osborne WJ. Heavy metal determination and aquatic toxicity evaluation of textile dyes and effluents using Artemia salina. Biocatalysis and Agricultural Biotechnology. 2020; 25(2020): 101574. doi: 10.1016/j.bcab.2020.101574.
- [43]Gauvin A, Smadja J, Aknin M, Faure R, Gaydou EM. Isolation of bioactive 5α, 8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000; 78(7): 986-92.
- [44]Rivera AP, Uy MM. In vitro antioxidant and cytotoxic activities of some marine sponges collected off Misamis Oriental Coast, Philippines. E- J. Chem. 2012 Jan 1; 9(1): 354-358.
- [45]Swantara MD, Rita WS, Suartha N, Agustina KK. Anticancer activities of toxic isolate of Xestospongia testudinaria sponge. Vet World. 2019; 12(9): 1434-1440. doi: 10.14202/vetworld.2019.1434-1440.
- [46]Hamidi MR, Jovanova B, Panovska TK. Toxicоlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced pharm bull. 2014; 60(1): 9-18.
- [47]Mioso R, Marante FJ, Bezerra RS, Borges FV, Santos BV, Laguna IH. Cytotoxic Compounds Derived from Marine Sponges. A Review (2010-2012). Molecules. 2017; 22(2): 208. doi: 10.3390/molecules22020208.
- [48]Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int. J. Drug Dev. & Res. 2011; 3(3):189-196.
- [49]Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004; 142(2): 231-55. doi: 10.1038/sj.bjp.0705776.
- [50]Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002; 2(10): 787-95.
- [51]Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017; 9(6): 7204-7218. doi: 10.18632/oncotarget.23208.
- [52]Ahmed AU. An overview of inflammation: mechanism and consequences. Frontiers in Biology. 2011; Front. Biol. 6(4): 274-281. doi: 10.1007/s11515-011-1123-9.
- [53]Kumar AN, Bevara GB, Laxmikoteswramma K, Malla RR. Antioxidant, cytoprotective and anti-inflammatory activities of stem bark extract of Semecarpus anacardium. Asian J Pharm Clin Res. 2013; 6(1): 213-219.
- [54]Elisha IL, Dzoyem JP, McGaw LJ, Botha FS, Eloff JN. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement Altern Med. 2016; 16(1): 307. doi: 10.1186/s12906-016-1301-z.
- [55]Aktas N, Genc Y, Gozcelioglu B, Konuklugil B, Harput US. Radical scavenging effect of different marine sponges from Mediterranean coasts. Rec. Nat. Prod. 2013; 7(2): 96-104.