Quantitative analysis of the factors influencing IDA and TSH downregulation in correlation to the fluctuation of activated vitamin D3 in women
Abstract
Anemia and thyroid disorders are global health issues that affect all ages but are more apparent in women. In this case, some serological components responsible for iron deficiency anemia (IDA) and thyroid-stimulating hormone (TSH) downregulation in women have been found actively regulated through a complex vitamin D3 mediated mechanism. This research has been investigated the correlation between activated vitamin D3 and the serological components responsible for IDA and dysregulation of TSH in childbearing and non-child-bearing women of different health conditions. Experimental sampling from 482 women suffering from both IDA and TSH dysregulation was taken, aged between 0 and 70 years. Serological parameters, such as iron, total iron-binding capacity, and ferritin, were assessed for IDA profiling, whereas thyroid-stimulating hormone and free thyroxin were for TSH profiling based on the individual’s serum vitamin D3 concentration. The resulting serological data were interpreted using sophisticated computer programming language and algorithms for quantitative biochemical analysis. The study resulted in a significant correlation between FT4 and vitamin D3 (p < 0.0001) for all age groups. TSH also showed strong interactions with the fluctuation of vitamin D3 levels (p < 0.0001), except for the children aged below 10 years (p < 0.063). The iron, TIBC, TSH, and FT4 showed phenomenal regulation with the steroidal-vitamin D3 concentration for congenital patients. Unlike the others, ferritin has a substantial connection with activated Vitamin D3 (p < 0.0064) fluctuation in the serum. To ratify, the concentrations of TSH, FT4, iron, TIBC, and ferritin were found to be significantly interconnected in terms of serum vitamin D3 concentration in women suffering from IDA and TSH downregulation simultaneously. In addition, the BMI condition of the patients can be a major factor in terms of correlating vitamin D3 with the regulatory factors of IDA and thyroid TSH as resulted in this research. To understand the accuracy and efficacy of the serum vitamin D3 in IDA and TSH downregulation, some other inflammatory markers and parathyroid hormone analysis of many samples can be conducted in continuation of this study.
Keywords
INTRODUCTION
Anemia is a global public health concern nowadays that affects developing countries and developed countries with the major consequences of human health hazards. According to the previous data, it affects one-quarter of the global population, with pregnant women and young children having a higher prevalence rate than men [1]. Turning to the factors, iron deficiency is the major cause of poor nutrition, which correspondingly results in severe Anemia with the consequence of both mother and child’s death [2]. Iron is vital to all biological functions, including DNA synthesis, respiration, cell proliferation, energy production, and so on [3]. Over 2 billion people are affected by iron deficiency worldwide [4,5], and the ubiquity of anemia among pregnant women and young children due to iron depletion has been well documented [6,7]. Age is also significantly correlated with IDA in childbearing females [8]. Additionally, premenopausal women who stick to a restrictive diet and usually intake a little amount of iron are mostly at risk of iron deficiency since they also lost iron during their menstrual cycle. However, in 2002, WHO reported iron deficiency anemia (IDA) as one of the most important factors in the global burden of disease [9]. Therefore, frequent screening of IDA is very significant. Numerous iron indicators are used to screen for IDA, and a potential example is how serum ferritin can be used as a diagnostic tool in clinical practice [10]. A combination of two serum transferrin markers is used in detecting IDA in regular hemodialysis anemic patients [11].
Thyroid gland dysfunction is also one of the most prevalent endocrine disorders worldwide [12]. Globally about 1.6 billion people are at risk of developing abnormal thyroid concentration due to iodine deficiency [13]. There are two types of thyroid disorder means- hyperthyroidism and hypothyroidism. Iron status in humans is inextricably related to thyroid gland function. IDA deteriorates thyroid metabolism and retards the physical and mental development of both young and adult individuals. Based on several investigations, IDA is interconnected with hypothyroidism which significantly increases serum thyroid-stimulating hormone (TSH) levels and decreases serum iron, serum ferritin, free T4 (FT4), transferrin, RBC count, and so on [14-18]. Ferritin is a universal protein that acts as an iron carrier, and serum ferritin level is negatively correlated with serum TSH levels [19,20]. In addition to iron and ferritin, values of total iron-binding capacity (TIBC), FT3, and FT4 have been reported as significantly lower in hypothyroid patients suffering from IDA [21,22]. This lower serum ferritin level is also associated with reducing sex hormones along with TSH, which exaggerates other endocrine dysfunctionalities [23]. The deficiency of iron can clinically produce hypothyroidism frequently in individuals [24]. Similarly, alterations in thyroid status change serum iron metabolism and hematological index phenomenally [16].
Vitamin D is a fat-soluble steroid hormone mainly produced in the skin when exposed to sunlight. Vitamin D may also be acquired from the ingested diet to a minor extent [25]. To explain further, vitamin D biosynthesis (25-hydroxyvitamin D) in the skin becomes initiated by UV rays of sun lights which convert 7-dehydrocholesterol to pre-vitamin D3; then, it is thermally isomerized to vitamin D3. However, vitamin D needs to be activated through a hydroxylation reaction mechanism, where a hydroxylase enzyme acts as the catalyst. In recent years vitamin D3 has taken close attention because its deficiency entails the risks of various human diseases such as gestation-associated disorders [26]. According to previous studies, insufficiency of vitamin D3 is prevalent in over one billion people globally [27,28]. The lower vitamin D3 levels are associated with higher serum TSH levels [25] and lower hemoglobin and ferritin levels [29]. Studies highly suggested the supplementation of vitamin D3 in case of hypothyroidism and Anemia, along with the high recommendation for the screening of vitamin D3 deficiency in all hypothyroid patients [25,30]. It is practically required for monitoring iron nutritional status as it exaggerates thyroid disorders in reproductive age and pregnant women [14]. Thyroid dysfunctions must also be considered by physicians treating anemia to ensure early detection and proper treatment [31]. Literature reveals that there is no study conducted yet on Bangladeshi patients suffering from iron deficiency anemia and thyroid hormone dysregulation, especially in women, where factors like lifestyle, sun exposure, dieting with proper vitamin D3 supplementation at both reproductive and nonreproductive conditions are also required to be studied.
Considering all the aforementioned facts, this current study aims to evaluate the impacts of vitamin D3 on the regulation of IDA and imbalanced TSH concentrations among the women of Bangladesh. In addition, a new dimension of studying the serological status of the anemic and thyroid patients depending on their vitamin D3 profiles can be achieved, through which it should be clinically suggestive as a serological parameter for assessing IDA, along with the ‘up and down-regulation’ of TSH.
MATERIALS AND METHODS
Clinical diagnosis of selective serological compounds
The current research started with diagnosing serum vitamin D3 levels [32] of the 482 women (452 are the test samples and 30 are the control samples) suffering from IDA and TSH simultaneously, where 56 of the diseased women have been taking medications (12.389%) for their thyroid issues to optimize their normal concentrations of TSH. To analyze the regulatory effects of vitamin D3 on the IDA and TSH disorders among the patients, several specific serological parameters were considered, such as serum iron (µg/dl), total iron-binding capacity (TIBC, µg/dl), and ferritin (ng/dl) for IDA confirmation. Whereas serum TSH (µIU/mL) and free thyroxine (FT4, ng/dl) were assessed to determine the TSH up and down regulations. The iron (µg/dl) and TIBC (µg/dl) levels of the patients’ serum were quantitatively analyzed using ‘Dimen-sion®IRON Flex® reagent cartridge (DF85)’ and ‘Dimension®Flex® Reagent IBCT’ (Sie-mens Healthcare Diagnostics Inc., USA) respectively, following the referred methodology [33,34]. The ferritin level (ng/dl) was tested quantitatively with ‘Beckman Coulter Access Ferritin Calibrators (S0-S5)’ considering its established protocol [34,35]. On the other hand, ‘ADVIA®Centaur™ TSH-3 Kit’ (Siemens, USA) and ‘ADVIA®Centaur™ FT4 Kit’ (Siemens, USA) were used to diagnose the concentrations of TSH (µIU/mL) and FT4 (ng/dl) respectively. Different patient groups were classified following their age, reproductive status, and past clinical history in all aspects. All those serum components were tested depending on their respective vitamin D3 concentrations to identify if there were any precise correlations with activated vitamin D3 or not. The kit used to detect vitamin D3 was ‘Human Vitamin D3 (VD3) ELISA kit; Cat No. MBS264661’. For transparent analysis, clinical data of the same serological tests and the vitamin D3 profiling of several normal women were also used as a control to compare with the IDA and thyroid patients. The BMI of all the control and clinical patients were taken were normal women considered below 25 (BMI<25). On the other hand, overweight and obese individuals were considered as 25<MBI<30 and 30<BMI<35 respectively. BMI of more than 35 was taken as morbidly obese [36]. Downregulation of TSH was considered depending on the increase of the patients’ BMI in this study.
The total research work was conducted under the Ethical Guidelines and Monitoring of Jashore Medical College (JMC), Bangladesh Medical and Dental Council (BMDC) in collaboration with the RPG Authority (Govt. Registration ID: 05-060-06021) under Project Category C2 (#Project EA No- 10/2021-2022). The ‘Ethical Clearance’ directly follows the Declaration of Helsinki and the Ministry of Health, Bangladesh. All necessary documents have been conserved by the corresponding author, which will be shared upon conditional requests. The authors are very cordial in publishing the manuscript and their consent is clear.
Post-diagnosis serological data analysis
To study the quantitative interactivity of the aforementioned serum components based on the individuals’ vitamin D3 concentration, different bioanalytical parameters were preferred, including- the two-way ANOVA test [37], Brown-Forsythe test [38,39], Bartlett’s test [40,41], and Tukey’s multiple comparisons test ‘p values’ as the majors [42-44]. In addition, least-square mean (LSM), mean difference (MD); standard error of the difference (SED); the difference between predicted means (DBPM), and 95% CI of difference (95% CID) were also analyzed. In this study, vitamin D3 was assigned to be considered as a significant serological analyzer only if its quantitative values belong to p < 0.05 in all aspects [45,46].
Software tools for the resulted data assessment
The biostatistical analysis and computational algorithms were performed using computational ‘R Programming Scripts’ (version R-4.0.2, for Linux) [47-49] and ‘GraphPad Prism’ (version 8.1.2, for Mac OS) [50-53] premium software packages.
RESULTS
In this research, the concentrations of TSH, FT4, iron, TIBC, and ferritin were found regulating in correlation to the vitamin D3 concentrations of the corresponding women suffering from IDA and TSH downregulation.
Quantitative analysis of the factors regulating TSH in response to Vit. D3
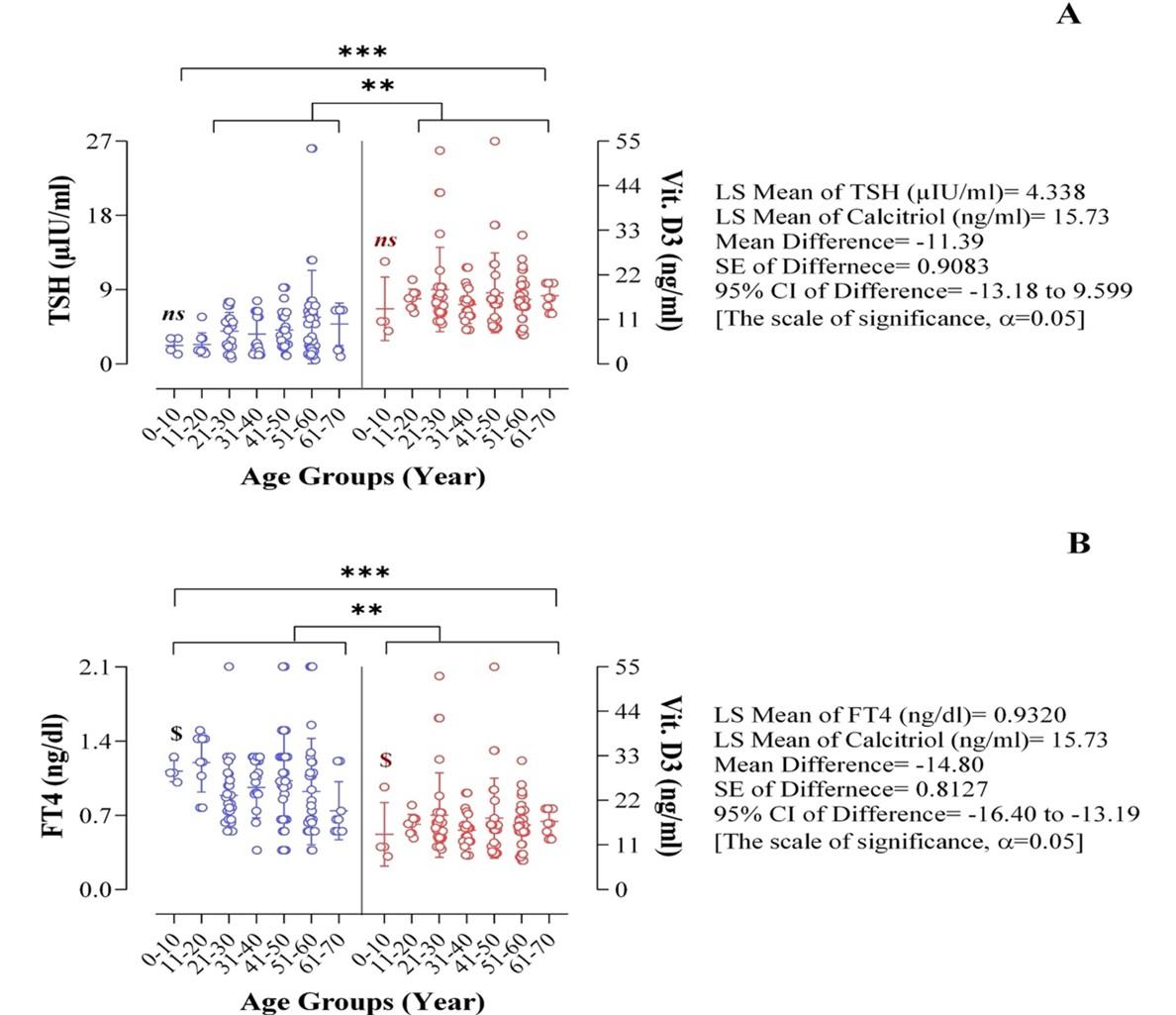
Individual patients belonging between 11 to 70 years possess a very strong correlation of both the TSH and FT4 level (p < 0.0001) with their respective vitamin D3 counts (in the scale of significance p < 0.05) (Figure 1). Though there is an insignificant relationship between the TSH and vitamin D3 (p < 0.063) among the patients ranging between 0 to 10 years (Figure 1A), the correlation between FT4 and vitamin D3 is highly significant (p < 0.0001) for the same age group (Figure 1B). The upper and lower values of TSH and FT4 are 3.1 (µIU/mL), 1.2 (µIU/mL), and 1.25 (ng/dl), 1.01 (ng/dl) respectively, for the women aged below 10 years. In both aspects, the activated vitamin D3 upper and lower values are- 25.3 (ng/mL) and 8.19 (ng/mL), respectively.
Quantitative assessment of the factors regulating IDA in response to Vit. D3
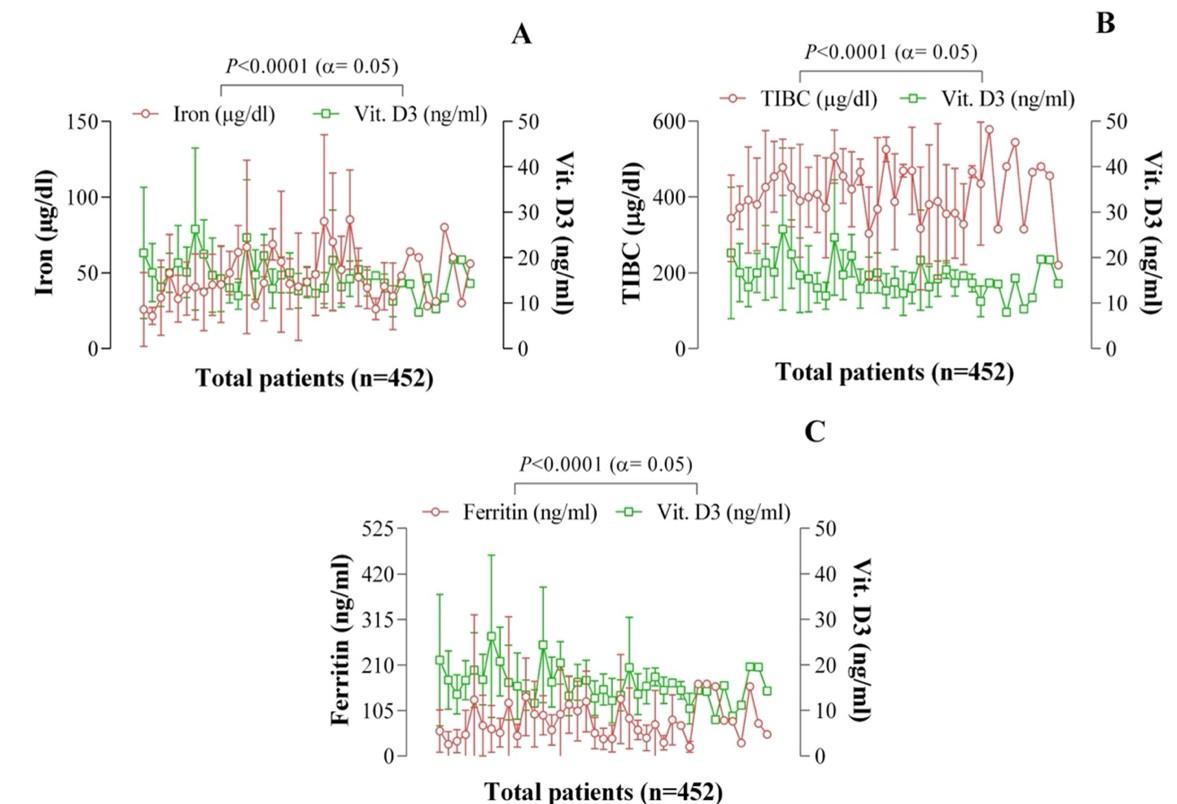
The serum iron, TIBC, and ferritin level fluctuate dramatically with the concentration of vitamin D3 present for all the selected patients from different age groups, resulting in the current serological quantitative analysis (Figure 2). The iron level increases (150µg/dl) and decreases (9µg/dl) with the ups and downs of vitamin D3 levels as 54.98 ng/mL and 8 ng/mL, respectively. The correlation between iron and vitamin D3 is found significant (p < 0.0001) among reproductive patients (Figure 2A). On the other hand, TIBC increased (580µg/dl) with the reduction of vitamin D3 (8ng/mL) and decreased (156µg/dl) with the progress of vitamin D3 concentration (52.7ng/mL), which means their serological profiles are reversible to each other (Figure 2B). Similarly, ferritin downregulates (5ng/mL) as the vitamin D3 level promotes (52.7ng/mL), resembling the findings of TIBC as well (Figure 2C) among reproductive women. In both the cases of TIBC and ferritin, the values are equally significant (p < 0.0001) when correlated with their vitamin D3 limits (Figure 2B and 2C). The correlation of iron, TIBC, and ferritin concerning the vitamin D3 level has also been found significant in nonproductive women (p < 0.0001 for each on the scale of significance p < 0.02), except for the women aged below fourteen years.
Impacts of Vit. D3 on the congenital patients of IDA and TSH downregulation
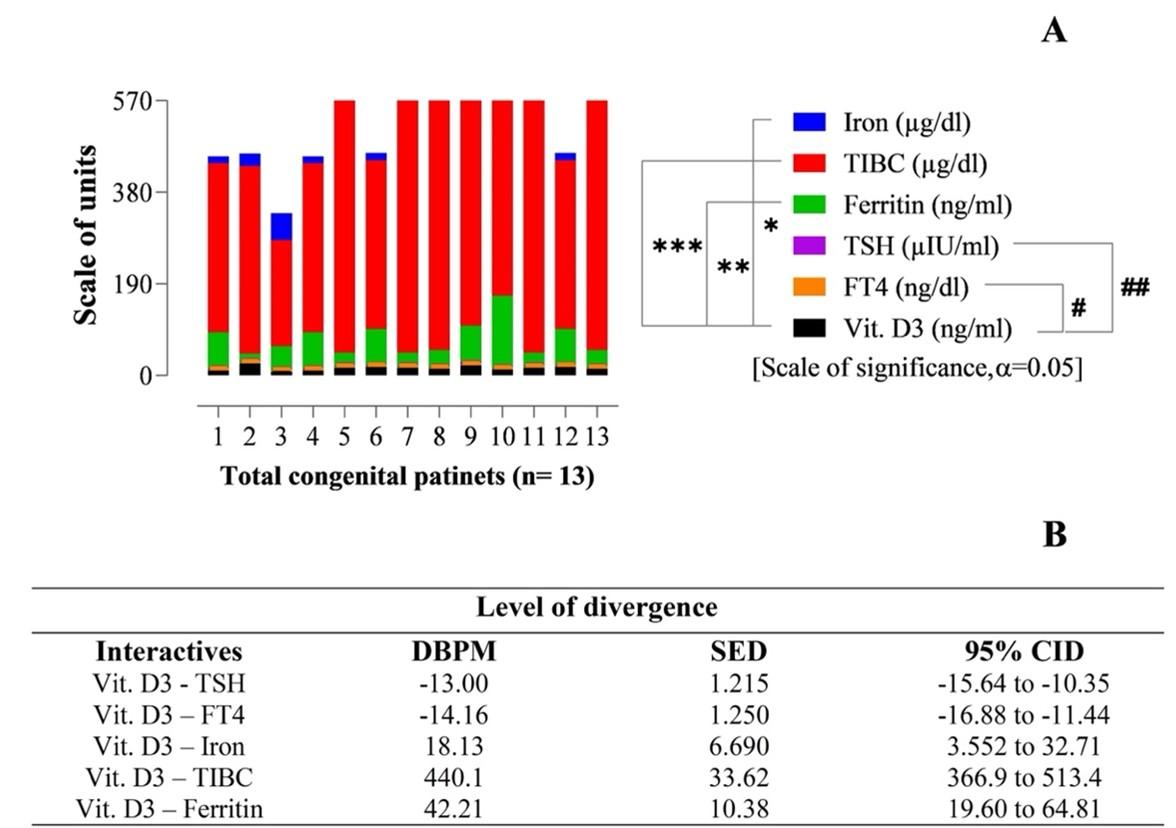
This study experienced 13 congenital cases of IDA and TSH disorders among all women. Surprisingly, according to their vitamin D3 profiles, all serological parameters have been found highly significant considering their two-way ANOVA and ‘Tukey’s multiple t-tests’ of variables (Figure 3). For the congenital patients, individual correlations of TIBC, TSH, and FT4 with their vitamin D3 concentration is exactly p < 0.0001 (Figure 3A). In contrast, ferritin shows a strong correlation but unlike the others with vitamin D3 concentration, means (p < 0.0064). The values obtained from the interactive components means iron, TIBC, ferritin, TSH, and FT4, with the vitamin D3, have been found very authentic considering their DBPM, SEM, and CID parameters, which established the findings as 95% confident (Figure 3B). It’s resulted that there are no null (‘0’) values in their 95% CID, which means the quantitative outputs are highly authentic and statistically significant. The ‘Z value’ for 95% confidence is 1.96 (Z = 1.96) as calculated from the statistical parameters (Figure 3).
Quantitative fluctuations of the patients’ IDA and TSH parameters as compared to the control group
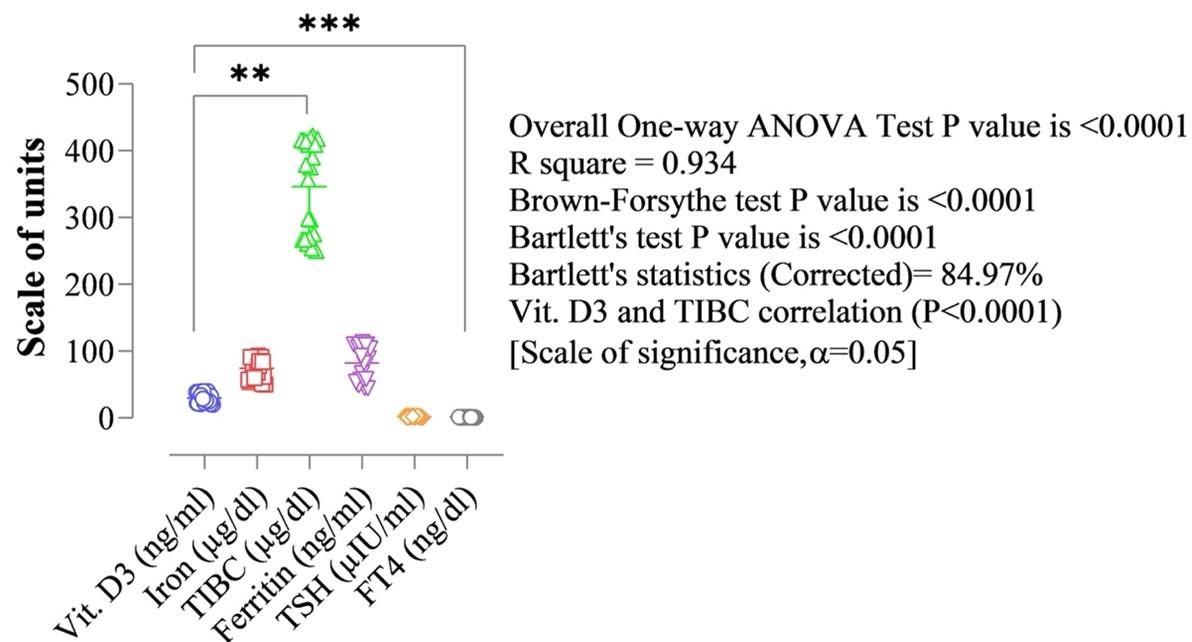
The overall results of each parameter were assessed based on the standard data generated by sampling the same serological parameters from thirty normal women as a control to compare their vitamin D3 profiles with the diseased women on that exact parameter. The vitamin D3 level of the normal women ranged between 20ng/mL to 40ng/mL. In response to which, their iron, TIBC, ferritin, TSH, and FT4 ranges were found as 50–93 µg/dl, 250–423 µg/dl, 45–113 ng/mL, 0.5–4.0 µIU/mL, and 0.70−1.67 ng/dl respectively (Figure 4). TSH levels of the participant normal pregnant women who were in the first trimester showed a significant positive correlation with BMI (r=0.253 and p=0.033) and those who were in the second trimester showed BMI (r=0.261 and p=0.029) of pregnancy in this research. The normal range of TSH levels in non-pregnant adult women was ranged between 0.8 and 5.0 mIU/L. Considering the TSH values, the BMI of the normal women was found below 25 (kg/m2) in this research meaning 21 to 24.6 (kg/m2). The clinically hypothyroid patients suffering from the IDA simultaneously ranged between 25.3 and 28.7 (kg/m2). Females aged less than 10 years, showed BMI showed 17 to 18.7 (kg/m2) suffering from hypothyroidism and IDA simultaneously. This is mainly observed among the congenital sufferers studied in this research. The normal females below 10 years showed 13.1 to 13.4 (kg/m2) of their BMI. Higher BMI levels were found to associate with lower concentrations of vitamin D3 in this research. Obesity was recognized strongly among the female patients who have been suffering from different stages of vitamin D3 deficiency in all the ages tested, which is another key finding of this research.
DISCUSSION
Vitamin D has long been recognized as an important hormone in regulating the musculoskeletal system as well as accounts for anemia risk and thyroid abnormalities. In the context of thyroid disorders, the antiproliferative and differentiating effects of the activated form of vitamin D are significant, and its function in modulating the immune system has been demonstrated in autoimmune thyroid disease (AITD) [54,55]. Several research studies have shown that vitamin D3 has vital role in maintaining bone health, immunity, and muscles. Many studies showed a link between vitamin D deficiency and thyroid downregulations [56]. IDA is a common problem and highly prevalent among Bangladeshi women, especially among pregnant women and females living in low iron water supplies [57]. Many IDA patients remain undiagnosed worldwide as the early stages show minor symptoms.
The function of several proteins, and metabolic activity, including imbalance of thyroid hormones, may occur due to iron deficiency. Changes in ferritin levels affect thyroid functions. Low levels of TSH and high levels of FT4 occur due to an imbalance in TSI (thyroid-stimulating immunoglobulin), leading to hyperthyroidism. FT4 does not bind to proteins, which is good for diagnosing thyroid problems [58].
The potential relationship of activated vitamin D3 with the regulation of TSH has been identified (Figure 1A). We have collected the TSH in µIU unit and vitamin D3 in ng/mL unit of serum concentration from 452 women blood who possessed the TSH and IDA disorders according to the age limit of 0 to 70. According to the current study, the age limit was conducted among the 10 years intervals for the higher frequency of random sample collection. After the statistical analysis of the relationship between TSH and vitamin D3, it showed (p < 0.063) among the patients ranged from 0 to 10 years. The values of the serological components for women over 10 years can act randomly because their hormonal and metabolic profile remained developmental like the neonatal, which can fluctuate insignificantly under any circumstances [59]. According to the result, the LS mean of TSH and vitamin D3 is 4.338 and 15.73, in the Mean Difference and SE Difference is −11.39 and 0.9083 (Figure 1A).
This study analyzed Free T4 (FT4) measurements that are not bound and can freely enter and affect the body tissues. FT4 normal values are 0.7 to 1.9ng/dL (Figure 4). Vitamin D3 can exert biological effects by binding with VDR (vitamin D receptor). The effects of vitamin D depend on VDR. Their polymorphic variants have been studied to a limited extent in the case of thyroid cancer [60]. In another study, there is an evaluation of the relationship between hypothyroidism and vitamin D, and their results indicated that patients with hypothyroidism suffered from hypovitaminosis D with hypocalcemia [30]. A potential relationship has been obtained between activated vitamin D3 and FT4 (Figure 1B) in this research. The statistical analysis significant relationship between vitamin D3 mediated FT4 regulation (p < 0.0001) among the patients ranged from 0 to 10 years. The upper and lower values of FT4 and vitamin D3 are 1.25 (ng/dl), 1.01 (ng/dl), and 25.3 (ng/mL), 8.19 (ng/mL), respectively; where the LS means of FT4 and vitamin D3 are 0.9320 and 15.73, in which the Mean Difference and SE of Difference are −14.80 and 0.8127. People who have hypothyroidism may not get enough vitamin D because of one of two possible reasons. Firstly, low vitamin D levels may be a result of inadequate vitamin D absorption from the gut. Secondly, the body may not adequately activate vitamin D [30]. The low value of vitamin D reduces the absorption efficiency of intestinal calcium, and the body responds by enhancing parathyroid hormone (PTH) release [61]. Increased blood PTH concentrations, particularly in the elderly, also occurred due to low calcium levels and an increased risk of fracture [62]. Furthermore, vitamin D therapy with calcidiol improves vitamin D while also dramatically lowering PTH levels, hence minimizing hyperparathyroidism [63]. The current investigation found vitamin D insufficiency in those aged 60 and more that may be linked to changes in mineral bone density and secondary hyperparathyroidism [64].
Iron-deficiency anemia is defined when blood levels of iron will be low, or less than 10 micromoles per liter (mmol/L) for both men and women (normally 10−30 mmol/L) (Figure 4). Iron deficiency anemia increases susceptibility to infectious disease, increased child mortality, slowed child development, and reduces scholastic performance [66]. There is an association between vitamin D deficiency with the regulation of a greater risk of anemia, lower mean hemoglobin (LMH), and higher usage of erythrocyte-stimulating agents [66]. The research results have been depicted a strong correlation between iron and vitamin D3 (p < 0.0001) among the patients (Figure 2A). The Vit. D3 level of the normal women ranged from 20ng/mL to 40ng/mL, and the iron level is 50−93 µg/dl (Figure 4). Here Iron level is increased (150µg/dl) and decreased (9µg/dl) with the increase and decrease of vitamin D3 levels as 54.98 ng/mL and 8 ng/mL, respectively. Total iron-binding capacity (TIBC) plays a pivotal role in indirectly measuring the percentage of transferrin situation involved in a positive correlation with vitamin D [67]. Transferrin and vitamin D levels decreased, whereas TIBC levels increased during iron-deficient anemic patients [68]. According to this study, the serum TIBC range for all the patients was between 220 µg/dL to 578 µg/dL with some fluctuations.
In contrast, the activated Vitamin D value was between 8 ng/mL and 26.29 ng/mL with a little bit of oscillation. In this research, it was found that serum TIBC result was increased. In contrast, serum vitamin D3 level was decreased in all the individual patients, consequently indicating iron deficiency anemia [69], as compared to the normal range (Figure 4). The ferritin level in the human body indicates the iron status and iron storage. Ferritin levels are lower in people who have iron-deficiency anemia, but they may be higher in people with inflammation and chronic disease-related Anemia [70]. However, in the present analysis, the range of the ferritin level was from 21 ng/mL to 166 ng/mL with some fluctuations, and the vitamin D3 level was between the ranges of 8 to 26.29 ng/mL respected to the normal control. It was noticed that serum vitamin D3 value was slightly higher, whereas serum ferritin values were significantly lower following the vitamin D3 scores for all the patients (Figure 2C). The current investigation found a positive association between serum vitamin D3 concentration and ferritin levels, consistent with previous findings [71,72].
It is mainly due to maternal risk factors such as inadequate dietary intake of vitamin D, insufficient exposure to sunlight, and pregnancy that occurs close to the people who suffer from congenital vitamin D deficiency [73]. Low maternal vitamin D levels may raise the risk of a newborn’s deficiency [74]. Besides, spontaneous hypothyroidism affects between 1% and 2% of the population and is more prevalent in older women [75]. Therefore, a poor pregnancy outcome is associated with vitamin D deficiency or insufficiency, which leads to several disorders such as low birth weight in newborns [76]. According to the current serological assessment, the value of vitamin D3 (8.19−25.3ng/mL) and iron (15−75 µg/dl) reduced significantly (Figures 3A and 3B) than the control group (20−40 ng/mL) and (50−93 µg/dl), respectively (Figure 4). Moreover, the FT4 level decreased slightly from the control 0.7−1.67 ng/dl to congenital 0.77−1.5 ng/dl. On the other hand, TIBC, Ferritin, and TSH indicate an upper range of 220−566 µg/dl, 17−150 ng/mL, and 1.2−5.7 µIU/mL than healthy people 250−423 µg/dl, 45–113 ng/mL, and, 0.5−4 µIU/mL, respectively. The results possessed a higher percentage of TIBC and a lower percentage of TSH. The TIBC, TSH, and FT4 have a significant relationship with their vitamin D3 concentration which is exactly p < 0.0001, whereas ferritin shows a strong correlation with vitamin D3 (p < 0.0064) (Figure 3A and 3B).
The research has described the values and regulatory relationships of the same serological parameters as iron, TIBC, ferritin, TSH, and FT4, with the serum vitamin D concentration of 30 normal women as a control group, where the range of vitamin D3 was found 20ng/mL – 40 ng/mL among the control group females, which was within the normal limit (Figure 4). On the other hand, the range of iron, TIBC, Ferritin, TSH, and FT4 were found 50−93mcg/dl, 250−423mcg/dl, 45−113n/mL, 0.5-4.0 mcgIU/ml, respectively, which were also within the normal ranges (Figure 4). The potential Vitamin D3 based regulation of the other serological parameters has been found in normal 30 women, used as the standard. Considering the biostatistical analysis, the overall one-way ANOVA test, Brown-Forsythe test, Barlett’s test, and the vitamin D3 correlation p-values were all exactly <0.0001 (in the scale of significance p < 0.05). The R square (R2) value was 0.9337, and Barrett’s statistics was 84.97%.
In clinical immunology, nutritional issues play a pivotal role in stimulating the secondary immune response when necessary. In the case of IDA, several nongenetic issues are involved including iron deficiencies as the major one. Application of proper food supplements especially the probiotic microorganisms and prebiotics, responsible for intestinal iron absorption can be very good preventives of IDA [77-80]. Passive immuniazation can imply a significant effect on the recovery of TSH downregulation among the patients [81], where the secondary immune response is activated due to systematic opsonization [82]. Besides, the research on the IDA and TSH downregulation among women is rapidly progressing over time globally with the radical advancements in the clinical molecular biology and medical biotechnology sectors.
CONCLUSION
It is reasonable to expect that significant vitamin D deficiency will occur in the majority of the women suffering from various forms of thyroid autoimmunity and iron deficiency anemia (IDA), based on our previous experience and the findings of our research study. The question remains on how to respond in such a circumstance. Based on the results of our serological research, it can be concluded that the concentrations of TSH, FT4, iron, TIBC, and ferritin were correlated with the levels of serum vitamin D3 in women suffering from TSH downregulation and IDA. Moreover, depending on a woman’s reproductive status, age, and BMI; vitamin D3 has can be suggested as a biomarker for tracking the status of IDA and TSH irregularities. However, more research with a larger sample size is required to understand better the serological profiles of patients with IDA and TSH disorders.
FUNDING
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A5A2019413).
ACKNOWLEDGMENTS
The authors are grateful to the RPG Interface Lab (Govt. License ID: 05-060-06021) authority for providing all types of technical support (under the Project of Category: C2; ID. #10-2021/22). At the same time, the authors are showing their gratitude to Prof. Dr. Md. Mohidur Rahman (Principal, JMC, Bangladesh), and Dr. Md. Azam Saklain (Associate Prof. and Head of the Pathology Department, JMC, Bangladesh) for arranging the ethical clearance certificate of this research unconditionally.
AUTHOR CONTRIBUTIONS
Conceptualization, Methodology, Supervision: Sharmin Ahmed; Project administration and Co-supervision: Salauddin Al Azad; Resources: Md. Abdul Rashid Mia, Data curation: Partha Biswas, Mithila Farjana; Writing original draft: All the authors participated equally; Visualization: Farzana Alam Arshe, Investigation: Sabrina Jahan Mily, Ananya Baidya Ankhi, Mahdi Mubin Shaikat; Validation and software: Salauddin Al Azad, Sabeeha Sultana, Kashfia Mawa, Zannatul Naim, Riazul Islam; Revision, and correspondence: Md. Ataur Rahman; Funding, and correspondence: Bonglee Kim.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.There is no conflict of interest among the authors.
References
- [1]Bieri JG. Second report of the ad hoc committee on standards for nutritional studies. The Journal of Nutrition. 1980;110(8):1726.
- [2]Macgregor MW. Maternal anaemia as a factor in prematurity and perinatal mortality. Scottish Medical Journal. 1963;8(4):134-40.
- [3]Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24-38.
- [4]McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public health nutrition. 2009;12(4):444-54.
- [5]Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR. A systematic analysis of global anemia burden from 1990 to 2010. Blood, The Journal of the American Society of Hematology. 2014;123(5):615-24.
- [6]da Costa AG, Vargas S, Clode N, Graça LM. Prevalence and risk factors for iron deficiency anemia and iron depletion during pregnancy: A prospective study. Acta medica portuguesa. 2016;29(9):514-8.
- [7]Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8(6):330.
- [8]Levi M, Simonetti M, Marconi E, Brignoli O, Cancian M, Masotti A, Pegoraro V, Heiman F, Cricelli C, Lapi F. Gender differences in determinants of iron-deficiency anemia: a population-based study conducted in four European countries. Annals of hematology. 2019;98(7):1573-82.
- [9]World Health Organization. The world health report 2002: reducing risks, promoting healthy life. World Health Organization; 2002.
- [10]Khan A, Khan WM, Ayub M, Humayun M, Haroon M. Ferritin is a marker of inflammation rather than iron deficiency in overweight and obese people. Journal of obesity. 2016;2016.
- [11]Suega K, Kandarini Y, Tubung J. Role of soluble transferrin receptor and transferrin receptor-ferritin index to detect iron deficiency anemia in regular hemodialysis patients. Open Access Macedonian Journal of Medical Sciences. 2019;7(1):97.
- [12]Unnikrishnan AG, Menon UV. Thyroid disorders in India: An epidemiological perspective. Indian journal of endocrinology and metabolism. 2011;15(Suppl2):S78.
- [13]Ansari MA. Thyroid disorders in Bangladesh-past, present, and future. Journal of Dhaka Medical College. 2014;23(2):151-2.
- [14]Luo J, Wang X, Yuan L, Guo L. Iron deficiency, a risk factor of thyroid disorders in reproductive-age and pregnant women: a systematic review and meta-analysis. Frontiers in endocrinology. 2021;12:93.
- [15]Refaat B. Prevalence and characteristics of anemia associated with thyroid disorders in non-pregnant Saudi women during the childbearing age: a cross-sectional study. Biomed J. 2015;38(4):307-16.
- [16]Rostaei Rad N, Vakili M, Zavar-reza J, Rezaie S, Shirvani AR. The relationship between thyroid hormone levels and body iron status in iranian hypothyroidism patients. International Journal of Medical Laboratory. 2016;3(3):176-84.
- [17]Sehat R, Shahabi Satlsar E, Hanachi P. Association between Iron, TIBC and Ferritin with Thyroid Hormones Levels in Patients Referred to Shouride Clinic Laboratory. Journal of Payavard Salamat. 2019;13(2):134-41.
- [18]Khatiwada S, Gelal B, Baral N, Lamsal M. Association between iron status and thyroid function in Nepalese children. Thyroid research. 2016;9(1):1-7.
- [19]Zilani M, Hasan N, Uddin SJ, Hossain H, Hazni H, Shilpi JA, et al. Chemical characterization and bioactivity of Trichosanthes dioica edible shoot extract. Oriental Pharmacy and Experimental Medicine. 2018;18(2):167-75.
- [20]He L, Shen C, Zhang Y, Chen Z, Ding H, Liu J, et al. Evaluation of serum ferritin and thyroid function in the second trimester of pregnancy. Endocrine Journal. 2018;65(1):75-82.
- [21]Shukla A, Agarwal S, Gupta A, Sarkar G. Relationship between body iron status and thyroid profile in an adult population: a hospital based study. Natl J Lab Med. 2017;2:1.
- [22]El-Masry H, Hamed AM, Hassan MH, FAyEd HM, Abdelzaher MH. Thyroid Function among Children with Iron Deficiency Anaemia: Pre and Post Iron Replacement Therapy. Journal of Clinical & Diagnostic Research. 2018;12(1).
- [23]Mostafa GG, Zahran FE, Omer SA, Ibrahim A, Elhakeem H. The effect of serum ferritin level on gonadal, prolactin, thyroid hormones, and thyroid stimulating hormone in adult males with sickle cell anemia. Journal of Blood Medicine. 2020;11:27.
- [24]Dahiya K, Verma M, Dhankhar R, Ghalaut VS, Ghalaut PS, Sachdeva A, Malik I, Kumar R. Thyroid profile and iron metabolism: mutual relationship in hypothyroidism. Biomedical Research. 2016;27(4):1212-5.
- [25]Al Azad S, Ahmed S, Biswas P, et al. Quantitative Analysis of the Steroidal Calcitriol-Mediated Regulation of the Serological Components Responsible for IDA and TSH Disorders in Reproductive and Nonproductive Women. Preprints.org. 2021; DOI: 10.20944/preprints202110.0007.v1.
- [26]Olmos-Ortiz A, Avila E, Durand-Carbajal M, Díaz L. Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients. 2015;7(1):443-80.
- [27]Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. Journal of pharmacology & pharmacotherapeutics. 2012;3(2):118.
- [28]Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition. 2008;87(4):1080S-6S.
- [29]Anwar E, Farhana N. Formulation and Evaluation of Phytosome-Loaded Maltodextrin-Gum Arabic Microsphere System for Delivery of Camellia sinensis Extract. Journal of Young Pharmacists. 2018;10.
- [30]Mackawy AM, Al-Ayed BM, Al-Rashidi BM. Vitamin D deficiency and its association with thyroid disease. International journal of health sciences. 2013;7(3):267.
- [31]Ashraf TS, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic anemia and thyroid function. Acta Bio Medica: Atenei Parmensis. 2017;88(1):119.
- [32]Garabédian M, Jacqz E, Guillozo H, Grimberg R, Guillot M, Gagnadoux MF, Broyer M, Lenoir G, Balsan S. Elevated plasma 1, 25-dihydroxyvitamin D concentrations in infants with hypercalcemia and an elfin facies. New England Journal of Medicine. 1985;312(15):948-52.
- [33]amanishi H, Iyama S, Yamaguchi Y, Kanakura Y, Iwatani Y. Modification of fully automated total iron-binding capacity (TIBC) assay in serum and comparison with dimension TIBC method. Clinical chemistry. 2002;48(9):1565-70.
- [34]Islam R, Akter KM, Rahman A, Khanam NN, Al Azad S, Islam MR, et al. The serological basis of the correlation between iron deficiency anemia and thyroid disorders in women: a community based study. J Pharm Res Int. 2021:69–81. https://doi.org/10.9734/jpri/2021/v33i19A31330.
- [35]Srinivasan B, Finkelstein JL, O’Dell D, Erickson D, Mehta S. Rapid diagnostics for point-of-care quantification of soluble transferrin receptor. EBioMedicine. 2019;42:504-10.
- [36]Milionis A, Milionis C. Correlation between body mass index and thyroid function in euthyroid individuals in Greece. International Scholarly Research Notices. 2013.
- [37]ZHAO H, WEN H, LIU Y, CAO L, DUAN Y, ZHENG X, HOU Z, LI X. The correlation between 25-hydroxy-vitamin D and serological indexes, immunological indexes in patients with rheumatoid arthritis. Chinese Journal of Rheumatology. 2019:95-101.
- [38]Erkan EP, Ströbel T, Dorfer C, Sonntagbauer M, Weinhäusel A, Saydam N, Saydam O. Circulating tumor biomarkers in meningiomas reveal a signature of equilibrium between tumor growth and immune modulation. Frontiers in oncology. 2019:1031.
- [39]Mantus G, Nyhoff LE, Kauffman RC, Edara VV, Lai L, Floyd K, Shi PY, Menachery VD, Edupuganti S, Scherer EM, Kay A. Evaluation of cellular and serological responses to acute SARS-CoV-2 infection demonstrates the functional importance of the receptor-binding domain. The Journal of Immunology. 2021;206(11):2605-13.
- [40]Komatsu N, Saijoh K, Kuk C, Liu AC, Khan S, Shirasaki F, Takehara K, Diamandis EP. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Experimental dermatology. 2007;16(6):513-9.
- [41]Robnett TJ, Machtay M, Hahn SM, Shrager JB, Friedberg JS, Kaiser LR. Pathological Response to Preoperative Chemoradiation Worsens with Anemia in Non—Small Cell Lung Cancer Patients. The Cancer Journal. 2002;8(3):263-7.
- [42]Alvarez-García G, García-Culebras A, Gutiérrez-Expósito D, Navarro-Lozano V, Pastor-Fernández I, Ortega-Mora LM. Serological diagnosis of bovine neosporosis: a comparative study of commercially available ELISA tests. Veterinary parasitology. 2013;198(1-2):85-95.
- [43]Arefin A, Ema TI, Islam T, Hossen MS, Islam T, Al Azad S, et al. Target specificity of selective bioactive compounds in blocking α-dystroglycan receptor to suppress Lassa virus infection: an in silico approach. Journal of Biomedical Research. 2021;35(6):459. https://doi.org/10.7555/JBR.35.20210111
- [44]Nipun TS, Ema TI, Mia MA, Hossen MS, Arshe FA, Ahmed SZ, et al. Active site-specific quantum tunneling of hACE2 receptor to assess its complexing poses with selective bioactive compounds in co-suppressing SARS-CoV-2 influx and subsequent cardiac injury. Journal of Advanced Veterinary and Animal Research. 2021;8(4):540-56. http://doi.org/10.5455/javar.2021.h544
- [45]Lüth S, Herkel J, Kanzler S, Frenzel C, Galle PR, Dienes HP, Schramm C, Lohse AW. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. Journal of clinical gastroenterology. 2008;42(8):926-30.
- [46]Vreugdenhil G, Baltus CA, Van Eijk HG, Swakk AJ. Anaemia of chronic disease: Diagnostic significance of erythrocyte and serological parameters in iron deficient rhuematiod arthritis pateints. Rheumatology. 1990;29(2):105-10.
- [47]Akter KM, Tushi T, Jahan Mily S, Mohona RA, Anis S, Chakraborty AK, et al. RT-PCR Mediated Identification of SARS-CoV-2 patients from particular regions of Bangladesh and the multi-factorial analysis considering their pre and post infection health conditions. Biotechnol J Int. 2020;24(6):43–56. https://doi.org/10.9734/bji/2020/v24i630121.
- [48]Dey D, Paul PK, Al Azad S, Al Mazid MF, Khan AM, Sharif MA, et al. Molecular optimization, docking, and dynamic simulation profiling of selective aromatic phytochemical ligands in blocking the SARS-CoV-2 S protein attachment to ACE2 receptor: an in silico approach of targeted drug designing. J Adv Vet Anim Res. 2021;8(1):24. https://doi.org/10.5455/javar.2021.h481.
- [49]Sharif MA, Hossen MS, Shaikat MM, Mashuk F, Haidary TI, Dey D, et al. Molecular optimization, docking and dynamic simulation study of selective natural aromatic components to block E2-CD81 complex formation in predating protease inhibitor resistant HCV influx. Int J Pharm Res. 2021;13(2) https://doi.org/10/318.38/ijpr/2021.13.02.408.
- [50]Al Azad S, Moazzem Hossain K, Rahman SM, Al Mazid MF, Barai P, Gazi MS. In ovo inoculation of duck embryos with different strains of Bacillus cereus to analyse their synergistic post-hatch anti-allergic potentialities. Veterinary medicine and science. 2020;6(4):992-9. doi: 10.1002/vms3.279
- [51]Rashaduzzaman M, Kamrujjaman M, Islam MA, Ahmed S, Al Azad S. An experimental analysis of different point specific musculoskeletal pain among selected adolescent-club cricketers in Dhaka city. Eur J Clin Exp Med. 2019;17(4):308–14. https://doi.org/10.15584/ejcem.2019.4.4.
- [52]Al Azad S, Farjana M, Mazumder B, Abdullah-Al-Mamun M, Haque AI. Molecular identification of a Bacillus cereus strain from Murrah buffalo milk showed in vitro bioremediation properties on selective heavy metals. Journal of advanced veterinary and animal research. 2020;7(1):62. doi: 10.5455/javar.2020.g394
- [53]Paul PK, Azad SA, Rahman MH, Farjana M, Uddin MR, Dey D, et al. Catabolic profiling ofselective enzymesin the saccharification of nonfood lignocellulose parts of biomassinto functional edible sugars and bioenergy: An in silico bioprospecting. J Adv Vet Anim Res. 2022; 9(1):19–32. http://doi.org/10.5455/javar.2022.i565
- [54]Kmieć P, Sworczak K. Vitamin D in thyroid disorders. Experimental and Clinical Endocrinology & Diabetes. 2015;123(07):386-93.
- [55]Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermato-endocrinology. 2013;5(1):51-108.
- [56]Mele C, Caputo M, Bisceglia A, Samà MT, Zavattaro M, Aimaretti G, Pagano L, Prodam F, Marzullo P. Immunomodulatory effects of vitamin D in thyroid diseases. Nutrients. 2020;12(5):1444.
- [57]Ahmed F, Khan MR, Shaheen N, Ahmed KM, Hasan A, Chowdhury IA, Chowdhury R. Anemia and iron deficiency in rural Bangladeshi pregnant women living in areas of high and low iron in groundwater. Nutrition. 2018;51:46-52.
- [58]Harjantini U, Dewi YL, Hanim D, Nurwati I. Correlation of dietary iron intake and serum iron with thyroid stimulating hormone (TSH) and free thyroxine (FT4) levels in adult hyperthyroid patients. Journal of basic and clinical physiology and pharmacology. 2021;32(4):571-6.
- [59]Esan AJ. Hematological differences in newborn and aging: a review study. Hematol Transfus Int J. 2016;3(3):178-90.
- [60]Kim D. The role of vitamin D in thyroid diseases. International journal of molecular sciences. 2017;18(9):1949.
- [61]Heaney RP. Toward a physiological referent for the vitamin D requirement. Journal of endocrinological investigation. 2014;37(11):1127-30.
- [62]Larijani B, Hossein-Nezhad A, Feizabad E, Maghbooli Z, Adibi H, Ramezani M, Taheri E. Vitamin D deficiency, bone turnover markers and causative factors among adolescents: a cross-sectional study. Journal of Diabetes & Metabolic Disorders. 2016;15(1):1-6.
- [63]Bañón S, Rosillo M, Gómez A, Pérez-Elias MJ, Moreno S, Casado JL. Effect of a monthly dose of calcidiol in improving vitamin D deficiency and secondary hyperparathyroidism in HIV-infected patients. Endocrine. 2015;49(2):528-37.
- [64]El Hilali J, de Koning EJ, van Ballegooijen AJ, Lips P, Sohl E, van Marwijk HW, Visser M, van Schoor NM. Vitamin D, PTH and the risk of overall and disease-specific mortality: results of the Longitudinal Aging Study Amsterdam. The Journal of steroid biochemistry and molecular biology. 2016;164:386-94.
- [65]Lee JA, Hwang JS, Hwang IT, Kim DH, Seo JH, Lim JS. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatric hematology and oncology. 2015;32(2):99-108.
- [66]Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, Rasgon SA. Vitamin D deficiency and anemia: a cross-sectional study. Annals of hematology. 2010;89(5):447-52.
- [67]Blanco-Rojo R, Pérez-Granados AM, Toxqui L, Zazo P, de la Piedra C, Vaquero MP. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. European journal of nutrition. 2013;52(2):695-703.
- [68]Raveendran AV, Shiji PV, Rajini P, Al Qassabi FS. Iron deficiency anemia: an update. BMH Medical Journal-ISSN 2348–392X. 2019;6(4):116-30.
- [69]Malczewska-Lenczowska J, Sitkowski D, Surała O, Orysiak J, Szczepańska B, Witek K. The association between Iron and vitamin D status in female elite athletes. Nutrients. 2018;10(2):167.
- [70]Kumari S, Swetha P, Nayak S, Singh S. The Association Between Ferritin and Vitamin D Levels in Premenopausal Fibroid Uterus Cases with Anemia. Cureus. 2021;13(2).
- [71]Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, Nayak A, Wesseling-Perry K, Westerman M, Hollis BW, Salusky IB. Suppression of iron-regulatory hepcidin by vitamin D. Journal of the American Society of Nephrology. 2014;25(3):564-72.
- [72]Seong JM, Yoon YS, Lee KS, Bae NY, Gi MY, Yoon H. Gender difference in relationship between serum ferritin and 25-hydroxyvitamin D in Korean adults. PloS one. 2017;12(5):e0177722.
- [73]Iyer KM. Metabolic and Endocrine Disorders. InGeneral Principles of Orthopedics and Trauma. 2019; 183-248.
- [74]Wang Y, Li H, Zheng M, Wu Y, Zeng T, Fu J, Zeng D. Maternal vitamin D deficiency increases the risk of adverse neonatal outcomes in the Chinese population: A prospective cohort study. PLoS One. 2018;13(4):e0195700.
- [75]Luster M, Duntas LH, Wartofsky L. The Thyroid and Its Diseases; Springer: pringer International Publishing AG. 2019.
- [76]Marei E, ُElmaghraby D, Gad AA. Vitamin D assessment in iron deficiency anemic pregnant women and their newborns. Egyptian Journal of Radiation Sciences and Applications. 2017;30(1):63-72.
- [77]Braun V, Hantke K. Recent insights into iron import by bacteria. Current opinion in chemical biology. 2011;15(2):328-34.
- [78]Brazaca SG, da Silva FC. Enhancers and inhibitors of iron availability in legumes. Plant Foods for Human Nutrition. 2003;58(3):1-8.
- [79]Abdullah-Al-Mamun M, Jakir Hasan M, Al Azad S, Giash Uddin M, Shahriyar S, Jyoti Mondal K. Evaluation of potential probiotic characteristics of isolated lactic acid bacteria from goat milk. Biotechnol J Int. 2016;14(2):1-7. https://doi.org/10.9734/BBJ/2016/26397
- [80]Dipta DE, Tanzila Ismail EM, Partha BISWAS SA, Shoeba Islam UR, Firoz M, Ahmed SZ, Salauddin AL, Rahman A, Afrin S, Mahedi RA, Badal MN. Antiviral effects of bacteriocin against animal-to-human transmittable mutated sars-cov-2: a systematic review. Front. Agric. Sci. Eng. 2021;8:603-22. DOI: 10.15302/J-FASE-2021397
- [81]Szabo M, Kovathana N, Gordon K, Frohmani LA. Effect of passive immunization with an antiserum to thyrotropin (TSH)-releasing hormone on plasma TSH levels in thyroidectomized rats. Endocrinology. 1978;102(3):799-805.
- [82]Al Azad S, Shahriyar S, Mondal KJ. Opsonin and its mechanism of action in secondary immune. Journal of Molecular Studies and Medicine Research. 2016;1(02):48-56. DOI: 10.18801/jmsmr.010216.06