Evaluation of antibacterial, antibiofilm activity of biosynthesis MgONPs and cellular immunity in rabbit
Abstract
Magnesium oxide (MgO) is one of the most promising nanoparticles due to its mono-metallic oxide group, High melting point, no toxicity issues, high hardness, and high purity. For those reasons, these materials were used in many fields including medicine, agriculture, electronics, energy, and environmental protection. Leuconostoc spp. was used as a facility to biosynthesize MgO nanoparticles, then optimized pH and Mg (NO3)2.6H2O concentration for its antibacterial activity and cellular immunity reactions including skin sensitivity through studying Interleukin 1-Beta (IL-1β), Interleukin 17 (IL-17) and Interleukin 2 (IL-2) responding. The study shows that the optimum pH for Magnesium Oxide (MgO) biosynthesis was 12, optimum Mg (NO3)2.6H2O concentration was 0.1 M, while antibacterial activity shows a high effect on Gram-negative bacteria with 125 µg/ml as minimum inhibiting concentration (MIC) and 500 µg/ml as minimum bactericidal concentration (MBC), the immunity study appeared that the MgO nanoparticles (MgONPs) altered the immunity response in skin test duration and diameter of MgONPs as 1.31±0.55, 9.33±1.15, 11.33±1.75 and 8.33±0.75 mm after 4,24,84 and 72 hours, respectively. IL-1β, IL-17 and IL-2 show significant differences compared to the control group at P-value ≤ 0.05 as 82.305±13.38, 101.444±16.943 and 49.781±5.264, respectively. MgONPs can be used as an alternative treatment for multidrug resistance MDR bacteria due to their high effectiveness against bacterial growth, MgONPs induced the cellular immunity response in rabbit tissues as delivery of immunogen.
INTRODUCTION
Magnesium oxide nanoparticles (MgONPs) have been widely used in a variety of applications: medical, electronical, energy and environmental studies [1]. Many studies in the last decades prepared metal oxides nanoparticles with large reactive surface areas possessing unique magnetic, electronical, optical, thermal, chemical and mechanical properties due to their special chemical and physical properties [2–4]. MgONPs showed excellent antibacterial effects against a wide range of pathogenic microorganisms for both Gram-positive and negative groups, so that may effectively help in medical fields as an alternative treatment, especially those multidrug resistance bacteria MDR [5]. Studies reported that the MgONPs activity against bacteria through the production of reactive oxygen species ROS which induce peroxidation of lipid in bacteria [6].
MgONPs have been evaluated for their effect on the immune system via an effect on humoral immune responses from the upward neutralizing antibodies, but due to commercial additives like Alum, which may lead to bad immune response consequences, but in general, there was no toxicity became discovered from using MgONPs as an adjuvant could be taken into consideration as a promising treatment [7].
Nanoparticles generally interact with many immune system components, which improves or drawbacks their interaction in the body [8]. Nanoparticles can dramatically activate the immune system like vaccination, Cytokines are cell to cell messengers just like hormones, which means nanoparticles can be used to maximize Cytokines activity by promoting a delivery carrier. Cytokines’ physiological role is widely recognized in tissue homeostasis, cell differentiation, delivery and immune tool response to trauma, inflammation, and infection [9,10].
Cytokines, as an anti-inflammatory was frequently measured to predict immune-modulation as a result of using nanomaterials in-vivo study as a response to measure the toxicity of nanomaterials, additionally alternate therapeutic effects of pharmaceutical substances delivered by nanoparticles [11]. NLR family pyrin containing 3 (NLRP3) expressed by macrophage, activation of NLRP3 triggered the immune response and act as an anti-inflammatory, nanoparticles can mediate NLRP3 activity to affect the secretion of IL-1β, which in turn double-walled carbon nanotube have reinforced the anti-inflammatory role of cytokine IL-1β released by monocyte through NLRP3 pathway [12,13].
Previous studies used E. coli as a pathogen model by collecting outer membrane vehicles OMV and coating them onto magnesium nanoparticles MgONPs to create what are called bacterial membrane magnesium nanoparticles BM-MgONPs which in turn activate rapidly the immune response of lymphocytes, results of this study show immune response higher than OMV only, so they used nanomaterials as vaccination. BM-MgONPs induce the production of interferon-gamma INFγ and interleukin 17 (IL-17) indicating the capability to generate strong Th1 and Th17 biased cell responses against bacterial infection [14].
MATERIALS AND METHODS
Ethical statements
Every volunteer has given written informed permission. This research received ethical approval (DSM/HO-1632) for scientific research from the Ministry of Health MOH and Ministry of Higher Education and Scientific Research MOHESR ethics committees in Iraq.
MgONPs synthesis from leuconostic spp.
MgONPs with 43.85 nm were prepared and characterized in a previous study [15]. MgONPs suspension at 4µg/ml concentration, where 4mg of the nanoparticles powder were dissolved in one ml of deionized distilled water and they were mixed well with vortex, then placed in a sonicator Ultra Sonic Bath and then filtered with a microfilter [16].
Optimization of MgONPs synthesis
The effect of various physicochemical parameters such as pH and Magnesium nitrate hexahydrate concentration were studied to determine the optimum growth condition of MgONPs synthesis [17,18]. The effects of pH were studied by incubating the broth of bacteria with Mg (NO₃)₂.6H₂O, pH (6, 8, 10, 12,14) was adjusted in a series of 5 tubes. The culture was harvested incubator at 40°C for 20min. Media then were incubated at room temperature for 10hr., then centrifuged to sediment the MgONPs and washed twice with DW and examined in UV-spectrophotometer. An experiment was designed for the detection of the optimum concentration of the substrate for biosynthesis of MgONPs. Two concentrations (1 and 0.1) M from Mg (NO₃)₂.6H₂O were examined for the optimum concentration for MgONPs biosynthesis. The optimum pH was used, and all the tubes were incubated in a shaking incubator at 40°C for 20min. Media then were incubated at room temperature for 10hr, then centrifuged to sediment the MgONPs and washed twice with DW and examined in a UV -spectrophotometer.
Antibacterial activity test of MgONPs by agar well diffusion method
The activity MgONPs as an antimicrobial against MDR human pathogen was tested against Gram-negative bacteria Salmonella Typhi, the test was carried out according to clinical and laboratory standards institute instruction [19,20]. In this study, we used the agar well diffusion method to assess bacterial responses to inhibition or resistance to MgONPs, so a serial dilution of MgONPs was prepared as (500, 250, 125, 62.5, 31.25, 15.6 µg/ml) in triplicates, the bacterial isolates were firstly incubated at 4°C for 15 minutes before transmitted to 37°C, measuring of inhibition zone was taken directly after the appearance of inhibition then recording time and inhibition zone diameter with a digital vernier calliper.
Minimal inhibitory and minimal bactericidal concentration (MIC and MBC) test of MgONPs
McFarland tube 0.5 was prepared as a standard for an estimate the bacterial growth, candidate bacterial isolates were incubated at 37°C overnight in a 10 ml nutrient broth medium, then inoculation each sample with 1ml of the microbial suspension (108 colony/ml), MgONPs were prepared as six dilutions (diluted in deionized water) as follow: 500, 250, 125, 62.5, 34.25 and 15.6 µg/ml, and same dilutions without MgONPs as a negative control, the MIC measured by spectrophotometer at 600 nm after incubation, well showed no turbidity in nutrient agar plates, bacterial colonies show no growth presented as MBC [21].
Preparation of MgONPs vs antibiotics combination
A mixture of our candidate MgO nanoparticles and other antibiotics were prepared by choosing a minimal active dose of MgONPs and antibiotic as 125 µg/ml MgONPs + 5mg/ml CIP, and 125 µg/ml MgONPs + 10mg/ml GN and inserted in every well of at room temperature for 1 hour then incubated for 24 hours at 37°C. Bacterial isolates standardized at 1.5*108 in 0.1 ml, agar medium drilled by cork borers [19,20].
Immunization of lab animals
Laboratory animals in this study included six New Zealand adult male rabbits Oryctolagus cuniculus in the animal house of the College of Sciences/the University of Babylon, fed with healthy no additives during the experiment, each rabbit was about 1-1.5 kg of weight at 3-5 months age. The animals were immunized intramuscularly with MgONPs synthesized by Leuconostoc spp. at 1 ml per 1 kg according to [22]. The layout of the experiment was carried out by immunization of three rabbits with MgONPs with 4 µg/ml of concentration at 1 ml per each 1 kg of animal weight, and the control group was injected with normal saline, the experiment was prolonged for three weeks and animals one dose per week [23].
Blood samples were collected by heart puncture with a sterile disposal syringe (about 8-10 ml) from six immunized rabbits with MgONPs and non-immunized animals (control group). Half of the blood volume about 2-4 ml of collected blood was left at room temperature till clotted, then centrifuged at 3000 rpm per 5 minutes. Serum was collected and divided into 0.5 ml in an Eppendorf tube and froze till the testing time, serum was divided into small groups to prevent any errors that may occur due to repeated freezing and thawing. The remaining half of the collected blood was placed in an EDTA tube to prevent coagulation [24].
Skin test
A skin test was carried out in the fourth week of the experiment when started by examining the intradermal skin layer for both groups (MgONPs injected and control groups) and recording the observed changes after 4, 24, 48 and 72 hours. This test was to find out the effect of MgO nanoparticles on the sensitivity of animals and the effect to stimulate the immune response of animals. Interleukin (IL-1β, IL-2 and IL-17) levels were measured according to manufacturer instruction of (Elabscience-China) by ELISA technique.
Statistical analysis
Data were analyzed and processed by SPSS 19 software performing a One-Way Analysis of Variance (ANOVA) test, Mean, Standard Deviation (SD), and P-value which was below 0.05 considered for statistical significance [25].
RESULTS
MgONPs biosynthesis optimization
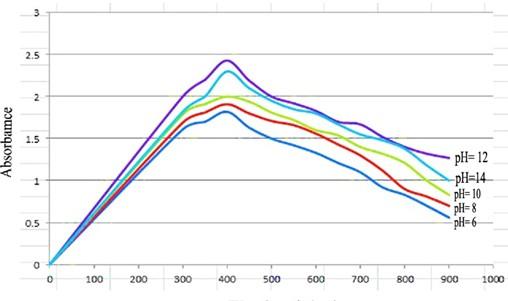
pH optimization, optimizing of pH important factor in nanoparticles biosynthesis due to its effect on the stability of nanoparticles structure which may affect the aggregation of the particle also the physical properties of nanoparticles, in this study we assess the optimum pH of MgONPs synthesized by a bacterial isolate by preparing pH as 6, 8, 10, 12 and 14 (buffering accomplished by NaOH 1% and HCl 1% to reach accurate pH value), the results depending on wavelength 350-700 nm which show that the pH=12 was the best pH value show optimum absorption by spectrophotometer as shown in figure 1.
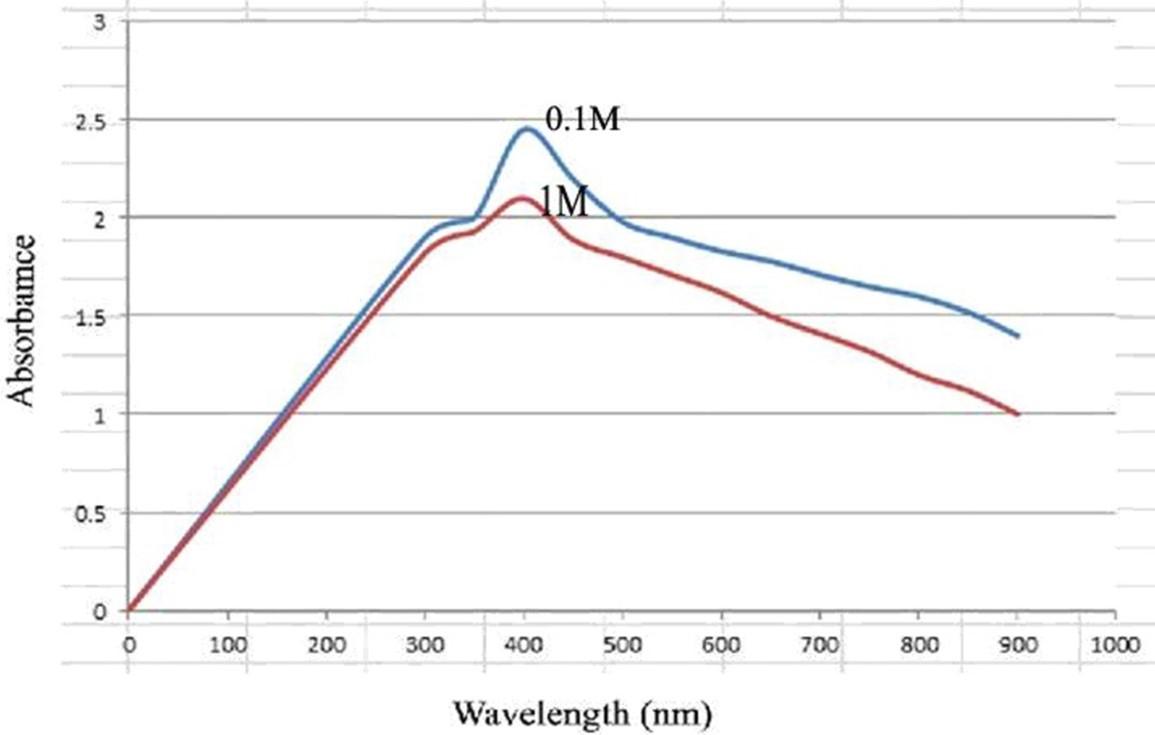
Nanoparticles salt magnesium-nitrate hexahydrates Mg (NO₃)₂.6H₂O concentration used as additives to the medium broth in this study as 0.05, 0.1 and 0.2 M as well as 0.05, 0.1 and 0.2 M for NaOH which prepared according to [9], the salt concentration shows best yield production was at 0.1 M when measured at 100-1000 nm of wavelength as shown in figure 2.
The experiment was carried out depending on the Taguchi method, by preparing different concentrations of Mg (NO3)2.6H2O were added to NaOH solution and stirred for 30, 60, and 90 minutes then separated by centrifugation, washing solution to remove any impurities then dried by the oven, Magnesium hydroxide powder was crystalized at 450°C in a furnace resulting white powder made from magnesium oxide nanoparticles [5,15,17].
Antibacterial activity of MgONPs against MDR bacteria and determination (MIC) and (MBC) test
The antimicrobial activity of candidate MgONPs was evaluated with four types of Salmonella Typhi isolates which were diagnosed biochemically by the Vitek-2 system. MIC and MBC were established with serial dilution by broth microdilution technique then the growth was measured with a spectrophotometer at 600 nm of wavelength as shown in table 1.
MIC of MgONPs result was 125 µg/ml and the MBC was 500 µg/ml these results show the effectiveness of magnesium oxide nanoparticles as an antimicrobial agent [26], MgONPs alone or in combination with other antimicrobial agents came with the same results which are reduction in the number of Gram-negative bacteria according to this study and a previous study [27,28]. As shown in table 2.
Table 1. MIC and MBC of MgONPs for Salmonella Typhi.
Table 2. Antibiotic susceptibility of Salmonella Typhi by agar well diffusion test.
Table 2 shows the increase of antibiotics concentration led to making bacteria more susceptible to antibiotics, diameter inhibition zone of MgONPs in Salmonella Typhi colonized Petri dish was about 22, 25, 30 and 31 mm from the centre of the inhibition zone (125 µg/ml) as shown in table 3, while the inhibition zone of CIP with different concentrations (diluted with distilled water) 5mg/ml was 31, 30, 26 and 28 mm in diameter of four isolates of Salmonella Typhi, for the GN 10mg/ml the diameter of inhibition zone was 30, 22, 25 and 25 mm for same isolates.
Table 3 revealed MgONP’s effectiveness in inhibition of Salmonella Typhi growth as many other previous studies [29] which discovered that the MgONPs show the best antimicrobial activity against Bacillus subtilis, Salmonella Typhi (Gram-negative bacteria) and E. coli (Gram-positive bacteria), table 4 show the activity of MgONPs as an antimicrobial agent in combination with GN and CIP antibiotics against Salmonella Typhi.
As shown in table 4 the combination of MgONPs 125 µg/ml + CIP 5mg/ml show inhibition zone as 33, 25, 30 and 25 mm for the 4 Salmonella Typhi isolates, while the combination of MgONPs 125 µg/ml + GN 10 mg/ml show inhibition zone diameter as 25,33, 30 and 31 mm.
The effect of antimicrobial activity of MgONPs against Salmonella Typhi clearly appears in figure 3, The results reveal that when antibiotics are combined with MgONPs, bacteria become more susceptible. These findings are consistent with those [30] who investigated the same hypothesis. MgONPs showed antibacterial activity against both gram-positive and gram-negative bacteria (Staphylococcus aureus and Escherichia coli, respectively) at a minimal inhibitory concentration. When antibiotics were combined with MgONPs, there was a substantial increase in bacterial sensitivity P ≤ 0.05. Many studies have indicated that the antibacterial mechanism of MgONPs is due to the formation of ROS such as superoxide anion (O2 −) [31]. Some studies reported that the increasement of MgONPs surface area led to increasing of O2 in solution which in its led to increasing damage to the bacterial cell walls [3,32].
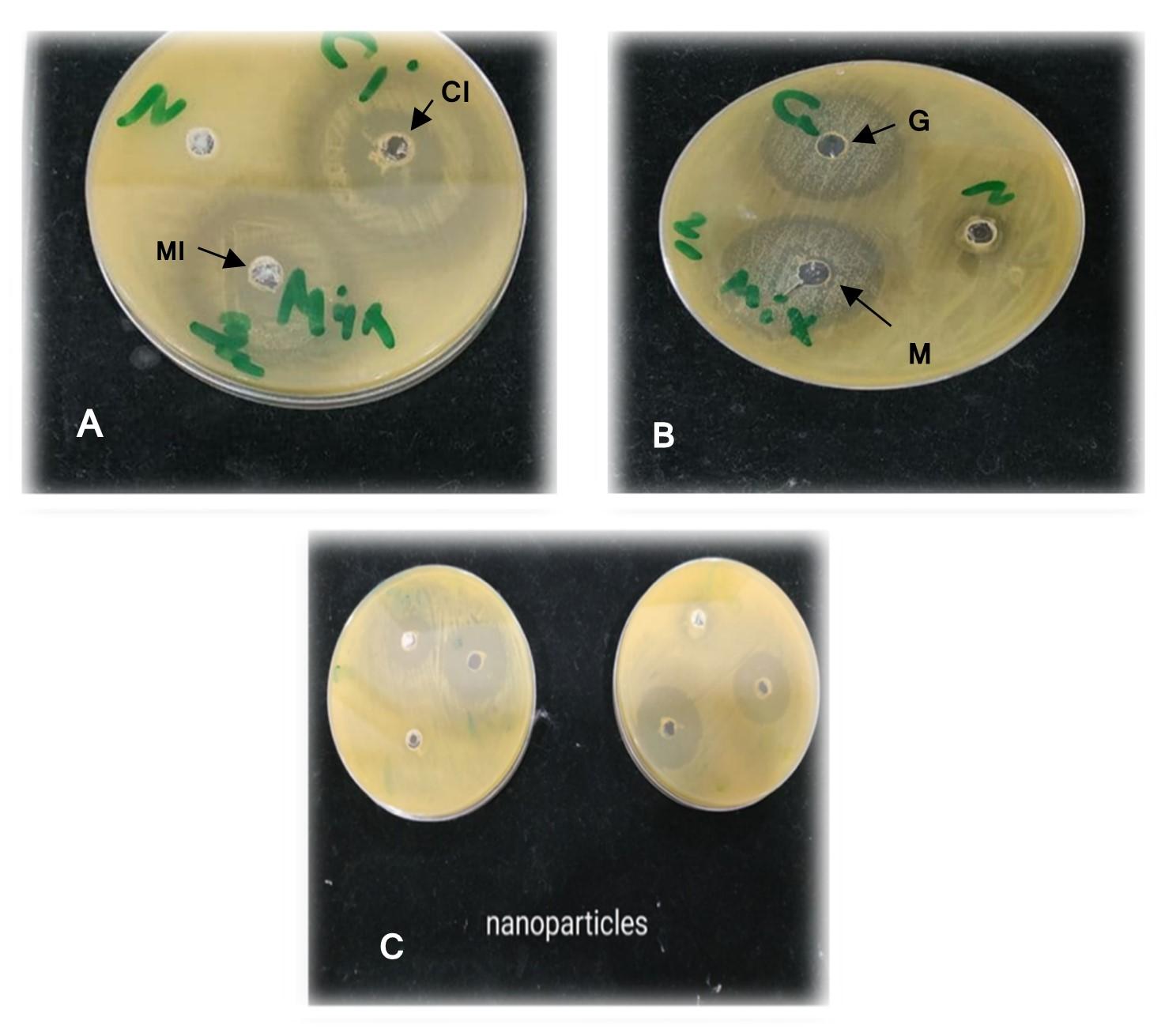
Table 3. Antibacterial activity of MgONPs against Salmonella Typhi by agar well diffusion test.
Table 4. Combined effect of MgONPs with antibiotic of Salmonella Typhi by agar well diffusion test.
Effect of MgONPs on cytokines
The skin test was measured in all the animals that were immunized, and the results were measured and compared to the control group. As shown in figure 4, the induration of skin was higher after 48 hrs. compared with other times. The skin appeared with erythema and induration and necrosis.
A skin test was performed to study the effect of MgONPs on cellular immunity, which erythematic, pus cell, induration and necrosis. The induration diameter of animal groups immunized with MgONPs that synthesis by isolate leuconostic spp. as shown in table 5 was 1.31 mm after 4 hrs. of immunization, after 24 hrs. of injection, the skin of rabbits immunized with MgONPs showed pus cell and induration diameter was 9.33 mm and showed a significant increase (P≤0.05) when compared to the mean of induration diameter after 4 hrs. Also, results showed after 48 hrs. of injection increases with MgONPs was shown pus cell and necrosis and the mean induration diameter was 11.33 mm and had significant differences when compared to the induration diameter after 24 hr., but after 72 hrs. of injection with MgONPs was shown erythema, pus cell and necrosis and had significantly decreased (P≤0.05) in the mean of induration diameter which reached 8 mm and 8.33 mm compared the mean of induration diameter after 48 hrs.
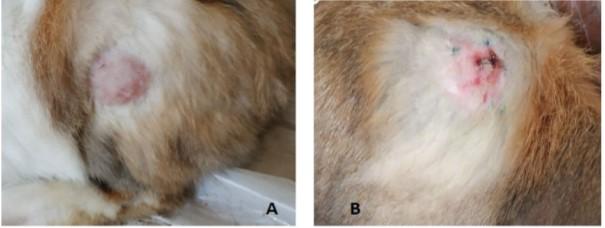
Table 5. Skin sensitivity of the immunized rabbits.
The results of the production of cytokines were estimated using the equation from the standard curve carried out with the same test. Statistical analysis of IL-1 β, IL-17 and IL-2 showed a mean value (17.700 ± 2.545, 50.310 ± 5.498 and 23.434±2.501) respectively, of the control group when compared to the immunized group with MgONPs the mean value was (82.305±13.38, 101.444±16.943 and 49.781±5.264) respectively, as shown in table 6.
Immune system drives the specialized immune cells T cells to the skin where they release chemical messengers called lymphokines, which have been sensitized by prior infection. These lymphokines produce induration at and around the injection site (a hard, elevated region with distinct borders) by promoting local vasodilation (increases in blood vessel diameter), producing oedema, fibrin deposition and other types of inflammatory cells [33].
Table 6. The concentration of IL-1β, IL-17 and IL-2 (pg/ml) in immunized and controlled rabbits.
DISCUSSION
The results show that pH 12 was the best pH value gave the maximum MgONPs yield production when measured by spectrophotometer at wavelength 350-450 nm, studies show that the pH value, when drooped to less than 10 show very low concentration of nanoparticles produced by microorganisms and that, occur due to unfavourable condition for bacterial growth which in turn lead to decrease metabolism levels of microorganisms [24]. Hydroxyl group in the surface of nanoparticles in pH 12 (alkaline) permit high absorption by UV light, pH value and many other factors that directly effect on biosynthesis process such as; stability of nanoparticles, chemical structure, application target, etc., these factors play important roles as a reducing agent as enzymes, carbohydrates, proteins and other molecules presents in biomass filtrate which can affect directly on the biosynthesis process [26].
The results of antimicrobial activity revealed that when antibiotics are combined with MgONPs, bacteria become more susceptible. These findings are consistent with those [30] who investigated the same hypothesis. MgONPs showed antibacterial activity against both gram-positive and gram-negative bacteria (Staphylococcus aureus and Escherichia coli, respectively) at a minimal inhibitory concentration. When antibiotics were combined with MgONPs, there was a substantial increase in bacterial sensitivity P≤0.05. Many studies have indicated that the antibacterial mechanism of MgONPs is due to the formation of ROS such as superoxide anion (O2 −) [31]. Some studies reported that the increasement of MgONPs surface area led to increasing of O2 in solution which in its led to increasing damage to bacterial cell walls [3,32].
The results of the skin test revealed that the DTH for the skin used as the indicator of cellular immunity in rabbits immunized by the different kinds of Salmonella antigens revealed a substantial increase in inoculated animals. Testing of the final result. Parallel with [34], who found that after 24 hours the skin response was higher [35,36]. The DTH response is characterized as a remembrance or T-cell reaction because it needs a certain antigen to be prematurely immunological responsive [31,35]. It can detect some antigens which have been already known to respond to immunity, providing an indicator for T-cells’ activity to recall antibodies. The major responses for DTH are human neutrophils, followed by fusion of mononuclear cells made up of macrophages and T cells, whereas the mouse reacts highly to DTH antigens in mice [36,37].
Results found by this study agree with the previous study Delay-type Hypersensitivity DTH was induced by induction of pro-inflammatory cytokines through Th1 responses by AgNPs in rabbits [37]. Lubberts (2015) referred that the diameter of the inflamed area of injected rabbits with the antigens after 4, 24, 48 and 72 hours show a significant increase in contrast to the control group (at P-value ≤ 0.05), these results refer that the 3 antigens types inducing the Delay-Type Hypersensitivity DTH which belong to IV type of hypersensitivities which act as a result from T-cells inflammation activity where antibodies not involved [38]. The inflammation response results from T-cell reacting and responding to antigens, soluble molecules play important role in clinical immunology as a group, then microphage digest or separate those soluble molecules to their precursors and detect the components to stimulate immune chemical signals to communicate with other cells, chemokines considered cytokines that induce leukocyte hemotoxins, there are several other stimulating activities initiated along with cytokines. The other function of interleukin’s IL-1β, IL-2 and IL-17 is the enhancement of immunological response, inducing T and B cells that were susceptible to IL-1β, while IL-2 was largely involved with lymphocytes [38,39], IL-17 is a pleiotropic cytokine that plays a crucial role in establishing autoimmune disease, IL-17 also enhance the fatality immune interaction via recruitment of inflammatory cells like neutrophils, T-cells and dendritic cells, on the other hand, this excessive elimination of immune cells activity to their target in the chronic inflammatory reaction is a harmful and may lead to autoimmune disease, therefore the high regulation roles is mandatory for immunological homeostasis maintenance [40].
Carol and his colleagues (2008) demonstrated that a significant inflammatory response has been observed in the release of IL-1β after 24 hours of exposure to 15 nm of nanoparticles, and IL-1β was the most intermediates of fever and other symptoms appearance which raise as a response to illness since that the macrophages stimulated by lipopolysaccharides to release [41,42]. Metal nanoparticles influence immunological activities as same as protein antigens but with different immune response pathways [42]. Virus-like nanoparticles (VLN) are molecules that resembled viruses but are non-infectious due to a lack of viral genetic materials, VLN can naturally synthesize by individual expression of viral capsid proteins, or from different or combinations of different capsid proteins viruses to create recombinant VLN [43,44]. VLN co-expressed IL-2 with many different anchors of membrane receptors, C-terminus of IL-2 fuse with glycosylphosphatidylinositol anchors receptors with 2 immunological-like domain structures of CD16 which lead to stimulation of T-cells and inducing the CD8 and T-cells effect in vivo [45]. The majority of antigen-specific T-cells react with IL-2 to liposomes after each dose injected, the repeating process increase T-cells toxicity by increasing activation and proliferation of T-cells [46,47].
CONCLUSIONS
From the results obtained, the use of Leuconostoc spp. for the biosynthesis of MgONPs is an effective and low-cost method. In addition, it can be used this nanoparticle as an anti-bacterial and anti-biofilm produced by bacteria, indicating the possibility of using them in both medical and non-medical applications, especially when in combination between these nanoparticles and antibiotics. When studying the skin test after injection with nanoparticles to laboratory animals, the skin response was high, and this indicates the activity of T cells stimulating antibodies. Also, measuring the level of cytokines after the injection led to an increase in DTH, which in turn leads to a harmful inflammatory reaction that leads to autoimmune diseases.
ACKNOWLEDGEMENT
The authors would like to thank Dr Yasir Al-Mawlah (DNA Research Center / University of Babylon) Pune for their kind support during this work and for providing us the lab facilities to make this work accomplished.
AUTHOR CONTRIBUTIONS
Conception and design of study: FrialAbd and Lubna Albayati. Drafting the manuscript: Duaa Hassan and FrialAbd. Analysis and/or interpretation of data: Duaa Hassan and Lubna Albayati.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Rajagopalan S, Koper O, Decker S, Klabunde KJ. Nanocrystalline metal oxides as destructive adsorbents for organophosphorus compounds at ambient temperatures. Chemistry – A European Journal. 2002; 8(11):2602-2607.
- [2]Obeid MM, Jappor HR, Al-Marzoki K, Al-Hydary IA, Edrees SJ, Shukur MM. Unraveling the effect of Gd doping on the structural, optical, and magnetic properties of ZnO based diluted magnetic semiconductor nanorods. RSC Advances. 2019; 9: 33207-33221.
- [3]Huang L, Li DQ, Lin YJ, Wei M, Evans DG, Duan X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. Journal of Inorganic Biochemistry. 2005;99(5): 986-993.
- [4]Zhang Y, Ma M, Zhang X, Wang B, Liu R. Synthesis, characterization, and catalytic property of nanosized MgO flakes with different shapes. Journal of Alloys and Compounds. 2014;590.
- [5]Khan A, Shabir D, Ahmad P, Khandaker MU, Faruque MRI, Din IU. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Materials Research Express. 2021; 8 (2021) 015402.
- [6]Tang ZX, Lv BF. MgO nanoparticles as antibacterial agent: Preparation and activity. Brazilian Journal of Chemical Engineering. 2014;31(3).
- [7]Xu Y, Tang H, Liu JH, Wang H, Liu Y. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicology Letters. 2013;219(1).
- [8]Hussain S, Vanoirbeek JAJ, Hoet PHM. Interactions of nanomaterials with the immune system. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(2):169-83.
- [9]Duque GA, Descoteaux A. Macrophage cytokines: Involvement in immunity and infectious diseases. Vol. 5, Frontiers in Immunology. 2014.
- [10]Simpson S, Kaislasuo J, Guller S, Pal L. Thermal stability of cytokines: A review. Vol. 125, Cytokine. 2020.
- [11]Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M. Impact of nanoparticles on the immune system. Journal of Biomedical Nanotechnology. 2011;7(1).
- [12]Sester DP, Thygesen SJ, Sagulenko V, Vajjhala PR, Cridland JA, Vitak N, Chen KW, Osborne GW, Schroder K, Stacey KJ. A novel flow cytometric method to assess inflammasome formation. The Journal of Immunology. 2015;194(1).
- [13]Meunier E, Coste A, Olagnier D, Authier H, Lefèvre L, Dardenne C, Bernad J, Béraud M, Flahaut E, Pipy B. Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine: Nanotechnology, Biology and Medicine. 2012 Aug 1;8(6):987–95.
- [14]Camacho AI, de Souza J, Sánchez-Gómez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29(46).
- [15]Hasan D, Gameel F, Abdulazeez L. Biosynthesis Method and Characterization of MgO Nanoparticles by Using Local Leuconostoc spp. Isolate. [Internet]. Annals of the Romanian Society for Cell Biology. 2021 [cited 2022 Mar 4]. p. 9072–7.
- [16]Naghsh N, Kazemi S. Effect of nano-magnesium oxide on glucose concentration and lipid profile in diabetic laboratory mice. Iranian Journal of Pharmaceutical Sciences. 2014;10(3).
- [17]Mohanasrinivasan V, Subathra Devi C, Mehra A, Prakash S, Agarwal A, Selvarajan E, Jemimah Naine S. Biosynthesis of MgO Nanoparticles Using Lactobacillus Sp. and its Activity Against Human Leukemia Cell Lines HL-60. Bionanoscience. 2018;8(1).
- [18]John Sushma N, Prathyusha D, Swathi G, Madhavi T, Deva Prasad Raju B, Mallikarjuna K, Kim HS. Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea—characterization and in vitro antioxidant studies. Applied Nanoscience (Switzerland). 2016;6(3).
- [19]Abdulazeem L, Alasadi YF, Al-Mawlah YH, M. Hadi A. A Mini review: Silver Nanoparticles (AgNPs) as Antimicrobial in Magical Socks. Journal of Pharmaceutical Research International. 2021 Nov 20;33(51):23–32.
- [20]Lubna Abdulazeem, Nashwan M. AL-Gburi, Mohammad Dyia, Yasir H. Al-Mawlah, Ahamed H. Rasheed. Antimicrobial resistance profiles of bacteria isolated from poultry droppings and treated by AgNPs green synthesis from Thymus kotschyanus. Plant Archives. 2020 Oct 10;20(2):5973–9.
- [21]Schneider E, Völcker C, Haude W. Age and sex dependence of phospholipid concentrations in human erythrocytes. Z Med Lab Diagn. 1990; 31(2):85-89
- [22]Ramesh Krishnan, Vijay Arumugam, Suresh Kumar Vasaviah. The MIC and MBC of Silver Nanoparticles against Enterococcus faecalis – A Facultative Anaerobe. Journal of Nanomedicine & Nanotechnology. 2015, 06(03).
- [23]Okoli AS, Iroegbu CU. Evaluation of extracts of Anthocleista djalonensis, Nauclea latifolia and Uvaria afzalii for activity against bacterial isolates from cases of non-gonococcal urethritis. Journal of Ethnopharmacology. 2004;92(1).
- [24]James S. Lewis II. Performance Standards for Antimicrobial Susceptibility Testing Performance Standards for Antimicrobial Susceptibility Testing Suggested Citation. CLSI document M02-A11. 2018;
- [25]George D, Mallery P. SPSS for Windows Step by Step: Answers to Selected Exercises. A Simple Guide and Reference. 2003.
- [26]Patra JK, Baek KH. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. Vol. 2014, Journal of Nanomaterials. 2014.
- [27]Haghshenas L, Amini A, Bashir Bahati A, Rahimi G. In vitro Antibacterial Biofilm effect of Magnesium Oxide Nanoparticles on Streptococcus mutans. Micro & Nano Biomedicine. 2016;1(1).
- [28]Strindelius L, Wikingsson LD, Sjöholm I. Extracellular antigens from Salmonella enteritidis induce effective immune response in mice after oral vaccination. Infection and Immunity. 2002;70(3).
- [29]Fouda A, Hassan SED, Abdel-Rahman MA, Farag MMS, Shehal-deen A, Mohamed AA, Alsharif SM, Saied E, Moghanim SA, Azab MS. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Current Research in Biotechnology. 2021;3.
- [30]Imani MM, Safaei M. Optimized Synthesis of Magnesium Oxide Nanoparticles as Bactericidal Agents. Journal of Nanotechnology. 2019;2019.
- [31]Hayat S, Muzammil S, Rasool MH, Nisar Z, Hussain SZ, Sabri AN, Jamil S. In vitro antibiofilm and anti-adhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria. Microbiology and Immunology. 2018;62(4).
- [32]Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. Journal of Radiation Research and Applied Sciences. 2016;9(1).
- [33]Creusot RJ, Mitchison NA. How DCs control cross-regulation between lymphocytes. Trends in Immunology. 2004;25(3).
- [34]Flemming HC, Wingender J. The biofilm matrix. Vol. 8, Nature Reviews Microbiology. 2010.
- [35]Shkodenko L, Kassirov I, Koshel E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Vol. 8, Microorganisms. 2020.
- [36]Jin T, He Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. Journal of Nanoparticle Research. 2011;13(12).
- [37]Raheem H, Althahab A, Jameel F. Immunological Study of Silver Nanoparticles Produced from Proteus mirabilis in Rabbit. Al-Kufa University Journal for Biology. 2016, 8(2): 177–187.
- [38]Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol [Internet]. 2015 Jul 30 [cited 2022 Mar 4];11(7):415–29. Available from: https://pubmed.ncbi.nlm.nih.gov/25907700/
- [39]Zwicky P, Unger S, Becher B. Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. Journal of Experimental Medicine. 2020;217(1).
- [40]Carlson C, Hussein SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. Journal of Physical Chemistry B. 2008;112(43): 13608–13619
- [41]Kozak W, Kluger MJ, Tesfaigzi J, Kozak A, Mayfield KP, Wachulec M, Dokladny K. Molecular mechanisms of fever and endogenous antipyresis. Annals of the New York Academy of Sciences. 2000;917: 121-134.
- [42]Zampronio AR, Melo MCC, Hopkins SJ, Souza GEP. Involvement of CRH in fever induced by a distinct pre-formed pyrogenic factor (PFPF). Inflamm Res. 2000; 49(9):473–479.
- [43]Wojta-Stremayr D, Neunkirchner A, Srinivasan B, Trapin D, Schmetterer KG, Pickl WF. CD8+T cell fate and function influenced by antigen-specific virus-like nanoparticles co-expressing membrane tethered IL-2. PLoS ONE. 2015;10(5).
- [44]Zeltins A. Construction and characterization of virus-like particles: A review. Vol. 53, Molecular Biotechnology. Molecular Biotechnology 2013; 53: 92–107.
- [45]Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. Journal of Controlled Release. 2013;172(2).
- [46]Frick SU, Domogalla MP, Baier G, Wurm FR, Mailänder V, Landfester K, Steinbrink K. Interleukin-2 functionalized nanocapsules for T cell-based immunotherapy. ACS Nano. 2016;10(10).
- [46]Raheem HQ, Almawla YH. Silver nanoparticles as antibacterial action against pseudomonas fluorescens isolated from burn infection. Annals of the Romanian Society for Cell Biology, 12578–12583.