Effects of dexamethasone induced stress on the intestinal morphology and morphometry in broiler chicken
Abstract
The intestine of poultry plays a significant role in the health and production through enzymatic and microbial digestion of feed as well as absorption of nutrients. The current study was designed to explore the gross and histological alterations in the broiler duodenum and cecum triggered by dietary dexamethasone (DEX). The study was conducted on four homogenous groups of one-day-old chicks (20 chicks/group) i.e. one control (Non-DEX) and three treatment groups (DEX-1, DEX-2, and DEX-3). The broilers were fed commercial broiler feed containing DEX at the rate of 0, 3, 5, and 7mg/kg feed in the Non-DEX, DEX-1, DEX-2, and DEX-3 groups, respectively. The gross morphologic and morphometric data were recorded immediately after the collection of samples on days 7, 14, 21, and 28. Then, the tissue samples were processed for histological investigation. In the gross morphometric study, the weight, length, and width of the intestine were found significantly less in the DEX groups. Histopathological study results showed degeneration of intestinal glands (duodenum), mucosa, and lymphatic nodules with loss of lymphatic nodules (cecum). The percentage of the degenerated nodule was also increased. The length, width, and surface area of the duodenal villi, thickness of the mucosal layer of the cecum, and diameter of the cecal lymphatic nodules were substantially decreased in all the DEX groups. The magnitude of the alterations was associated with both the dose and duration of DEX treatment. However, the current study results indicate that DEX treatment significantly alters the morphologic and morphometric characteristics of the broiler intestine.
INTRODUCTION
Poultry meat is a popular source of protein in the human diet which has made the poultry sector economically valuable over the last few decades. It was predicted that global food consumption will be doubled by 2050, and per capita meat consumption would rise with the increasing purchasing capacity of the consumers in developing countries [1]. In comparison to the traditional husbandry techniques, modern poultry strains are genetically modified for rapid growth in a shorter period [2]. In this regard, nutrient digestibility and absorbability of the gut play a crucial role in actualizing its genetic potentiality [3, 4].
In the field of veterinary research, broiler ‘gut health’ is a widely discussed topic due to its role in health and growth performance [4, 5]. Duodenum and cecum are the major gastrointestinal parts for the digestion and absorption of nutrients from the diet. The duodenum receives the digestive enzymes and bicarbonates produced by the pancreas as well as the bile produced by the liver, which plays a major role in the digestion of protein and fat. The cecum plays a significant role in its growth as it harbors a diversified bacterial community and can ferment a wide variety of feed ingredients like starch, cellulose, hemicellulose, polysaccharides, fructooligosaccharides, and lignin to different final products [6]. The undigested compounds which are not absorbed in the small intestine, finally end up in the cecum, where they are digested by an anaerobic fermentation process [7]. As the proximal region of the cecum is continuously exposed to extra cecal bacterial or other antigenic invasions, the lymphatic nodules of the cecum play a critical function against these foreign invaders [8]. Thus, the cecum impedes the colonization of pathogenic microorganisms and detoxifies harmful elements to maintain its optimum digestive and absorptive functions [3]. So, the importance of the duodenum and cecum in broiler growth and development is indisputable. In this context, understanding the role of the gastrointestinal tract is crucial, as it has an enormous impact on the growth performance and health of broiler. However, stress, both biological and nutritional, is one of the major concerns in broiler production [9, 10, 11].
Exposure to different stressors leads to the secretion of corticosteroids (CORTs) by the hypothalamic-pituitary-adrenocortical (HPA) axis, hence it is known as stress hormone [12, 13]. Synthetic CORT dexamethasone (DEX) administration mimics the negative impacts of increased CORT [12]. Besides inducing stress, corticosteroids can also influence digestive function by markedly decreasing the digestibility of protein and carbohydrates [10]. A great deal of research was performed earlier focusing on the immunosuppressive effect of CORT [7, 9, 14, 15, 16]. According to the previous study report, supplementation of steroid growth promoters like DEX with diet does not improve the growth rate of broiler [12, 17]. DEX reportedly damages the kidney by vacuolation of kidney tissue, shrinking the tubules, and decreasing the number of nephrons [18, 19]. A high dose of DEX alters the morphology of breast and thigh meat, and also results in the developmental arrest of immune organs in the broiler [15, 17]. DEX also reportedly alters the liver morphology [20]. The effects of prednisolone related to gastrointestinal motility, intestinal histology, and mucosal mast cells were studied in rats [21].
The enteric system is a major body system that is responsible for the digestion and absorption of dietary nutrients. So, any alteration in its morphology affects the functions of other organs or body systems. Nonetheless, the adverse effects of stress induced by different doses of dietary DEX on morphology and morphometry of the enteric systems of broiler are not yet well-documented. Therefore, the administration of analogs of CORT like DEX can be an effective tool in investigating the adaptation in intestinal morphology in broiler in response to DEX induced stress.
MATERIALS AND METHODS
Experimental animals
This experiment was ethically approved by the “Animal Welfare and Experimentation Ethics Committee” of Bangladesh Agricultural University, Bangladesh [Approval No.- AWEEC/BAU/2021(3)]. A total of 80 one-day-old healthy broiler chicks of Cobb-500 strain were used in this study.
Housing and feeding management
The broiler chicks were then randomly assigned to four groups i.e. one control (Non-DEX) and three experimental or treated groups as DEX-1, DEX-2, and DEX-3. The broilers were fed commercial broiler feed (Nourish Poultry and Hatchery Limited, Bangladesh) containing CORT (DEX, BP 0.5 mg, Opsonin Limited) at the rate of 3mg/kg, 5mg/kg, 7mg/kg in group DEX-1, DEX-2 and DEX-3 respectively. The broilers were fed starter feed for the first 14 days, and then shifted to grower feed for the rest of the experiment. A constant supply of an adequate amount of feed and fresh drinking water was ensured. Standard rearing conditions were maintained throughout the experiment [19].
Gross morphology and morphometry
Five broilers from each group were sacrificed manually on each sampling day (7, 14, 21, and 28 days of the experiment) by the cervical subluxation method. Duodenum and right cecum were collected from each broiler immediately after dissection. Color, weight, length, and width of the cecum were considered for the gross morphologic and morphometric study. The color of the duodenum and cecum was compared between the Non-DEX and DEX groups by visual inspection. Weight (gm) was measured using a high precision balance (FGH Series, AND Company Limited, Korea). The length and width were measured by a graded scale (cm). The width of the cecum was measured from the mid-region of the blind sac.
Histopathology
About one cm of the duodenal and cecal segments (from blind sac) were collected from each broiler and fixed in 10% buffered formalin and then routinely processed into slides with Hematoxylin and Eosin (H&E) stain according to the protocol described in the earlier study [15]. For this, the formalin-fixed tissues were dehydrated in the ascending grades (70%, 80%, 90%, 100% – I, II, and III) of alcohol followed by clearing in three changes in xylene. Then, the tissues were infiltrated with three different grades of melted paraffin (49 °C, 55 °C, and 58 °C) for 30 minutes each. The tissues were then embedded in paraffin (58 °C). Finally, 5 µm thin sections were cut using a sliding microtome (MIC 509, Euromex, Japan). The cut sections were floated in a warm (37°C) water bath for stretching followed by mounting on the adhesive (50% egg albumin and 50% glycerol) painted glass slides. Then the tissue sections were dried on a slide warmer (37°C).
All the stained tissue sections were examined and analyzed blindly to avoid any biases. The histomorphological attributes of the duodenal and cecal tissues were studied under a light microscope (Leica DMR; Leica Microsystems, Wetzlar, Germany) at 100X and 400X magnifications. Length (measurements were taken from the tips of the villi to the villi-crypt junctions) and width (measured from the mid-region of the villi) of duodenal villi, mucosal thickness, and diameter of lymphatic nodules of cecum were measured in micrometer (µm) using a calibrated stage micrometer. The surface area of the villi was calculated using the following formula- ; where π = 3:1416 [22]. The number of intact and degenerated lymphatic nodules in the cecum was counted and the percentage was calculated. Measurement of all the variables was done from 10 randomly selected focuses at 100X magnification.
Necessary photographs were captured from ten randomly selected focuses at 100X and 400X magnifications for better illustration of the obtained results. All the images were captured by photomicroscope (Model: CX41U-LH50HG, Olympus Corporation, Tokyo, Japan).
Statistical analyses
All the data obtained in this study were analyzed using IBM SPSS Statistics 22. Differences among the groups of birds were compared using one-way ANOVA with posthoc Duncan’s multiple range test where P < 0.05 was considered significant. In all trials, data were expressed as mean ± standard error of the mean (SEM).
RESULTS
Gross morphologic and morphometric profile of duodenum
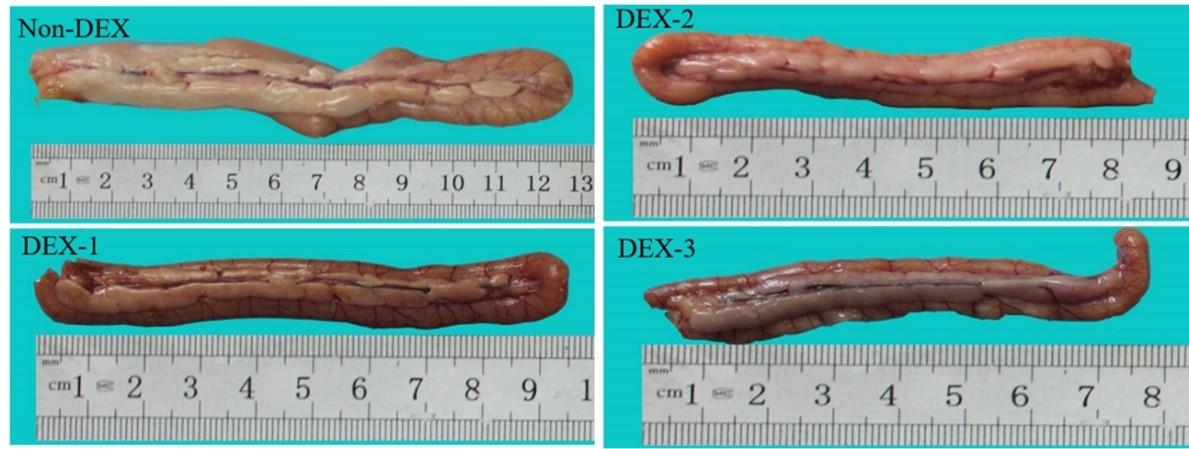
The gross attributes and alterations of the duodenum are shown in Figure 1. The duodenum of the Non-DEX group appeared light greyish color whereas the DEX treated groups revealed slightly darker color with a reddish tinge.
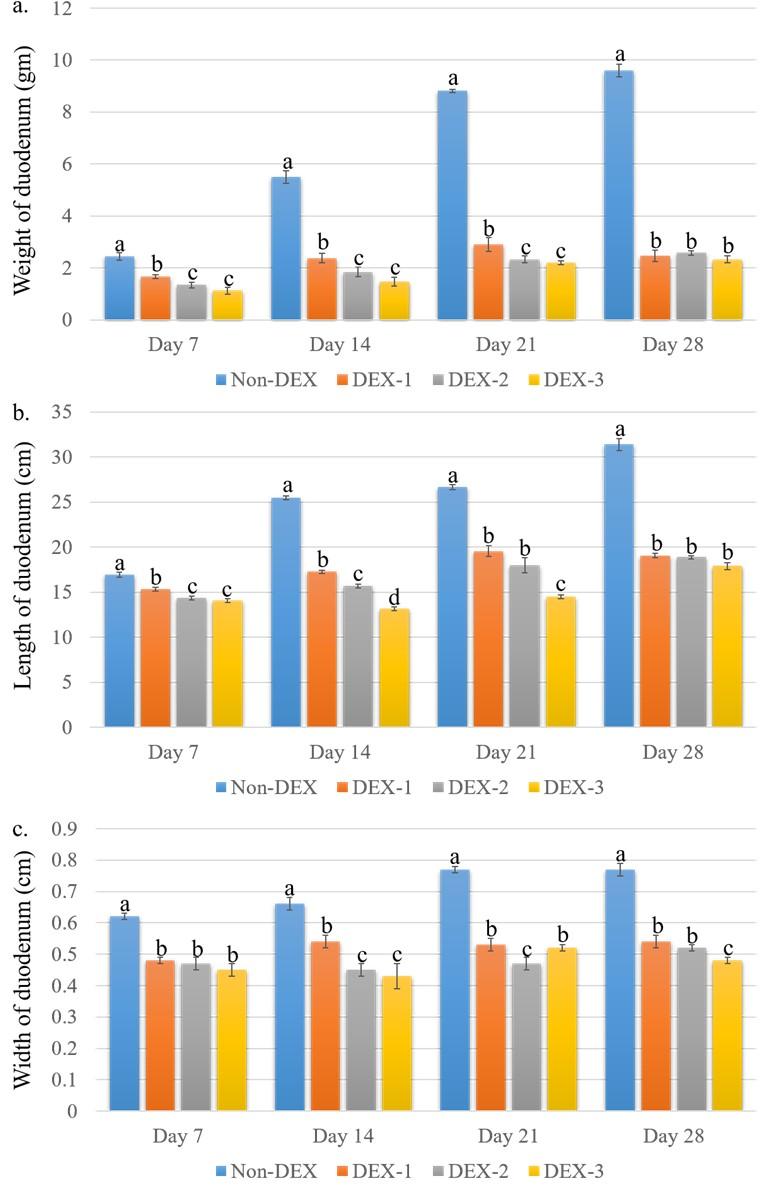
The gross morphometric parameters of the duodenum are shown in Figure 2. The weight of the duodenum was significantly (P < 0.05) less in the DEX treated groups compared to the Non-DEX group. Among the DEX treated groups, the highest weight was found in the DEX-1 group and the lowest weight was found in the DEX-3 group on all days of the experiment. Similarly, the length and width were also significantly (P < 0.05) less in the DEX treated groups. On day 7, the length was found significantly (P < 0.05) less in the DEX-2 and DEX-3 groups compared to the Non-DEX group. The highest length was found in the DEX-1 group and the lowest length was seen in the DEX-3 group. On day 28, there was no significant (P > 0.05) difference in length between the DEX groups. There was a substantial (P < 0.05) difference in width on day 7 among the DEX treated groups. However, a significant (P < 0.05) difference in width was found from days 14 to 28 among the DEX treated groups.
Histopathological profile of duodenum
The histo-architecture of the duodenum of Non-DEX and DEX treated groups is shown in Figure 3. The Non-DEX group revealed general histological architecture. However, the DEX treated groups showed marked microscopic alterations, especially in the duodenal glands (crypt of Lieberkuhn) and villi. In the DEX-1 group, the intestinal glands showed no alterations though they started to degenerate in the DEX-2 group. In the DEX-3 group, almost all the intestinal glands were degenerated, resulting in the reduction of the intestinal gland population.
The histomorphometric data of the duodenum are shown in Table 1. The histomorphometric study revealed that both the length and width of duodenal villi were significantly (P < 0.05) less in the DEX groups as compared to the Non-DEX group. However, among the DEX treated groups, the highest length and width of duodenal villi was found in the DEX-1 group and the lowest value was found in the DEX-3 group. The surface area of the villi was also substantially (P < 0.05) decreased in the DEX treated groups which were also dose dependent.
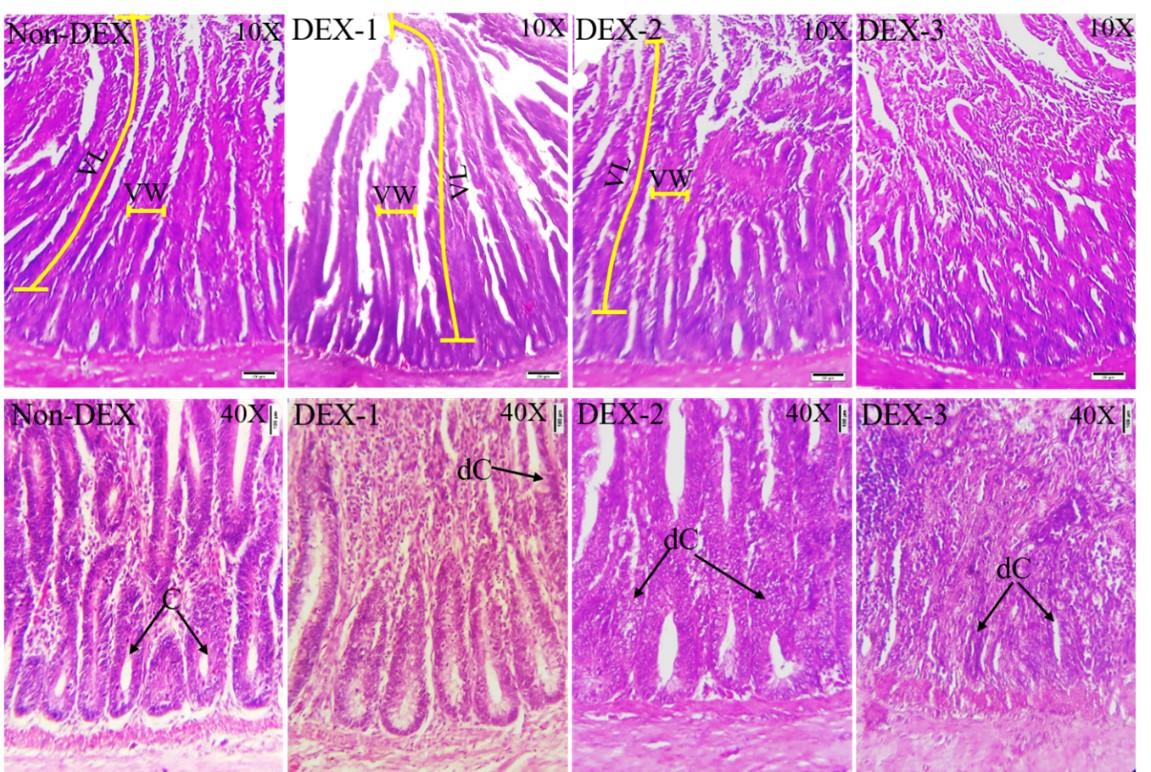
Table 1. Histomorphometric data on duodenal villi length, width, and surface area, in Non-DEX and DEX groups.
Gross morphologic and morphometric profile of cecum
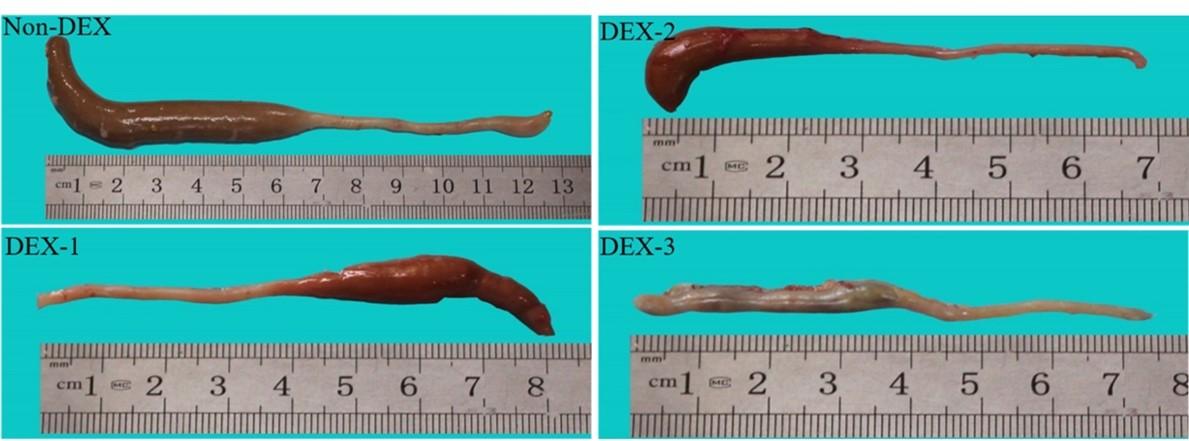
The gross attributes and alterations of the cecum are shown in Figure 4. The cecum was a paired organ with a lower diameter at the origin but gradually increased in size until it formed a blind end terminally. The gross morphometry distinguished three distinct regions in the cecum. The cecum of the Non-DEX group was greenish-grey in color whereas the cecum of the DEX treated groups was greyish color with a slight reddish tinge.
The weight of the left cecum in an individual group of broilers on different days of the experiment is shown in Figure 5. The weight was significantly (P < 0.05) less in the DEX treated groups as compared to the Non-DEX group. Significant (P < 0.05) differences among the DEX treated groups were seen only on day 28 where the DEX-2 group was significantly (P < 0.05) different from the DEX-1 and DEX-3 groups. The length and width of the left cecum in the individual group of broilers on different days of the experiment are shown in Figure 5. Both the length and width were significantly (P < 0.05) less in the DEX treated groups as compared to the Non-DEX group. There were also significant (P< 0.05) differences among the DEX treated groups.
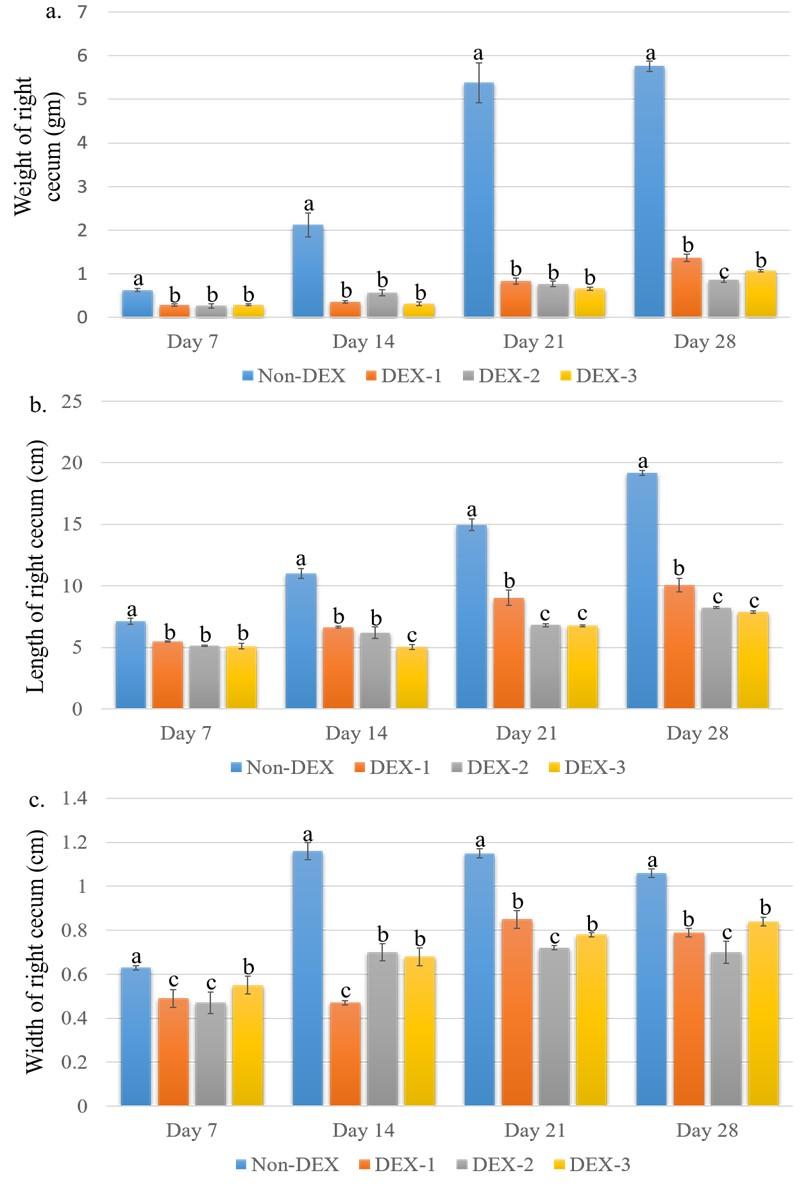
Histopathological profile of cecum
The histo-architecture of the cecum of Non-DEX and DEX treated groups is shown in Figure 6. The Non-DEX group revealed general histological architecture. On the other hand, marked disruption of mucosal epithelial cells was seen in all the DEX treated groups. Mucosal degeneration was also noticed which was more extensive in the high dose groups. Degeneration of mucosa started from the tip of the mucosal layer to the base. The lymphatic nodules showed degenerative changes in the treatment groups from day 7 which increased afterward. Degenerative changes were increased significantly in the high-dose groups. On day 28, the histological architecture was almost completely lost in the DEX-3 group. The thickness of the muscular layer also decreased in the DEX treated broilers. Some extent of degeneration in the muscular layer was also seen.
The histomorphometric data of mucosal height, the greater and lesser diameter of lymphatic nodules are shown in Table 2. Mucosal height was significantly (P < 0.05) less in the DEX treated groups as compared to the Non-DEX group. Both the greater and lesser diameter of the lymphatic nodules was also significantly (P < 0.05) less in the DEX treated broilers. There were also significant (P < 0.05) differences among the DEX treated groups as the values were decreased gradually with the increased dose of DEX.
The percentage of degenerated lymphatic nodules is shown in Figure 7. The percentage was increased with the progression of both age and DEX dose. The maximum percentage (92.31%) of degenerated lymphatic nodules was seen on day 28 in the DEX-3 group.
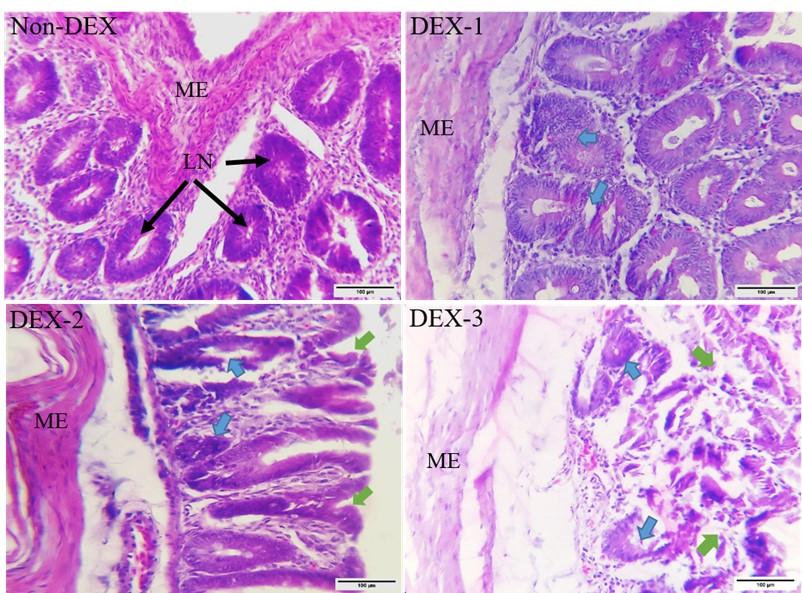
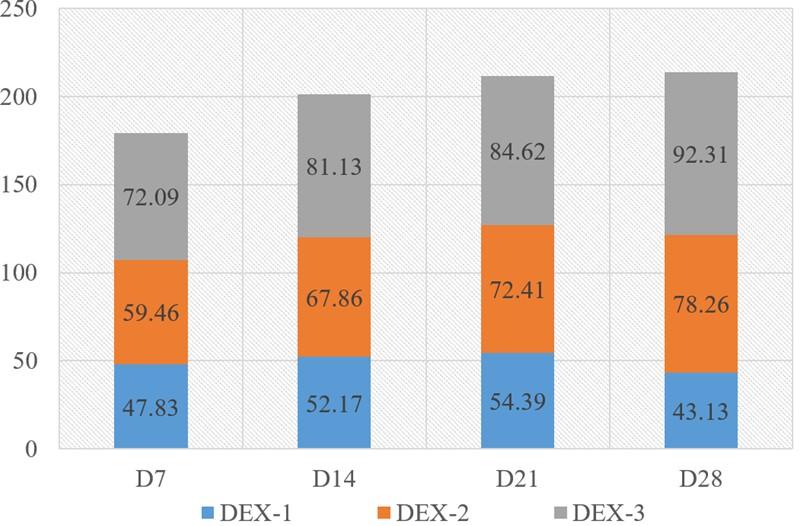
Table 2. Histomorphometric data on mucosal height and diameter of lymphatic nodule in control and DEX groups.
DISCUSSION
Any sort of stress activates different biological mechanisms in the body in order to maintain homeostasis and physiological activities. As the broilers are reared for massive meat production purposes, they are constantly exposed to different stresses, i.e. climate change, heat, high stock density, nutritional constraints, etc. during their very short lifetime [9, 10, 23]. Exposure to these stressors activates the physiological stress response through activation of the HPA axis which leads to a rise in the level of the corticosteroid, a stress hormone [12, 13, 14]. DEX administration induces oxidative stress in poultry and thus can mimic the adverse effects of increased levels of natural corticosteroids [12, 24]. DEX had been used to induce stress in broilers in the previous studies which demonstrated that it could exert effects homologous to the natural corticosteroids secreted while exposed to different stresses [12, 13, 14, 24, 25]. The morphology and morphometric attributes of the intestine possess a significant role in maintaining gut health, nutrient digestibility, and ultimately in the growth performance of the broiler. Height and width of villi, the thickness of the mucosa, crypt depth, etc. are commonly measured to assess gut digestibility and nutrient absorption [4, 27]. In the current study, we induced stress in broiler chickens with dietary DEX at three different doses to investigate the adaptations in the intestinal morphology and morphometry in the DEX exposed broilers.
In the current study, we found that the color of the DEX-1 and DEX-3 groups are quite different from the Non-DEX group where the color of the DEX-1 group was found quite darker. The findings of gross morphometric measurements of the Non-DEX group on different days of the experiment are also almost similar to the earlier report [2]. However, in the DEX groups, all the gross morphometric parameters were decreased significantly in the DEX treated groups compared to the Non-DEX group. Intestinal sizes are closely related to body sizes [28]. Dietary DEX reduces the growth performance and weight gain in the broiler [12]. The previous study reports also suggest that corticosteroids like DEX negatively affect organ weight gain and reduce organ size [15, 20, 29]. However, the effects of DEX on intestinal volume and weight gain had not been documented previously. Feed efficiency and the growth of broilers greatly depend on the enzymatic activities in the duodenum and the diversified microbial community that inhabits the caeca. Dietary lipid particles are mostly digested and emulsified in the duodenum by pancreatic enzymes and hepatic bile acids [30]. Nutrients, such as undigested starch, protein, and fiber enter the caeca bypassing the small intestine [6]. The blind end of the cecum helps to retain the digesta for longer periods and thus enhances nutrient absorption [30, 31, 32]. So, the reduced intestinal size may adversely affect the overall growth rate of the broiler.
In the histomorphological study, the duodenum and cecum of the Non-DEX groups on different days of the experiment revealed general histological characteristics as described in the previous studies [2, 8]. On the other hand, significant alterations were seen in the DEX treated groups. The length of the villi was found significantly less in the DEX treated groups which matches the findings of the previous study report [7]. The length of the villi is one of the prominent indices of the intestine’s capacity to absorb dietary nutrition [26]. The reduction of the surface area of the villi might also explain the lower weights of the duodenum and cecum due to reduced nutrient absorption. The crypts of Liberkuhn lie between the intestinal villi and assist in the protein digestion as well as protection of the host from enteric pathogens [32]. According to earlier studies, CORT decreases the digestion of protein and carbohydrates [10, 28]. Crypt depth is considered an indicator of intestinal epithelium maturation [26]. CORT treatment reportedly slows down intestinal epithelial cell proliferation and thus decreases intestinal villi length and crypt depth which impairs nutrient absorption in the intestine of broilers [26]. In the current study, the crypts have almost degenerated in the higher dose group which may affect the overall gut health and performance.
Marked disruption of the mucosal surface epithelial cells along with mucosal degeneration was seen in the cecum of DEX treated broilers. Continuity of the intestinal lining epithelium is crucial for maintaining intestinal permeability which is mainly regulated by tight junction distribution and integrity. The epithelial cells overlying the mucosa are derived from progenitors like stem cells residing within the crypt [33]. The undifferentiated epithelial cells exit the base of the crypt and migrate through the lumen and mature into highly specialized absorptive enterocytes [34]. If the mucosal epithelial cells get damaged or destroyed due to adverse conditions in the intestine, the stem cells located in the crypt repair the epithelial layer by reproducing new undifferentiated epithelial cells [35]. So, in case of degeneration or loss of crypt, the damaged villous epithelial layer can’t regenerate which may greatly alter the intestinal functionality. The extent of intestinal nutrient uptake is modulated not only by the digestive secretions but also by the absorptive surface area of the intestine [4]. So, the integrity of the mucosal layer is crucial for proper intestinal function and thus the mucosal morphological features correspond with increased feed efficiency and growth rate in broiler [36]. Histomorphometric investigation showed that the mucosal height declined significantly in the treated broilers. This finding is similar to the results reported by the earlier studies [13, 21]. The major role of the caeca is to separate the intestinal contents into a nutrient-rich fluid fraction that enters the caeca for digestion and absorption [6]. The villi of the mucosa extend into the lumen of the intestine and thus increase the absorptive surface area [35]. So, the decrease of the mucosal surface area will lead to the reduction of absorptive surface area in the cecum which may negatively affect the feed efficiency and ultimately the growth rate and health of the broiler.
Besides these, the degeneration and loss of lymphatic nodules were also seen in the current study. Lymphatic nodules are among the gut-associated lymphoid tissues in poultry, which is one of the main components of the lateral immune system. These gut-associated lymphoid tissues react with the gut microflora and play a pivotal role in controlling the incidence of poultry enteric disorders through its immunological functions [37]. Hence, the structural integrity of lymphatic nodules is crucial for proper functioning and achieving optimal production performance through digestion, absorption of nutrients, and immunity.
The thickness of the muscularis layer was also seen to be decreased in the treated broilers with scattered degenerative changes. DEX decreases protein synthesis, as well as increases protein catabolism and proteolytic activities in the muscularis layer leading to muscular atrophy [38]. However, the exact mechanism of the muscle degeneration and reduction of the thickness of the muscularis layer of the broiler cecum is ambiguous.
CONCLUSIONS
Looking at an overview of the obtained results from the current study, it is evident that DEX treatment induces stress in broilers which significantly affects the morphology and morphometry of the broiler intestine. The adverse effects of DEX induced stress on the intestinal mucosa, glands, and lymphatic nodules may lead to impaired digestion of food particles, reduced absorption of nutrients, and diminished gut immunity which may affect the health and production of broiler. However, further study is recommended to investigate the enzymatic activity in the duodenum as well as the microbial population in the cecum of stressed broilers induced by dietary DEX.
ACKNOWLEDGMENTS
We appreciate the research assistance from the Department of Parasitology, Faculty of veterinary science, Bangladesh Agricultural University, Bangladesh. We also acknowledge the technical support from the Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Bangladesh.
AUTHOR CONTRIBUTIONS
NS conceptualized and supervised the experiment. RI performed the experiment. RRD assisted in the sample collection and gross data recording. A provided research assistance for histomorphometric data collection. SB and RI analyzed the data and interpreted the results. NS and RI drafted the manuscript. MRI and ZH critically revised and edited the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work in ensuring that questions relating to the accuracy or integrity of any part of the work.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Godfray HC, Crute IR, Haddad L, Lawrence D, Muir JF, Nisbett N, et al. The future of the global food system. Philos. Trans R Soc B Biol Sci. 2010; 365: 2769-2777.
- [2]Tan Z, Luo L, Wang X, Wen Q, Zhou L, Wu K. Characterization of the cecal microbiome composition of Wenchang chickens before and after fattening. PloS One. 2019; 14: e0225692.
- [3]Nain S, Renema RA, Zuidhof MJ, Korver DR. Effect of metabolic efficiency and intestinal morphology on variability in n-3 polyunsaturated fatty acid enrichment of eggs. Poult Sci. 2012; 91: 888–898.
- [4]Diaz Carrasco JM, Casanova NA, Fernández Miyakawa ME. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019; (10): 374.
- [5]Svihus B, Choct M, Classen HL. Function and nutritional roles of the avian caeca: a review. World’s Poult Sci J. 2013; 69: 249-264.
- [6]Carvalho D, Herpich JI, Chitolina GZ, Gava MS, Moraes LB, Furian TQ, et al. Characterization of immune and enteric systems of broilers after immunosuppression with dexamethasone. Acta Sci Vet. 2018; 46: 1606.
- [7]Majeed MF, Al-Asadi FS, Nassir AA, Rahi EH. The morphological and histological study of the cecum in broiler chicken. Bas J Vet Res. 2009; 8: 19-25.
- [8]Calefi AS, Quinteiro-Filho WM, Ferreira AJ, Palermo-Neto J. Neuroimmunomodulation and heat stress in poultry. World’s Poult Sci J. 2017; 73: 493-504.
- [9]Puvadolpirod S, Thaxton JP. Model of physiological stress in chickens 4. Digestion and metabolism. Poult Sci. 2000; 79: 383-390.
- [10]Scanes CG. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult Sci. 2016; 95: 2208-2215.
- [11]Ademu LA, Erakpatobor-Iyeghe GT, Barje PP, Daudu OM, Wafar RJ. Response of broiler chickens under dexamethasone induced stress conditions. Asian J Res Anim Vet Sci. 2018; 1: 1-9.
- [12]Berenjian A, Sharifi SD, Mohammadi-Sangcheshmeh A, Bakhtiarizadeh MR. Omega-3 fatty acids reduce the negative effects of dexamethasone-induced physiological stress in laying hens by acting through the nutrient digestibility and gut morphometry. Poult Sci. 2021; 100: 100889.
- [13]Mehaisen GM, Eshak MG, Elkaiaty AM, Atta AR, Mashaly MM, Abass AO. Comprehensive growth performance, immune function, plasma biochemistry, gene expressions and cell death morphology responses to a daily corticosterone injection course in broiler chickens. PLoS One. 2017; 12: e0172684.
- [14]Sultana N, Amin T, Afrose M, Rony SA, Rafiq K. Effects of Dexamethasone on the Morphometry and Biometry of Immune Organs of Broiler Chicken. Int J Morphol. 2020a; 38: 1032-1038.
- [15]Yang J, Liu L, Sheikhahmadi A, Wang Y, Li C, Jiao H, et al. Effects of corticosterone and dietary energy on immune function of broiler chickens. PLoS One. 2015 10: e0119750.
- [16]Akter F, Sultana N, Afrose M, Kabir A, Islam R, Sikder MH. Adaptations of muscular biology in response to potential glucocorticoid treatment in broiler chicken. J Adv Biotechnol Exp Ther. 2021; 4: 1-8.
- [17]Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: mechanisms. Nat Rev Endocrinol. 2014; 10: 403-411.
- [18]Sultana N, Islam R, Akter A, Ayman U, Bhakta S, Rony SA, et al. Biochemical and morphological attributes of broiler kidney in response to dietary glucocorticoid, dexamethasone. Saudi J Biol Sci. 2021; 28: 6721-6729.
- [19]Sultana N, Afrose M, Rafiq K. Effects of steroid growth promoter on morphological and biochemical adaptations in liver of broiler. Vet World. 2020b; 13: 2330-2337.
- [20]Lima MBD, Gama LA, Hauschildt AT, Dall’Agnol DJR, Corá LA, Americo MF. Gastrointestinal motility, mucosal mast cell, and intestinal histology in rats: effect of prednisone. Biomed Res Int. 2017; 2017: 1-8.
- [21]Moghaddam HN, Alizadeh-Ghamsari AH. Improved performance and small intestinal development of broiler chickens by dietary L-glutamine supplementation. Journal of Appl Anim Res. 2013; 41: 1-7.
- [22]Yin LY, Wang ZY, Yang HM, Xu L, Zhang J, Xing H. Effects of stocking density on growth performance, feather growth, intestinal development, and serum parameters of geese. Poult Sci. 2017; 96: 3163-3168.
- [23]Vahdatpour T, Nazer Adl K, Ebrahim Nezhad Y, Mahery Sis N, Riyazi SR, Vahdatpour S. Effects of corticosterone intake as stress-alternative hormone on broiler chickens: performance and blood parameters. Asian J Anim Vet Adv. 2009; 4: 16-21.
- [24]Hu X, Guo Y. Corticosterone Administration Alters Small Intestinal Morphology and Function of Broiler Chickens. Asian-Australasian J Anim Sci. 2008; 21: 1773–1778.
- [25]Li Y, Cai HY, Liu GH, Dong XL, Chang WH, Zhang S, et al. Effects of stress simulated by dexamethasone on jejunal glucose transport in broilers. Poult Sci. 2009; 88: 330-337.
- [26]Berrocoso JD, Kida R, Singh AK, Kim YS, Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult Sci. 2017; 96: 1573-1580.
- [27]Nasrin M, Siddiqi MNH, Masum MA, Wares MA. Gross and histological studies of digestive tract of broilers during postnatal growth and development. JBAU. 2012; 10: 69-77.
- [28]Metzler-Zebeli BU, Magowan E, Hollmann M, Ball ME, Molnár A, et al. Differences in intestinal size, structure, and function contributing to feed efficiency in broiler chickens reared at geographically distant locations. Poult Sci. 2018; 97(2): 578-591.
- [29]Tschanz SA, Makanya AN, Haenni B, Burri PH. Effects of neonatal high-dose short-term glucocorticoid treatment on the lung: a morphologic and morphometric study in the rat. Pediatr Res. 2003; 53: 72-80.
- [30]Bauer E, Jakob S, Mosenthin R. Principles of physiology of lipid digestion. Asian-Australas J Anim Sci. 2005; 18: 282-295.
- [31]Hunt A, Al-Nakkash L, Lee AH, Smith HF. Phylogeny and herbivory are related to avian cecal size. Sci Rep. 2019; 9: 1-9.
- [32]Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013; 75: 289-311.
- [33]Barker N, Van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007; 449; 1003-1007.
- [34]Edelblum KL, Turner JR. Epithelial Cells: Structure, Transport, and Barrier Function. Mucosal Immunol. 2015; 1: 187-210.
- [35]Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009; 337: 1-35.
- [36]Yamauchi KE. Review on chicken intestinal villus histological alterations related with intestinal function. J Poult Sci. 2002; 39: 229-242.
- [37]Callaway TR, Edrington TS, Anderson RC, Harvey RB, Genovese KJ, Kennedy CN, et al. Probiotics, prebiotics, and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev. 2008; 9: 217-225.
- [38]Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013; 45: 2163-2172.