Antimicrobial and phytochemical screening of selected wild mushrooms naturally found in Garhwal Himalayan region, Uttarakhand, India
Abstract
Natural products contain several ingredients that can treat number of ailments. Due to the increase in antibiotic-resistant microorganisms, natural resources are being looked at as an alternative source to combat harmful microbes. This can reduce the effects on harmful microbes by obtaining antibacterial compounds derived from natural resources. The aim of present study is to explore some new potent varieties of unexplored wild mushroom species to investigate their effects on microbial activity. In this study hexane, chloroform, methanol, 70% ethanol, and hot water extracts of Cantharellus cibarius, Phellinus pectinatus, Laccaria laccata, Trametes versicolor, and Gloeophyllum sepiarium were tested for antibacterial activity against nine bacterial strains namely Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aerouginosa, Acinetobacter baumannii, Pseudomonas fluorescens, Enterobacter aerogenes, Proteus mirabilis by disk diffusion method. The present study showing that Phellinus pectinatus and Gloeophyllum sepiarium mushroom species methanol and Ethanol extracts are most active against Bacillus subtilis, Klebsiella pneumoniae, Acinetobacter baumannii, Enterobacter aerogenes and Pseudomonas aerouginosa bacterial strains. The present study reveals important secondary metabolites compounds including alkaloids, flavonoids, carbohydrates, glycosides, etc. were present in wild mushroom extracts. Out of 5 extracts, methanol and ethanol extract have been shown a great potential as antimicrobial secondary metabolites compared to other extracts. The result of present research is expressing the high potency of extracts to stop the growth of bacteria and this extract can be further suggested for medical utilizations and could be used as natural antimicrobial source.
INTRODUCTION
Unlike other fungi, mushrooms (macrofungi) have a large fruiting body that is visible by naked eyes. Some mushrooms are edible, while others are non-edible. The nutritional value of some mushrooms makes them functional foods, while other mushrooms have been used expansively in traditional medicament, and as a source of development of drugs and nutritious medicinal substances [1].
There are approximately 0.14 million mushroom species worldwide, 14,000 of which are known species, 7000 of which are edible, 20,000 of which are protected, and 700 of which are said to have substantial pharmacological capabilities. A wide range of medicinal and sanitary properties are found in wild mushrooms viz., antibacterial, antifungal, antiviral, antiparasitic, antioxidant, anticancer, anti-inflammatory, anti-HIV, antitumor, antidiabetic, cytotoxic, anticoagulant, hepatoprotective, hypocholesterolemic, antiproliferative [2,3].
The fruiting body of mushrooms contains various types of bioactive compounds like terpenoids, steroids, flavonoids, polyketides, alkaloids, dietary fibers, polyphenol, and polysaccharides (especially β-glucans). Mushrooms contain highly healthy most valuable nutritional compounds such as proteins, minerals, vitamins (vitamins B complex, vitamin C and D2), true elements, as well as low calories, low fat, and limited amounts of cholesterol [4].
The development of new drugs or finding natural products to support antibiotics has become essential since antimicrobial resistance has spread around the globe. According to a World Health Organization report, antibacterial resistance is a threat to the prevention and treatment of infections caused by microbes. A real threat to society is the development of resistant strains such as Staphylococcus aureus, Klebsiella pneumonia, and Escherichia coli.
The threat of infectious diseases has become a significant issue for public health worldwide. In recent years, antibiotics have proven to be very valuable in treating infections caused by a variety of pathogens. In the meantime, there is increasing resistance to conventional antibiotics, contributing to decreased morbidity and mortality, extended hospital stays, and higher hospital charges [5]. Mushrooms are among the natural resources that have been exploited in the past years and might serve as a source of new antimicrobials.
Prophylactic and therapeutic use of antimicrobials has been around for decades. A major clinical problem in treating infectious diseases has been the resistance of microorganisms to antibiotics. The goal of the present study must look at the antibacterial activities of five wild mushrooms found in Uttarakhand Himalayan, namely Cantharellus cibarius, Phellinus pectinatus, Laccaria laccata, Trametes versicolor, and Gloeophyllum sepiarium. The present result in this article and some of the investigation may help to guide future investigation to discover new compounds that may be safe, effective, and potent in fighting microbes.
MATERIALS AND METHODS
Collection of specimens
Five species of wild mushrooms Cantharellus cibarius, Phellinus pectinatus, Laccaria laccata, Trametes versicolor, and Gloeophyllum sepiarium were obtained from diverse local woodlands in the Himalayan region Uttarakhand. Several trips were made to the Uttarakhand Himalayas between July 2019 and January 2020 to collect fresh fruiting bodies of these mushrooms, the morphology characteristics were observed and recorded in the field. For further investigation, the mushrooms were wrapped in paper bags and brought to the laboratory for extraction. The identification was also done through the available field guide of macrofungi, monographs, review of relevant literature like Adhikari, 2000; Vishwakarma et al., 2011; Bhatt et al., 2018; Singha et al., 2020; Khadka and Aryal, 2021 and available web resources mycokey.com, MykoWeb, www. mushrooms, etc. and identified based on macro– morphological [6]. All the identified specimens have been submitted to SGRRU Patel Nagar Microbiology Department in Dehradun, Uttarakhand, India, for further examination (Figure 1-2, and Table 1).

Table 1. Summary of wild mushrooms selected from Garhwal Himalayan region.
Extraction of mushroom specimens
The powders of each mushroom were extracted sequentially in hexane, chloroform, methanol, 70%, ethanol, and hot water. For this, the material was taken in a conical flask and the flask was covered with aluminum foil. It was then left in a rotary shaker for 72h. So that the contents can be mixed well. After complete extraction, the extracts were centrifuged at 3000 rpm for 15min. After that, the extract was filtered with the help of Whatman no 1 filter paper. Rotary evaporation was then used to evaporate and dry the extract. After drying the extract was stored at 4°C [7,8] as shown in Figure 3 and Table 2).
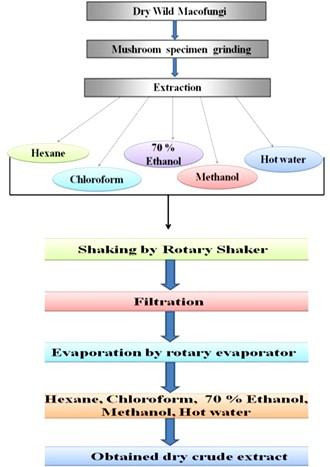
Table 2. Solvents used for extraction: physico-chemical properties [9].
Test strains
A total number of nine bacterial strains, including Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aerouginosa, Acinetobacter baumannii, Pseudomonas fluorescens, Enterobacter aerogenes, Proteus mirabilis. were used in this study. These strains are obtained from the Department of Microbiology, SGRR School of Basic & Applied Sciences, SGRR University Dehradun, Uttarakhand, India.
Evaluation of antimicrobial activity
Antimicrobial testing was conducted by the disc diffusion method on sterilized Mueller Hinton Agar medium (MHA). Luria Bertani broth was prepared and taken up to 5 ml in each culture tube and followed by autoclave at 121°C for 15 min. Each tube containing the LB broth were then inoculated separately with selected bacterial strains. Turbidity of 0.5 McFarland standard 1 ×10 8 CFU was obtained after 24 h of incubation at 37°C. On each Mueller Hinton agar (MHA) plate, the bacterial suspension (100 µl) containing 1 ×10 8 CFU/ml was poured, respectively. Then, Whatman filter paper (6 mm in diameter) was impregnated with 100 µl of each mushroom extract and placed them evenly on the surface of Mueller-Hinton agar plate. All the plate is incubated at 37°C for 24 – 48 hours. After the incubation period, the diameter of a well-defined inhibition zone was measured by ruler [10]. Streptomycin was used as positive control.
Qualitative phytochemical analysis
The most active extracts methanol and ethanol were used for Phytochemical analysis such as alkaloids, steroids, glycosides, flavonoids, carbohydrates, etc. The procedure described by [11-13] was followed.
Test for alkaloids
Mayer’s test: A few drops of Mayer’s reagent were added to 5ml of the extract on the side of the test tube. Positive findings were observed in the form of a white creamy precipitate.
Dragendorff’s test: 1 or 2 mL of Dragendorff’s reagent were added to 5 mL of mushroom extract. Positive results are denoted by a conspicuous orange and yellow precipitate.
Wagner’s test: 1-2 drops of Wagner’s reagent are added to 5 ml of the extract by the side of a test tube. The reddish-brown precipitate ensures the test is positive.
Test for terpenoids: 4-5 ml of extract is taken in a test tube. After that 2 ml of chloroform is added and again conc. sulfuric acid is carefully poured through the side of the test tube. The presence of terpenoids is indicated by a reddish-brown color.
Test for steroids: (a) 2 ml of chloroform was added to the extract and concentrated sulfuric acid was poured through the side of the test tube. Red color formation on the lower surface indicates the presence of steroids. (b) In two ml of chloroform, two ml of concentrated sulfuric acid, and two ml of acetic acid, were added to the extract. The presence of green color indicates the steroids.
Test for glycosides
Libermann Burchard’s test: 2ml chloroform and 2 ml acetic acid were added to the extract. after that, the mixture was cooled in the ice. After cooling, sulfuric acid was added to it. A change in color from violet to blue and from blue to green indicates the presence of glycosides.
Keller-Killiani test: One milliliter of glacial acetic acid, a few drops of ferric chloride solution, and concentrated sulfuric acid are slowly added to the extract from the sides of the test tube. The presence of a glycoside is indicated by a reddish-brown ring at the interface.
Test for flavonoids
Ferric chloride test: A few drops of 5% ferric chloride are added to 2-3ml of the extract. the presence of dark green color indicates flavonoids.
Alkaline reagent test: A few drops of sodium hydroxide are added to the test solution. if after this a dark yellow color is formed, then a few drops of dilute acetic acid are added, after which it becomes colorless, which indicates the presence of flavonoids.
Test for phenolic compounds
Ferric chloride test: A few drops of 5% ferric chloride are added to 2-3ml of the extract. the presence of bluish-black precipitate indicates phenolic compounds.
Lead acetate test: A few drops of 10% lead acetate reagent are added to the test solution. The presence of white precipitates indicates the presence of phenolic compounds.
Test for carbohydrates
Fehling’s test: Reagents fehling’s A and fehling’s B are mixed with each other in equal amounts and then added to1- 2 ml of extract and boiled slowly. The presence of a brick red color indicates the reducing sugar.
Benedict’s test: The extract is treated with 1-2 ml of benedicts reagent and then gently heated. The presence of reducing sugar is indicated by the formation of an orange-red precipitate.
Molisch’s test: A few drops of α – naphthol are added to 2ml of extract. After that two ml of concentrated sulfuric acid is added slowly from the sides of the test tube. The formation of a purple ring at the junction indicates the presence of carbohydrates.
Barfoed’s test: A sample of 2 ml extract is treated with 1 ml of barfoed’s reagent and then heated. Precipitate with a red-orange color indicates the presence of non-reducible sugar.
Test for proteins (amino acids)
Ninhydrin test: The solution is treated with a few drops of Ninhydrin reagent. The presence of blue color signifies a positive result.
Millon’s test: A few drops of Millon’s reagent are added to a 2 ml extract. The presence of proteins is shown by a white precipitate.
Xanthoproteic test: A few drops of concentrated nitric acid added to the extract. Yellow coloration indicates the presence of protein.
Test for saponins
Foam test: 1 ml of extract is boiled with 5-6 ml distilled water and after that, it is shaken rapidly. The formation of foam indicates the presence of saponins.
Test for organic acid
Malic acid test: Two to three drops of 40% FeCl are added to the test solution. The presence of yellow color indicates organic acids.
Test of inorganic acid
Carbonate test: for test solution add dilute HCL. If CO gas is liberated it indicates the presence of carbonate.
Statistical analysis
All tests were carried out in a triplicate manner. Means ± standard deviations (SDs) are used to represent experimental results. IBM SPSS 2019 software was used for the statistical analysis.
RESULTS
Effect of mushrooms extract on microbial activity
A total of five wild mushrooms species were taken to examine their effects on microbial activity.
Significant microbial activity was shown by five wild mushrooms Cantharellus cibarius, Phellinus pectinatus, Laccaria laccata, Trametes versicolor, and Gloeophyllum sepiarium (Table 3-7, and Figure 4). For this, the extracts of these mushrooms were extracted in different solvent systems viz. Hexane, chloroform, methanol, 70% ethanol, and hot water. After that, the microbial activity of mushroom extracts was observed against nine bacterial strains respectively Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aerouginosa, Acinetobacter baumannii, Pseudomonas fluorescens, Enterobacter aerogenes, Proteus mirabilis.
Out of five extracts of Cantharellus cibarius species of mushroom, ethanol showed higher antimicrobial potency. In second place chloroform and methanol recorded moderate antimicrobial potency. Other solvents showed little or negative antimicrobial efficacy. Maximum 29.6 ±1.72 mm and 29.6±1.45mm zone of inhibition was recorded against the Staphylococcus aureus and Klebsiella pneumoniae in the ethanol solvent. A minimum zone of inhibition was observed in Escherichia coli, Pseudomonas aeruginosa in the hexane solvent system. In addition, chloroform and ethanol solvent systems showed low inhibition zones against Pseudomonas fluorescens.
Mushroom Phellinus pectinatus was also extracted with a five solvent system Hexane, chloroform, methanol, 70% ethanol, and hot water respectively. The highest effect of mushroom extracts on microbial activity was observed in ethanol and methanol solvents of Phellinus pectinatus. After these, the moderate effect on microbial activity was recorded in hexane, chloroform, and hot water. Hexane, chloroform, 70% ethanol, methanol, and hot water showed the highest activity against Klebsiella pneumoniae among all solvent systems. Hexane, 70% ethanol, methanol, and hot water showed a high zone size i.e 22 ±1.13mm, 16.6±1.45 mm, 28 ±2.26mm, 26.3±1.72mm, respectively against Acinetobacter baumannii. The lowest activity was observed against Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Enterobacter aerogenes. No activity was recorded in Pseudomonas aeruginosa, Pseudomonas fluorescens and Proteus mirabilis.
High antimicrobial potency against Pseudomonas aeruginosa was observed in the methanol and hot water extract of Laccaria laccata also recorded a good zone of inhibition against Acinetobacter baumannii in ethanol and hot water extract. A zone of low inhibition was recorded against Bacillus subtilis, staphylococcus aureus, Enterobacter aerogenes. No inhibition zones were recorded in Klebsiella pneumoniae, Pseudomonas fluorescens, and Proteus mirabilis.
Ethanol extract of Trametes versicolor mushroom showed high microbicidal effect against Bacillus subtilis and Staphylococcus aureus up to 28 ±0.95 and 30.2±0.84mm, respectively. The chloroform extract showed moderate microbial activity in Staphylococcus aureus, Pseudomonas aeruginosa, and Enterobacter aerogenes strains. Zone of inhibition was recorded at the lowest levels in Escherichia coli and Acinetobacter baumannii. Furthermore, no antimicrobial potency was recorded for Klebsiella pneumoniae, Pseudomonas fluorescens, and Proteus mirabilis in any of the mushroom extracts.
Areas of high inhibition against Bacillus subtilis were observed in ethanol, methanol, and hot water extract of Gloeophyllum sepiarium mushroom respectively 28.8 ±0.86, 29.5 ±0.85, and 20 ±1.13. Pseudomonas aeruginosa also recorded a region of higher inhibition in ethanol and methanol extracts. The zone of inhibition was recorded in ethanol and methanol extracts at moderate levels for Enterobacter aerogenes. Acinetobacter baumannii showed the lowest inhibition zone in the extract of hexane and chloroform. No regions of inhibition were recorded in Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas fluorescens, and Proteus mirabilis. Streptomycin antibiotic used was a positive control and exhibits moderate to high activity effect, like mushroom extract (zone of inhibition moderate 20.1±1.42 and high 28.9 ±0.70). Thus, mushroom extract worked simultaneously like antibiotic and can be used in place of antibiotic (Table 3-7, and Figure 4).
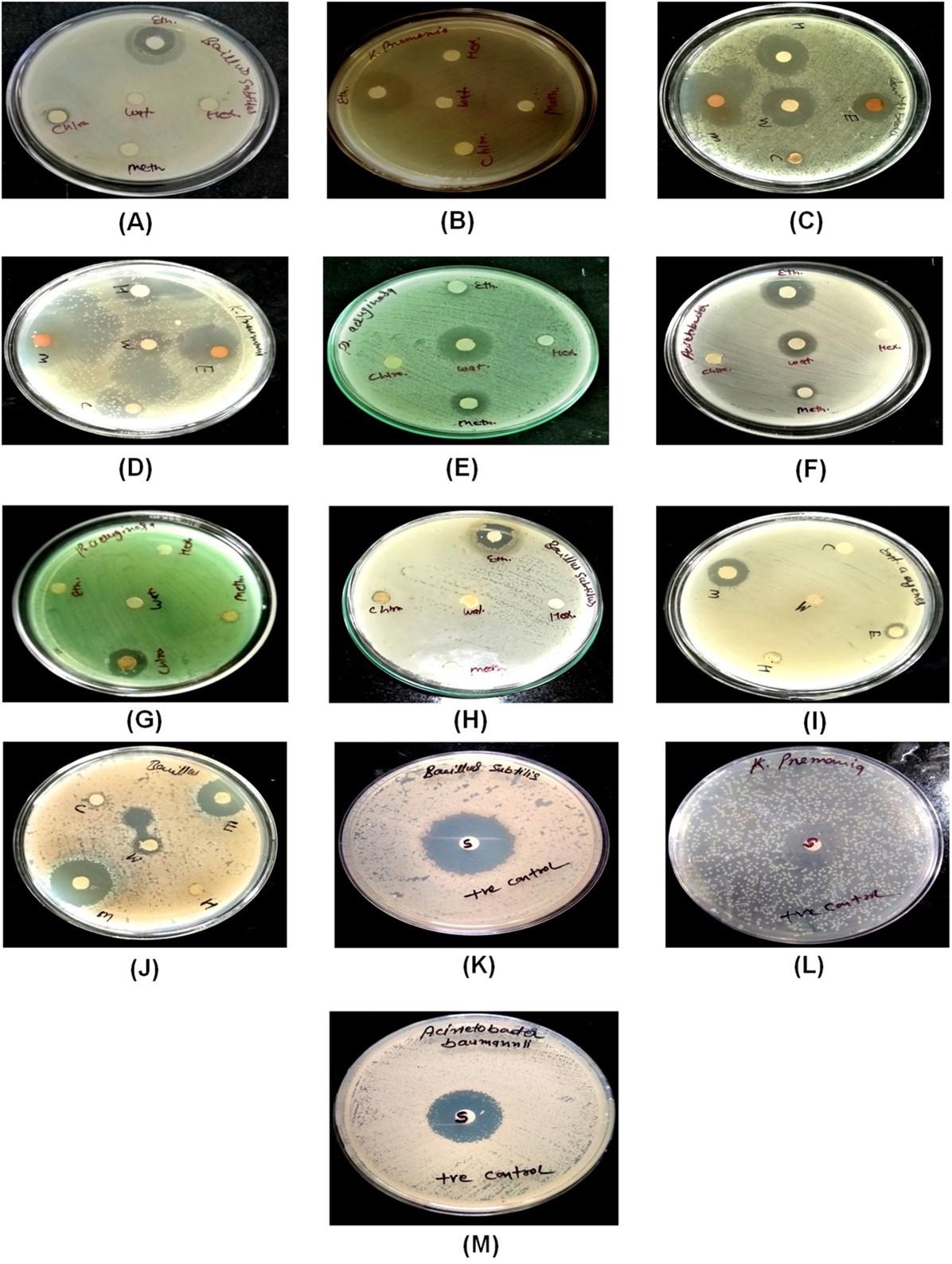
Table 3. Effect of Cantharellus cibarius extracts on microbial activity.
Table 4. Effect of Phellinus pectinatus extracts on microbial activity.
Table 5. Effect of Laccaria lacta extracts on microbial activity.
Table 6. Effect of Trametes versicolor extracts on microbial activity.
Table 7. Effect of Gloeophyllum sepiarium extracts on microbial activity.
Phytochemical analysis
The most active solvents of the methanol and ethanol extracts of Cantharellus cibarius, Phellinus pectinatus, Laccaria laccata, Trametes versicolor, and Gloeophyllum sepiarium mushrooms were contained most active phytoconstituents.
Among the compounds found in Cantharellus cibarius (Ethanol extract) are terpenoids, steroids, glycosides, while alkaloids, flavonoids, phenolic compounds, carbohydrates, amino acids, saponins, and inorganic acids are found to be negative. A methanol extract of Phellinus pectinatus was found positive for terpenoids, steroids, glycosides (Keller-killiani test), flavonoids, phenolic compounds (ferric chloride test), carbohydrates, and amino acids. Alkaloids (Wagner’s test), glycosides (Libermann Burchard’s test), phenolic compounds (lead acetate test), amino acids (Ninhydrin test, Millon’s test), saponins, and inorganic acids were all found negative. Laccaria laccata (ethanol extract) terpenoids, steroids, glycosides (Libermann Burchard’s test), flavonoids (Ferric chloride test), carbohydrates (Barfoed test), and amino acids (Ninhydrin test) were found positive while alkaloids, glycosides (Keller-Killiani test), flavonoids (Alkaline reagent test), phenolic compounds (Lead acetate test), carbohydrates, amino acids (Millon’s test and xanthoproteic), saponins, and inorganic acids was not present in extract. The tests for terpenoids, steroids, glycosides, flavonoids, carbohydrates, and amino acids were found positive for Trametes versicolor (ethanol extract); the tests for alkaloids, flavonoids, phenolic compounds, carbohydrates (Benedict’s test, Barfoed test), amino acids (Millon’s test, Xanthoproteic), saponins, and inorganic acids were negative. alkaloids, terpenoids, steroids, glycosides (Keller-Killiani test), flavonoids (alkaline reagent test), carbohydrates and amino acids (xanthoproteic) were positively detected in Gloeophyllum sepiarium (methanol extract). Whereas glycosides (Libermann Burchard’s test), flavonoids (ferric chloride test), phenolic compounds, amino acids (ninhydrin test, and millon’s test), saponins and inorganic acids were not present in the extract (Table 8).
Table 8. Phytochemical analysis for five wild macrofungi.
DISCUSSION
A growing number of infectious diseases are increasingly being treated with reduced success due to the emergence of multidrug-resistant organisms. Due to this, we urgently need to develop new and effective antibiotics against the present antibiotic-resistant pathogens [14]. The present study has outlined all five mushroom genera which assessed and demonstrated antimicrobial properties.
Different levels of antimicrobial activity were observed for selected wild mushrooms against the tested bacterial strains. Ethanol, methanol, and chloroform extracts were found to be able to reduce the growth effect of various bacteria strains as compared to the hexane and hot water extracts of Cantharellus cibarius.
All extracts of Phellinus pectinatus have shown prominent effects against bacterial strains, except Pseudomonas aeruginosa, Pseudomonas fluorescens, Proteus mirabilis. The study suggests that all extracts of Phellinus pectinatus have the ability to reduce microorganisms.
The ethanol and hot water extract of Laccaria laccata (were found to be more capable of reducing the growth effects of microbial strains. While other extracts of the species showed moderate to lowest antimicrobial effects.
Ethanol and chloroform extracts of Trametes versicolor showed significant effect on microbial activity. Other extracts showed very minimal antimicrobial activity. This shows that ethanol and chloroform extracts of Trametes versicolor are capable of inhibiting microorganisms.
Ethanol and methanol extracts of Gloeophyllum sepiarium showed wide spectrum antimicrobial effect. Whereas other extracts showed a moderate level of antimicrobial activity. From this, it is known that methanol and ethanol extracts of Gloeophyllum sepiarium may be able to kill more bacterial strains. Significant antimicrobial activity was observed in all species of mushrooms.
As a discussion, we see here that ethanol and methanol extract gave the highest bacterial growth inhibition, and it was followed by chloroform and hexane. The least amount of antibacterial activity was demonstrated by the water extract. Studies show that mushrooms contain active components that affect the growth of highly sensitive bacteria that may be better soluble in organic solvents than in aqueous solvents. These findings are consistently described in the literature indicating that organic solvent extracts exhibit higher consistent antibacterial activity than water extracts [15,16]. It was found that ethanol extracts and methanol extracts were more effective than other extracts. This indicates that they have a broad spectrum of antibacterial effects.
Several investigations have shown that the methanol and ethanol extract have antibacterial properties against bacterial infections [1,17-19]. Numerous researchers have reported that mushroom extracts in a variety of solvent systems possess an excellent antimicrobial activity [20-22]. This study suggests that mushrooms can be a potential source of antimicrobials that are good and sustainable.
A preliminary qualitative phytochemical study was conducted on the most active solvents after antimicrobial activity. Ethanol was selected from Cantharellus cibarius, Laccaria laccata, and Trametes versicolor, and methanol was selected from Phellinus pectinatus and Gloeophyllum sepiarium as the most active solvents. The results obtained from phytochemical analysis are indicated in (Table 8).
In Phytochemical analysis, the higher presence of alkaloids, terpenoids, steroids, glycosides, flavonoids, carbohydrates were seen in Phellinus pectinatus and Gloeophyllum sepiarium, and after this, the second place was recorded in Cantharellus cibarius, Laccaria laccata, and Cantharellus cibarius. The absence of saponin and inorganic acid was observed in all mushroom extracts. Possibly many of these compounds exhibit antibacterial activity that can be used as an alternative to antibiotics to treat infectious diseases caused by microbes.
A vast variety of flavonoids have antioxidant properties that are effective against a wide spectrum of microorganisms. Flavonoids are commonly present in a human meal as polyphenolic compounds and are found everywhere in natural sources [23]. Flavonoids have antioxidant, anti-inflammatory, anticarcinogenic, and antiviral qualities as well as anti-diarrheal, anti-allergic, and antibacterial capabilities [24].
Alkaloids are a class of chemical substances that occur in nature and most of which are basic nitrogen compounds. Which generally have the disrupts the functioning of the DNA of microorganisms [25]. Antiparasitic, Antioxidative, Anti-HIV, Antibacterial, and Anticorrosive activity are also found in alkaloids. Molecular structures of terpenoids contain a carbon backbone composed of isoprene. Terpenoids have been shown to provide a variety of pharmacological advantages, including anti-bacterial, anti-malarial, anti-inflammatory, and anti-cancer properties [26]. Glycosides are chemical substances that contain a sugar group and a non-sugar group (glycon and aglycon) attached to a glycosidic chain. The glycoside class of drugs serves as analgesics, antirheumatics, antibiotics, antibacterials, antifungals, cardiotonics, antioxidants, demulcents, anticancer agents, antitumoriferous agents, and purgatives [27]. Several autoimmune diseases and inflammations can be treated with steroids. The use of steroids can induce anesthesia [28]. And used for traumatic spinal cord injury, aspiration pneumonitis, ambulatory surgery, hyperreactive airway. Carbohydrates found in mushrooms are one of the powerful and common compounds that exhibit many health-benefiting activities. Mushrooms carbohydrates have been shown to have antitumor, immunomodulatory, and anti-inflammatory activities, one of three health benefits investigated by researchers. A high proportion of cardiovascular and hematological diseases are treated with carbohydrates-based therapeutics [29].
The present study suggests that the secondary metabolites appear to be enriched in the obtained mushrooms [30]. Preliminary studies indicate the presence of bioactive compounds. There is substantial evidence that these active metabolites cure a variety of human diseases such as diarrhea, gastroenteritis, dysentery, menstrual deformation, liver disease, spasmodic, chronic eczema, psoriasis [31].
Through antimicrobial assays and phytochemical screening, ethanol and methanol extract yielded showed best results. Solvents dissolve endogenous compounds primarily because they are capable of dissolving them [32], so it is good preparation for the curing of diseases.
CONCLUSION
At present time, the rate of bacterial resistance is increasing day by day due to excessive use of antibiotics. Considering the increasing antimicrobial resistance, it becomes imperative to find an alternative solution to combat infectious diseases. In such a situation, there is a need for such compounds that can reduce the effect of bacterial resistance. Wild mushrooms can be an option in this direction. From the present study, we find that the mushrooms extracts provide potential antimicrobial agents and bioactive active components that can be used as sources of antimicrobial agents that may exhibit a variety of therapeutic activities. Studies suggest that these mushrooms may be a good source of some natural antibiotics and bioactive compounds.
ACKNOWLEDGMENT
The authors would like to express their gratitude to Professor and Head Department of Microbiology at Shri Guru Ram Rai University Dehradun, Uttarakhand, who assisted in numerous ways during this investigation.
AUTHORS CONTRIBUTION
The experiments were designed and conceived by KS. GK performed the experiments and analyzed the data. The manuscript was drafted by KS. It was critically reviewed by KS for its intellectual content. The final version to be published was approved by KS and GK.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012; 78(16):1707-18.
- [2]Elkhateeb WA, Elnahas MO, Paul W, Daba TG. Fomes fomentarius and polyporus squamosus models of marvel medicinal mushrooms. Biomed. Res. Rev. 2020; 3(1):1-4.
- [3]Venkatachalapathi A, Paulsamy S. Exploration of wild medicinal mushroom species in Walayar valley, the Southern Western Ghats of Coimbatore District Tamil Nadu. Mycosphere. 2016;7(2):118-30.
- [4]Sánchez, C. Bioactives from Mushroom and Their Application. In: Puri, M. (eds) J. Food Bioact. Springer, Cham, 2017, pp 23-57.
- [5]Thabit AK, Crandon JL, Nicolau DP. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 2015; 16(2):159-77.
- [6]Kothiyal G, Singh K, Kumar A, Juyal P, Guleri S. Wild macrofungi (Mushrooms) diversity occurrence in the forest of Uttarakhand, India. Int. J. Botany Stud. 2022; 7(1): 567-578.
- [7]Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB. Investigation of antioxidant and antimicrobial activities of different extracts of Auricularia and Termitomyces species of mushrooms. Sci. World J. 2019 Jul 24;2019.
- [8]Habyarimana T, Cyuzuzo P, Yamukujije C, Yadufashije C, Niyonzima FN. Phytochemical and Antimicrobial Activity of Ocimum suave Against Selected Human Pathogenic Bacteria. J. Drug Deliv. Ther. 2022;12(1):123-8.
- [9]Adam OA, Abadi RS, Ayoub SM. The effect of extraction method and solvents on yield and antioxidant activity of certain sudanese medicinal plant extracts. Int. J. Phytopharm. 2019; 8:248-52.
- [10]Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Int. J. Phytopharm. 2016 Apr 1;6(2):71-9.
- [11]Adebayo EA, Ishola OR. Phytochemical and antimicrobial screening of crude extracts from the root, stem bark, and leaves of Terminalia glaucescens. Afr. J. Pharmacy Pharmacol. 2009; 3(5):217-21.
- [12]Kokate CK. A Textbook for Practical Pharmacognosy. Vallabh prakashan 5’’ edn. 2005; 107 -111.
- [13]Joyce Priyakumari C, Aparna K, Sagaya Jansi R. Phytochemical Analysis of Didymocarpus pedicellatus. Int. J. Pharm. Sci. Rev. Res. 2014; 25(1): 307-309.
- [14]Otieno OD, Onyango C, Onguso JM, Matasyoh LG, Wanjala BW, Wamalwa M, et al. Genetic diversity of Kenyan native oyster mushroom (Pleurotus). Mycologia. 2015; 107(1):32-8.
- [15]Kamra A, Bhatt AB. Evaluation of antimicrobial and antioxidant activity of Ganoderma lucidum extracts against human pathogenic bacteria. Int. J. Pharm. 2012;4(2):359-62.
- [16]Reid T, Kashangura C, Chidewe C, Benhura MA, Mduluza T. Antibacterial properties of wild edible and non-edible mushrooms found in Zimbabwe. Afr. J. Microbiol. Res. 2016;10(26):977-84.
- [17]Suliaman SQ, AL-Abbasi SH, Mahmood YH, AL-Azzawi HA. Antimicrobial activity of four selected wild mushrooms in iraq. Biochem. Cell. Arch. 2021; 21: 4533-4537.
- [18]Ranadive KR, Belsare MH, Deokule SS, Jagtap NV, Jadhav HK, Vaidya JG. Glimpses of antimicrobial activity of fungi from World. J. New Biol. 2013;2(2):142-62.
- [19]Gashaw G, Fassil A, Redi F. Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020; 2020: 9312489.
- [20]Jose Alves M, CFR Ferreira I, Dias J, Teixeira V, Martins A, Pintado M. A review on antifungal activity of mushroom (basidiomycetes) extracts and isolated compounds. Curr Top Med Chem. 2013;13(21):2648-59.
- [21]Wasser SP. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011;89(5):1323-32.
- [22]Pala SA, Wani AH, Ganai BA. Antimicrobial potential of some wild Macromycetes collected from Kashmir Himalayas. Plant Sci. Today. 2019;6(2):137-46.
- [23]Spencer JP. Flavonoids: modulators of brain function?. Br. J. Nutr. 2008;99(E-S1): ES60-77.
- [24]Cushnie TT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38(2):99-107.
- [25]Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plant Res.. 2010;4(9):7537.
- [26]Thoppil RJ, Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011;3(9):228.
- [27]Hossen SM, Hossain MS, Yusuf AT, Chaudhary P, Emon NU, Janmeda P. Profiling of phytochemical and antioxidant activity of wild mushrooms: Evidence from the in vitro study and phytoconstituent’s binding affinity to the human erythrocyte catalase and human glutathione reductase. Food Sci. Nutr. 2022;10:88–102.
- [28]Shaikh S, Verma H, Yadav N, Jauhari M, Bullangowda J. Applications of steroid in clinical practice: a review. Int. Sch. Res. Notices. 2012; 2012: 985495. doi:10.5402/2012/985495.
- [29]Kilcoyne M, Joshi L. Carbohydrates in therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 2007; 5(3):186-97.
- [30]Kalaw SP, Albinto RF. Functional activities of Philippine wild strain of Coprinus comatus (OF Müll.: Fr.) Pers and Pleurotus cystidiosus OK Miller grown on rice straw-based substrate formulation. Mycosphere. 2014;5(5):646-55.
- [31]umar VS, Sathishkumar G, Sivaramakrishnan S, Sujatha K, Razia M. Evaluation of phytoconstituents, in vitro antioxidant and antimicrobial activities of edible white button mushroom Agaricus bisporus. Int. J. Pharm. 2016;8(3):67-71.
- [32]Campos AR, Albuquerque FA, Rao VS, Maciel MA, Pinto AC. Investigations on the antinociceptive activity of crude extracts from Croton cajucara leaves in mice. Fitoterapia. 2002;73(2):116-20.