Chemical characterization, antimicrobial, antioxidant and larvicidal activities of certain fungal extracts
Abstract
Fungal extracts are considered a promising source of bioactive compounds that represent the basic core of the drug industry. This study aimed at exploring the antimicrobial, antioxidant and larvicidal activities of three ethyl acetate extracts of three fungal isolates Fusarium oxysporum, Aspergillus fumigatus and Penicilluim griseofulvum. These fungi were isolated from sediment samples collected in water courses of three Egyptian governorates; Giza, Qalubeya and Gharbeya, during a year from January to December 2019, molecularly identified using 18Sr RNA technique and were grown on rice medium for 14 days and extracted with ethyl acetate. GC-MS examination of the extracts led to identification of 15, 29 and 24 compounds, respectively. Hexadecanoic acid was detected as a main component in the investigated extracts. Moreover, in Folin-Ciocalteu’s assay, the tested fungal extracts showed noticeable total phenolic contents (TPCs) values of 115.84, 88.24 and 73.22, respectively for Aspergillus fumigatus, Penicillium griseofulvum and Fusarium oxysporum. Additionally, Aspergillus fumigatus extract exhibited strong total antioxidant capacity (TAC) value of 409.46 mg AAE/g dry extract, followed by Penicillium griseofulvum and Fusarium oxysporum extracts with TAC values of 299.28 and 281.31 mg AAE/g dry extract, respectively. All extracts exhibited antimicrobial activities against Staphylococcus aureus (G+ve bacterium), Escherichia coli (G-ve bacterium), Candida albicans (yeast) and Aspergillus niger (Fungus). Also, the extracts showed high larvicidal activity against miracidia and cercariae of Schistosoma mansoni. In conclusion, Fusarium oxysporum, Aspergillus fumigatus and Penicilluim griseofulvum are excellent sources of bioactive compounds which have multiple biological effects.
INTRODUCTION
The phenomenon of oxidative stress caused by over-production of free radicals inside human body leads to many serious diseases such as cancer, cardiovascular, and inflammation. Moreover, the harmful effects of this phenomenon can be reduced via using naturally occurring antioxidant compounds as free radical scavengers [1-4]. Recently, pathogenic microbial strains have shown a great ability to resist antibiotics, which leads to the possibility of infection with numerous infectious diseases. Therefore, scientists turned to discovering new antimicrobial compounds from natural sources such as the secondary metabolites of fungi as an alternative to current antimicrobial agents [5-7]. Sediment fungi are a diverse group of organisms grow in extreme and unique habitats that provide them the capability to produce unique secondary metabolites which have various biological activities [8]. Fungi have appeared recently as novel antioxidants sources as a part of their bioactive secondary metabolites [9]. In addition to antioxidants, fungi exhibit various bioactivities and functions like; antifungal, antimicrobials and larvicidal activities [6,10]. Fusarium, Aspergillus, and Penicillium species are the most famous producers of biologically active secondary metabolites due to their ability to produce alkaloids, peptides, steroids, terpenoids, phenols, quinines, and flavonoids, with antimicrobial and antioxidant activities [11,12]. Numerous active components have been isolated from Aspergillus clavatus, A. fumigatus, A. giganteus, A. terreus, Penicillium expansum , P. terreus, Penicillium expansum, P. urticae, P.urticae, and P. griseofulvum وغيرها. griseofulvum [13]. Moreover, destroying the larval stages in schistosomiasis prophylaxis is very important to break the transmission cycle. The isolates of Aspergillus, Penicillium, Fusarium, Rhizopus and Coelomycete species were reported to have toxic effect against miracidia and cercariae of Schistosoma mansoi [14, 15].
Therefore, the aims of the current study were to isolate, identify molecularly the Fusarium oxysporum; Aspergillus fumigatus; Penicilluim griseofulvum and to evaluate the bioactive compounds produced by fungi by studying the antimicrobial, antioxidant and larvicidal activities of their extracts. GC-MS/MS investigation, determination of total phenolic content and identification of their bioactive secondary metabolites were also studied.
MATERIALS AND METHODS
Fungal isolation and purification
F. oxysporum, A. fumigatus and P. griseofulvum were isolated from sediment samples collected in water courses of three Egyptian governorates; Giza, Qalubeya and Gharbeya during a year from January to December 2019. 25 g of each sediment sample were transferred to 250 ml of sterilized tap water in 500 ml Erlenmeyer flasks fitted with cotton plugs for fungal isolation [16]. At room temperature and constant speed (150 rpm) for 15 minutes the flasks were shaken. Then, they were left until complete sedimentation of the soil. Serial decimal dilutions were made from the original concentration to reach dilutions 1/100 and 1/1000. 0.5ml volumes were added into Sabouraud agar media. 3 replicate plates were prepared for each concentration and were incubated for one week at 28°C (±2°C). After that, rhea growth of fungal colonies was counted and recorded in a colony forming unit per milliliter (cfu/ml). Isolated species were sent for molecular confirmation.
Molecular identification of isolated fungal strains
Molecular identification has been recognized by DNA extraction, PCR and sequencing (18SrRNA). The isolated fungi were identified using the nuclear ribosomal internal transcribed spacer (ITS) region 1, 2, along with the short structural gene (5.8 S) as they have been investigated in the supporting information of previous studies. The region ITS was selected to identify fungi because it has been recently recognized as a common marker for fungal identification [17,18]. The PCR products purification was done to remove separate PCR primers and dNTPs from PCR products by using Montage PCR Clean up kit (Millipore). Sequencing was achieved by means of Big Dye terminator cycle sequencing kit (Applied BioSystems, USA). to determination of sequencing products, the applied Biosystems model 3730XL automated DNA sequencing system (Applied BioSystems, USA) were used. ITS1 (5′- TCC GTA GGT GAA CCT GCG G-‘3) and ITS4 (5’- TCC TCC GCT TAT TGA TAT GC-‘3) were the Primer sequences used for the identification in the current study.
Cultivation and extraction of fungi
The three fungal strains; F. oxysporum, A. fumigatus and P. griseofulvum were cultured in 3 Erlenmeyer flasks (1 L volume) containing 50 g rice and 50 mL distilled water for each fungus and sterilized for 20 min at 121°C (15 lb). Each flask was inoculated with spore suspension from 1 slant (10 days old). After incubation for 15 days at 30°C, the medium was extracted with ethyl acetate several times till exhaustion and by acetone followed by ethyl acetate [6].
GC-MS analysis
According to the reported procedures [19], GC-MS investigation was carried out, using a Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.251 mm, 0.1 mm film thickness). An electron ionization system with ionization energy of 70 eV was used; Helium gas was used as the carrier gas at a constant flow rate of 1ml/min for GC-MS detection. MS transfer line temperature and the injector was set at 280°C. The oven temperature was programmed to an initial temperature of 50°C (hold 2 min) to 150°C at an increasing rate of 7°C/min, then to 270°C at an increasing rate of 5°C/min (hold 2 min) then to 310°C as a final temperature at an increasing rate of 3.5°C/min (hold 10 min). The quantification of all the identified components was investigated using a percent relative peak area. A tentative identification of the compounds was performed based on the comparison of the irrelative retention time and mass spectra with those of the NIST, WILLY library data of the GC-MS system.
Estimation of total phenolic content (TPC)
According to the method described by [20], all determinations were carried out in triplicate and the total phenolic content was determined using Folin-Ciocalteu’s reagent. The reaction mixture was composed of (100 µl) of extract, 500 µl of the Folin-Ciocalteu’s reagent and 1.5 ml of sodium carbonate (20%). Then mixture was shaken and made up to 10 ml using distilled water. Gallic acid was used as standard and the mixture was allowed to stand for 2 h, then the absorbance was measured at 765 nm. The total phenolic content was expressed as mg gallic acid equivalent (GAE) per g extract [20].
Estimation of total antioxidant capacity (TAC)
All experiments were carried out in triplicate and phosphomolybdenum method was used for determination the antioxidant activity of each extract using ascorbic acid as standard, which based on the reduction of Mo (VI) to Mo (V) by the sample analyte and subsequent formation of a green colored [phosphate=Mo (V)] complex at acidic pH with a maximal absorption at 695 nm. In this method, 0.5 ml of each extract (200 µg /ml) in methanol was combined in dried vials with 5 ml of reagent solution (0.6 M sulfuric acid, 4 mM ammonium molybdate and 28 mM sodium phosphate). The vials containing the reaction mixture were capped and incubated in a thermal block for 90 min at 95°C. The absorbance was measured at 695 nm against a blank after the samples had cooled at room temperature. The blank consisted of all reagents and solvents without the sample, and it was incubated under the same conditions. The antioxidant activity of the sample was expressed as the number of ascorbic acid equivalent (AAE) [21].
Antimicrobial activities of the fungal extracts
Four different test microbes namely: Staphylococcus aureus (G+ve), Escherichia coli (G-ve), Candida albicans (yeast) and Aspergillus niger (fungus) were used for the investigation of the antimicrobial activity of different fungal extracts by using the agar disc diffusion method. In case of bacteria and yeast, nutrient agar plates were heavily seeded uniformly with 0.1ml of 105-106 cells/ml. Antifungal activities evaluation, potato dextrose agar plate seeded by 0.1ml the fungal inoculum. Filter paper discs (0.5cm), loaded with 1mg from each extract, were placed on the surface of inoculated plates. At low temperature (4°C) for 2-4 hours the plates were kept allowing maximum diffusion. The plates were then incubated at 37°C for 24 hours for bacteria and at 30°C for 48 hours in upright position to allow maximum growth of the organisms. The antimicrobial activity of the test agent was determined by measuring the diameter of zone of inhibition expressed in millimeter (mm). The experiment was carried out more than once and mean of reading was recorded [22].
Toxicity of the fungal extracts to Schistosoma mansoni larval stages (Miracidia & Cercariae).
The miracidicidal and cercaricidal activities of F. oxysporum, A. fumigatus and P. griseofulvum extracts were studied on S. mansoni miracidia and crecariae. S. mansoni ova and cercariae were obtained from Schistosome Biological Supply Center (SBSC), Theodor Bilharz Research Institute (TBRI). The concentrations used were 50, 100, 150 and 200 ppm from each extract. For 10 ml of each concentration about 100 miracidia or cercariae were introduced. Another 10 ml of dechlorinated tap water containing about 100 miracidia or cercariae was used as control. Microscopical examination of the miracidial or cercarial movement was carried out after different intervals of time either in control and fungal extracts (1/4, 1/2, 3/4,1, 2 and 3 hours). Stationary miracidia or cercariae was assumed to be dead and the total number of miracidia or cercaria was counted at the end of the experiment after adding Iodine solution [23].
Statistical analysis
All data were presented as mean ± S.D. using SPSS 13.0 program (SPSS Inc. USA).
RESULTS
Molecular identification of fungal species
The isolated fungi were molecularly identified by applying the obtained sequences from DNA of fungal isolates into BLAST search, it was found that, the fungal isolates had a similarity of 99.62, 99.48, 100% with the previously identified fungi Fusarium proliferatum isolate 2463 (acc. no. EU821469.1), A. fumigatus isolate PRN1 (acc.no. MZ328890.1) and P. griseofulvum strain P-1707 (acc. no. JQ316516.1), respectively. The phylogenic tree of the fungal isolates was also constructed (Figures 1, 2 & 3). Based on the above identification techniques, our local fungal isolates were identified as F. oxysporum f. sp. cucumerinum isolate MT1, A. fumigatus isolate MT21 and P. griseofulvum isolate MT6 with the Gene Bank accession numbers of OM722087.1, OM722121.1 and OM722119.1, respectively. The fungal strain (MM1) culture was deposited in the Microbial Chemistry Department Collection of Microorganisms.
GC-MS investigations of the ethyl acetate extracts of tested fungal species
GC-MS examination of F. oxysporum f. sp. Cucumerinum extract led to identification of 15 compounds (Figure 4). The total peak areas of the identified ingredients constitutes 36.54%, the prospects of the chemical structures of the identified compounds are recorded in Table (1). The main detected compounds are Hexadecanoic acid (10.54%) and (P, P, R)-Dimethyl-5,5′-dihydroxy-1,1′, 12, 12′-tetramethyl[6,6′]bi(benzo[c] phenanthrenyl)-8,8′ dicarboxylate (3.32%). Moreover, GC-MS/MS examination of A. fumigatus extract led to identification of 29 compounds (Figure 5). The total peak areas of the identified ingredients constitutes 77.24%, the prospects of the chemical structures of the identified compounds are recorded in Table (2). The main detected compounds are Hexadecanoic acid (15.77%), (R)-(-)-14-Methyl-8-hexadecyn-1-ol (15.64%), Hexadecanoic acid, methyl ester (5.78%), 9-Octadecenoic acid, methyl ester, (E) (5.25%), Hexadecanoic acid, ethyl ester (4.02%), Bicyclo[8.1.0]undecane (3.73%), 17-Pentatriacontene (2.04), and Tetradecanal (1.83%). While GC-MS/MS examination of P. griseofulvum extract led to identification of 24 compounds (Figure 6). The total peak areas of the identified ingredients constitute 78.32%, the prospects of the chemical structures of the identified compounds are recorded in Table (3). The main detected compounds are Hexadecanoic acid (27.59%), 9,12-Octadecadienoic acid (Z, Z) (16.65%), 9,12-Octadecadienoic acid (Z, Z), methyl ester (5.50%), and 5,6-Dihydro-4-methyl-2H-pyran-2-one (4.74%).
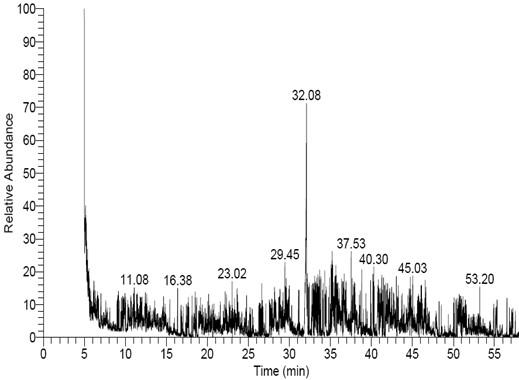
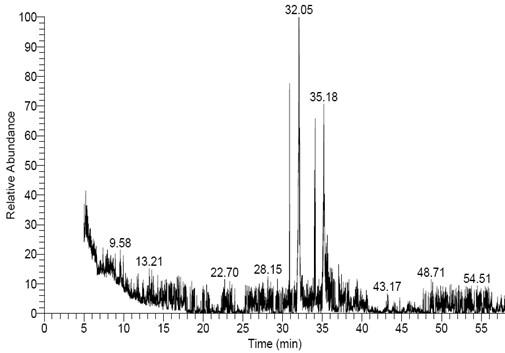
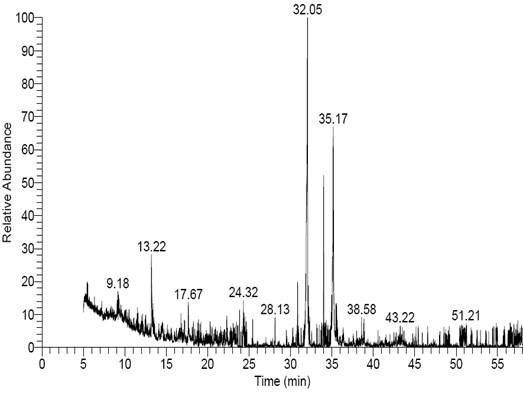
Table 1. Chemical composition of the ethyl acetate extract of Fusarium oxysporum f. sp. Cucumerinum.
Table 2. Chemical composition of the ethyl acetate extract of Aspergillus fumigatus.
Table 3. Chemical composition of the ethyl acetate extract of Penicillium griseofulvum.
Total antioxidant capacity and total phenolic content
As shown in Table 4, the ethyl acetate extract of A. fumigatus exhibited strong TAC value of 409.46 mg AAE/g dry extract, followed by P. griseofulvum and F. oxysporum extracts with TAC values of 299.28 and 281.31 mg AAE/g dry extract, respectively. Moreover, in Folin-Ciocalteu’s assay, the tested fungal extracts showed noticeable TPC values of 115.84, 85.24 and 73.22, respectively for A. fumigatus, P. griseofulvum and F. oxysporum (Table 4).
Table 4. Total antioxidant capacity and total phenolic content of ethyl acetate extracts of Fusarium oxysporum, Aspergillus fumigatus and Penicillium griseofulvum.
Antimicrobial activities of the fungal extracts
Results in Table (5) and Figure (7) evaluated the antimicrobial activities of three ethyl acetate extracts of three fungal isolates (F. oxysporum, A. fumigatus and P. griseofulvum) grown on rice medium. It has been found that all extracts exhibited antimicrobial activities against all test microbes. Extracts of F. oxysporum, A. fumigatus and P. griseofulvum showed anti Gram-positive bacterial test bacterium S. aureus with inhibition values of 12, 16 and 14mm, respectively. For the Gram-negative bacterial test microbe E. coli, extracts of F. oxysporum, A. fumigatus and P. griseofulvum exhibited inhibition values of 7, 13 and 11mm, respectively. Moreover, samples 1, 2 and 3 revealed anti yeast activities against C. albicans with inhibition values of 12, 18 and 18mm, respectively. In addition, antifungal activities were noticed for all extracts against A. niger with inhibition values of 13, 8 and 9mm, respectively. Purely isolated endophytic fungi were cultivated on rice as a medium at 30°C for 3-4 weeks. The secondary metabolites produced by fungi were extracted by ethyl acetate (EtOAc) as a solvent.
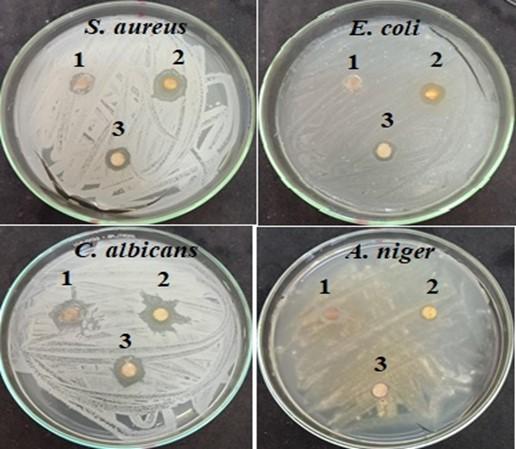
Table 5. Antimicrobial activity of different isolated fungi grown on rice medium against different test microbes.
Effect of fungal extracts on free larval stages of Schistosoma mansoni (Miracidia & Cercariae)
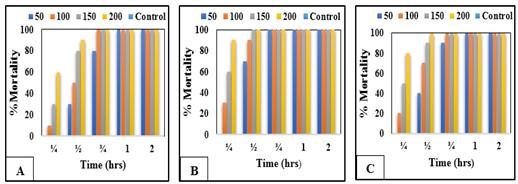
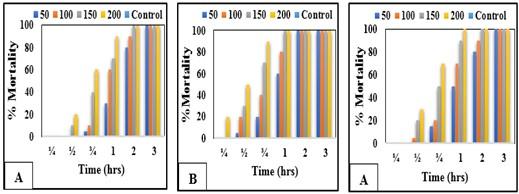
The data revealed that A. fumigatus excreted high mortalities of miracidia (Figure 8A, B and C) and cercariae (Figure 9A, B and C) followed by P. griseofulvum and F. oxysporum extracts. All fungal extracts exerted miracidial effect after 1/4 hour of their exposure to 50, 100, 150 and 200 ppm of the experimental extracts. Also, 100% of S. mansoni miracidia were killed after 3/4 hour of exposure to 100, 150 and 200 ppm of the tested extracts. While the mortality rate of cercariae reached 100% after 2 hours of exposure. P. griseofulvum and F. oxysporum showed cercaricidal effect after 1/2 hrs of exposure to 100 and 150 ppm, while Aspergillus fumigatus after 1/4 hrs of exposure to 200ppm. In general, the miracidia are more sensitive towards the toxic action of the tested agents than cercariae and the mortality percent of miracidia and cercariae is directly proportional to the time and the tested concentrations.
DISCUSSION
The present study was conducted to assess the antimicrobial, antioxidant and larvicidal activities of bioactive compounds produced by F. oxysporum; A. fumigatus; P. griseofulvum fungal ethyl acetate extracts. The isolated fungi were molecularly identified using 18Sr RNA technique and were grown on rice medium for 14 days and extracted with ethyl acetate. Traditional fungal identification protocols have been commonly applied and several new species till now are identified according to this method which was considered as time consuming and not accurate [24].
Moreover, GC-MS investigation, determination of total phenolic content and identification of their bioactive secondary metabolites were also studied. The identification was achieved via using computer search user-generated reference libraries, incorporating mass spectra [19, 22, 25, 26]. GC-MS investigation of F. oxysporum led to identification of some metabolites including methyl tetradecanone; oleic acid; eicosyl ester; methyl stearate; and bis(2-ethylhexyl) phthalate [27]. Moreover, different Penicillium species are characterized by the presence of biologically active compounds among them are polyketides [28-30] and alkaloids [31]. In the same context, GC-MS investigation of fermentation strain Snef1216 (Penicillium chrysogenum) led to identification of ten compounds like benzoic acid, 2-methoxy-, methyl ester; oxime-, methoxy-phenyl; cyclotrisiloxane, hexamethyl; undecane; benzoic acid, methyl ester; isopropyl myristate; hexadecanoic acid, methyl ester; 1,2-benzenedicarboxylic acid, butyl octyl ester; 8-octadecenoic acid, methyl ester (E) and heptadecanoic acid, 16-methyl-, methyl ester [32]. On the other side, GC-MS analysis of the ethyl acetate extract of Aspergillus fumigatus 269 isolated from Sungai Pinang Hot Spring, Riau, Indonesia led to identification of twenty-four components, the main identified constituents are eicosane, eicosane 2-methyl, phenol, 2,6-bis (1,1-dimethyl ethyl)-4-methyl, hexadecane 2, and 11-octadecenoic acid, methyl ester [33]. Different isolates from A. fumigatus isolated from fruit pulps were investigated for their chemical ingredients using GC-MS analysis led to identification of a set of fatty acids namely, 8,11-octadecadienoic acid, pentadecanoic acid, 4,7-octadecadienoic acid, hexadecenoic acid, heptadecanoic acid, cyclopropaneoctanoic acid, 5,9-octadecadienoic acid, 9,12-octadecadienoic acid and 6,8-octadienoic acid [34]. 17 compounds were detected by GC-MS analysis in the A. fumigatus culture filtrate including phenylethyl alcohol; 4-Hexyl-2,5-dioxo-2,5-dihydro-3-furanyl)acetic acid; phenol, 2,4-bis-(1,1-dimethylethyl); [(7-chloro-2,3-dihydro-1H-inden-4-yl)oxy](trimethyl)silane; 1-hexadecene; 4H-benzo[DE][1.6] nahthyridine,5,6-dihydro-8,9-dimethoxy; phenol,2,4-di-t-butyl-6-nitro-cis-4B,5; 1,4-benzenediol,2,6-bis(1,1-dimethylethyl)-; 6,9,12,15-docosatetraenoic acid, methyl ester; 12-hydroxy 14 methyl-oxa-cyclotetradec-6-en-2-one; 12-oxotricyclo[5.3.1.1(2,6)]dodeca-3,8-diene,11-acetoxy-4,5,9-trichloro; 2,4-oxazolidinedione, 3-(3,5-dichlorophenylo-5,5-dimethyl; (4-Methoxy-3-nitrophenyl) methano1, dimethylpentafluorophenylsilyl ether; 2h-1,4-benzodiazepin-2-one,7-chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy; (2e)-3-(3-chlorophenyl)-1-(4-chlorophenyl)-2-propen-1-one; 1-(3-chlor-phenyl)5-(2-methoxy-phenyl)-vinyl]-4,5-dihydro-1H-pyrazole;and picrotoxinin [35]. GC-MS analysis of A. fumigatus methanolic extract revealed the presence of 9-hexadecenoic acid; 2,4-dimethyl-5-methylthiopent-4-en-2-ol; E-11-hexadecenoic acid, ethyl ester; D-glucose; 6-O-α-D-galactopyranosyl; α-D-glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-; 6-acetyl –β-d-mannose , 4H-pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl-; 5-hydroxymethylfurfural, β-D-glucopyranoside; methyl, tetraacetyl-d-xylonic nitrile; n-hexadecanoic acid; 9-octadecenoic acid , (2-phenyl-1,3-dioxolan-4-yl)methyl ester; octadecanoic acid; 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol,(3β,5Z,7E)- and pyrimidin-2-ol,4-(3,4-dimethoxyphenyl)-6-phenyl [36].
The tested fungal extracts showed high antioxidant activity and noticeable total phenolic contents (TPCs). Fungal extracts are known for their vital bioactivities [9, 37] among them are antioxidant activities [38-40]. The ethanolic extracts of Penicillium chrysogenum and Penicillium fumiculosum showed total antioxidant activity values of 3.874 and 3.171 μg AA/g, respectively. Moreover, the extracts exhibited total phenolic content values of 2.859 and 2.109 mg GAE/g, respectively [41]. Additionally, fermentation strain Snef 1216 of P. chrysogenum showed total phenolic content value of 135.77 mg GAE/g [32]. Furthermore, VLC fractions from the ethyl acetate extract of Penicillium sp. SAM16‑EGY showed TAC values in the range from 212.53 to 687.56 mg AAE/g fraction [38]. In the same context, fungal extract of A. fumigatus displayed DPPH scavenging activity with LC50 value of 5μg/ mL [35].
By using the agar diffusion method [42], the antimicrobial activity of the extracts was tested against Staphylococcus aureus, Escherichia coli, and Candida albicans. For production of secondary metabolites, Curvularia sp. (RTFs-6) and Aspergillus sp. (RTL-6) were selected when grown in rice medium, extracted with ethyl acetate, and screened for their antimicrobial activity by agar well diffusion method. Fungal isolates were screened for antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium and Candida albicans. The identification of the prospective endophytic fungi [43].
The data revealed that the tested fungal extracts exerted high mortality against miracidia of S. mansoni faster than cercariae and the mortality percent is directly proportional to the time and the tested concentrations. These observations are in agreement with the study of [44] on Kelthane that killed miracidia faster than cercariae. Also, [45] showed that miracidial mortality was greater than that of cercariae during application of Hinsan after the same time intervals. Results of the current study also revealed that A. fumigatus excreted high mortalities of miracidia and cercariae followed by P. griseofulvum and F. oxysporum extracts. This effect was directly proportional to the time and the concentrations tested. These findings are in accordance with those obtained by [15, 46, 47]. They reported that the mortality rate of S. mansoni miracidia and cercariae increased by increasing the concentration and exposure period of Abamectin, the herbicides Butachlor and Fluazifop-p-butyl, butanol extract of Agave lophantha, the insecticide (fenitorthion) and the fungal extract of Aspergillus fumigatus.
CONCLUSION
This study revealed that the A. fumigatus ethyl acetate extract is very promising as it showed the highest activities as antimicrobial, antioxidant and larvicidal agent followed by P. griseofulvum and F. oxysporum. Thus, it can be concluded that these fungi play an essential role in the production of several bioactive secondary metabolites which have multiple biological effects.
ACKNOWLEDGEMENTS
Not applicable. This research received no external funding.
AUTHOR CONTRIBUTIONS
Asmaa Abdel-Motleb, Mosad A. Ghareeb, Mohamed S. Abdel-Aziz and Maha A.M. El-Shazly: Conceived, designed the study, performed the experiments, analyzed, and interpreted the data, wrote the first draft of the manuscript. All authors revised the manuscript and formulated the final version. All authors revised the manuscript and formulated the final version. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ghareeb MA, Mohamed T, Saad AM, Refahy LA, Sobeh M, Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J Pharm Pharmacol. 2018a; 70: 133-142.
- [2]Ghareeb M, Sobeh M, Rezq S, El-Shazly A, Mahmoud M, Wink M. HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-Inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molec. 2018b; 23(12): 3238.
- [3]Ghareeb M, Saad A, Ahmed W, Refahy L, Nasr S. HPLC-DAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities in vitro. Pharmacogn Res. 2018c; 10: 368.
- [4]Sobeh M, Mahmoud MF, Hasan RA, Abdelfattah MAO, Sabry OM, Ghareeb MA, El-Shazly AM, Wink M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci Rep. 2018; 8: 9343.
- [5]Ghareeb MA, Refahy LA, Saad AM, Osman NS, Abdel-Aziz MS, El-Shazly MA, Mohamed AS. In vitro antimicrobial activity of five Egyptian plant species. J of Appl Pharma. Sci. 2015; 5: 045-049.
- [6]Abdel-Aziz MS, Ghareeb MA, Saad AM, Refahy LA, Hamed AA. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY.Act Chromatog. 2018; 30(4): 243-249.
- [7]Mohammed HS, Abdel-Aziz, MM, Abu-baker, MS, Saad, AM, Mohamed, MA, Ghareeb, MA. Antibacterial and potential antidiabetic activities of flavone C-glycosides isolated from Beta vulgaris subspecies cicla L. var. flavescens (Amaranthaceae) cultivated in Egypt. Curr Pharma Biot. 2019; 20(7): 595-604.
- [8]Nejad EMA, Abtahi A, Zareian G. Evaluation of physical and chemical properties of soils of Doroudzan dam region of Marvdasht province with respect to drainage conditions and elapsed time. Euro J Exp Biol. 2013; 3(5): 213-217.
- [9]Abdel-Aziz M.S., Ghareeb M.A., Hamed A.A., Rashad E.M., El-Sawy E.R., Saad I.M. Ghoneem K.M. Ethyl acetate extract of Streptomyces spp. isolated from Egyptian soil for management of Fusarium oxysporum: the causing agent of wilt disease of tomato. Bioc Agri Biot. 2021; 37: 102185.
- [10]Hamed AA, Abdel-Aziz MS, Abd El Hady FK. Antimicrobial and antioxidant activities of different extracts from Aspergillus unguis SPMD-EGY grown on different media. Bull Nat Res Cen. 2018; 29.
- [11]Bhattacharyya PN, Jha DK. Optimization of cultural conditions affecting growth and improved bioactive metabolite production by a subsurface Aspergillus strain TSF 146. Intern. J Appl Biol Pharm Tech. 2011; 2: 133-143.
- [12]Kusari S, Hertwek C, Spiteller M. Chemical ecology of endophytic fungi:Origins of secondary metabolites. Chem Biol. 2012; 19: 792-798.
- [13]Bayman P, Baker JL. Ochratoxins: a global perspective. Mycop. 2006; 162 (3): 215–223
- [14]Abrito SP, Capuyan DTC, Sy JPU, Torres DF. Molluscicidal activity of Aspergillus, Penicillium, Rhizopus and Coelomycete species on eggs of golden apple snail. Philip Sci Hig Sch (PSHS). 2003; 37 (3): 213-219.
- [15]Osman GY, Mohamed AM, Abdel Kader A, Mohamed AA. Biological and biochemical impacts of the fungal extract of Aspergillus fumigatus on Biomphalaria alexandrina snails infected with Schistosoma mansoni. Res Revi Biosc. 2013; 7(12): 473-484.
- [16]Waksman S A. A Method for Counting the Number of Fungi in the Soil. J Bacteriol. 1922; 7(3): 339–34.
- [17]Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Consortium FB. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci Unit States Am. 2012; 109: 6241–6246.
- [18]El-Hady FKA, Abdel-Aziz MS, Shaker KH, El-Shahid ZA, Ghani MA. Coral-derived fungi inhibit acetylcholinesterase, superoxide anion radical, and microbial activities. Int J Pharm Sci Rev Res. 2014; 26: 301–308.
- [19]Madkour HMF, Ghareeb MA, Abdel-Aziz MS, Khalaf OM, Saad AM, El-Ziaty AK Abdel-Mogib M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts from Senna italica. J App Pharm Sci. 2017; 7: 023-032.
- [20]Kumar KS, Ganesan K, Rao PV. Antioxidant potential of solvent extracts of Kappaphycus alverezii (Doty). Edible seaweed. Food Chem. 2008; 107: 289-295.
- [21]Prieto P, Pineda M, Aguilar M. Spectrophotometric quantation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999; 269: 337- 341.
- [22]Abdel-Wareth MTA, El-Hagrassi AM, Abdel-Aziz MS, Nasr SM, Ghareeb MA. Biological activities of endozoic fungi isolated from Biomphalaria alexandrina snails maintained in different environmental conditions. Intern J Envi Stud. 2019; 76(5), 780-799.
- [23]Mohamed AM, Metwally NM, Mahmoud SS. Sativa seeds against Schistosoma mansoni different stages. Memór do Institu Oswal Cruz. 2005; 100(2): 205-211.
- [24]Zhao J, Kong F, Li R, Wang X, Wan Z, Wang D. Identification of Aspergillus fumigatus and Related Species by Nested PCR Targeting Ribosomal DNA Internal Transcribed Spacer Regions. J Clin Microbiol. 2001; 39(6): 2261–2266.
- [25]Shawky BT, Nagah M, Ghareeb MA, El-Sherbiny GM, Moghannem SAM, Abdel-Aziz M S. Evaluation of antioxidants, total phenolics and antimicrobial activities of ethyl acetate extracts from Fungi grown on rice straw. J Renew Mater. 2019; 7(7): 667-682.
- [26]Khalaf OM, Abdel-Aziz MS, El-Hagrassi AM, Osman AF, Ghareeb MA. Biochemical aspect, antimicrobial and antioxidant activities of Melaleuca and Syzygium species (Myrtaceae) grown in Egypt. J Physics: Conference Series. 2021; 1879(2): 022062.
- [27]Sangeetha C, Krishnamoorthy AS. Production of volatile organic compounds of Fusarium oxysporum f. sp. lycopersici on coinoculation with the metabolites of Chaetomium globosum. Mad Agric J. 2021; 108(4-6): 1-7.
- [28]Marinho AMR, Rodrigues-Filho E, Moitinho MLR, Santos LS. Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia Azedarach. J Braz Chem Soc. 2005; 16(2): 280-283.
- [29]Pastre R, Marinho AMR, Rodrigues-Filho E, Souzam AQL, Pereira JO, Diversity of polyketides produced by Penicillium species isolated from Melia azedarach and Murraya paniculata. Quimica Nova. 2007; 30: 1867-1871.
- [30]Luo H, Qing Z, Deng Y, Deng Z, Tang X, Feng B, Lin W. Two polyketides produced by endophytic Penicillium citrinum DBR-9 from medicinal plant Stephania kwangsiensis and their antifungal activity against plant pathogenic fungi. Natur Prod Communic. 2019;1-6.
- [31]Lai D, Brötz-Oesterhelt H, Müller WEG, Wray V, Proksch P. Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterap. 2013; 91: 100-106.
- [32]Sikandar A, Zhang M, Wang Y, Zhu X, Liu X, Fan H, Xuan Y, Chen L, Duan Y. Mycochemical screening and analysis, antioxidant activity, and biochemical composition of fermentation strain Snef1216 (Penicillium chrysogenum). J Analy Meth Chem. 2020; 3073906: 1-8.
- [33]Octarya Z, Novianty R, Suraya N, Saryono O. Antimicrobial activity and GC-MS analysis of bioactive constituents of Aspergillus fumigatus 269 isolated from Sungai Pinang Hot Spring, Riau, Indonesia. Biodiversitas. 2021; 22(4): 1839-1845.
- [34]Asci F, Aydin B, Akkus GU, Unal A, Erdogmus SF, Korcan SE, Jahan I. Fatty acid methyl ester analysis of Aspergillus fumigatus isolated from fruit pulps for biodiesel production using GC-MS spectrometry. Bioengineered. 2020; 11(1): 408-415.
- [35]Almanaa TN, Yassin MA, El-Mekkawy RM, Ahmed NS, Rabie GH. ,Anticancer and antioxidant activity by secondary metabolites of Aspergillus fumigatus. Adv Anim Vet Sci. 2021; 9(2): 265-273.
- [36]Al-Jassani MJ, Mohammed GJ, Hameed IH. Analysis of bioactive chemical compounds of Aspergillus fumigatus and evaluation of antifungal activity using twenty seven medicinal plants. Int J Pharm Bio Sci. 2016; 7(3B):191-204.
- [37]Elkhouly HI, Sidkey NM, Ghareeb MA, El Hosainy AM Hamed AA. Bioactive Secondary Metabolites from Endophytic Aspergillus terreus AH1 Isolated from Ipomoea carnea Growing in Egypt. Egypt J Chem. 2021a; 64(12): 7511-7520.
- [38]Ghareeb MA, Hamed MM, Saad AM, Abdel-Aziz MS, Hamed AA, Refahy, LA. Bioactive secondary metabolites from the locally isolated terrestrial fungus, Penicillium sp. SAM16-EGY. Phcog Res. 2019; 11:162-70.
- [39]Hamed AA, Soldatou S, Qader MM, Arjunan S, Miranda KJ, Casolari F, et al. Screening fungal endophytes derived from under-explored Egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorg. 2020; 8: 1617.
- [40]Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Nagwa M, Sidkey M. Bioactive secondary metabolite from endophytic Aspergillus Tubenginses ASH4 isolated from Hyoscyamus muticus: Antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacog J. 2021b; 13(2): 434-442.
- [41]Jakovljević VD, Milićević JM, Stojanović JD, Solujić SR, Vrvić MM. Antioxidant activity of ethanolic extract of Penicillium chrysogenum and Penicillium fumiculosum. Hem Ind. 2014; 68(1): 43-49.
- [42]Handayani D, Ananda N, Muh, AMA, Ruslan R, Fadriyanti O, Tallei T E. Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. J Appl Pharmaceut Sci. 2019; 9(03): 009-013.
- [43]Alurappa R, Chowdappa S. Antimicrobial activity and phytochemical analysis of endophytic fungal extracts isolated from ethno pharmaceutical plant Rauwolfia tetraphylla L. J Pure Appl Microbiol. 2018; 12(1): 317-332.
- [44]Mahmoud, MB. Effect of certain pesticides on B. alexandrina and the intramolluscan larval stages of S. mansoni. M. Sc. Thesis, Fac. of Science, Cairo, Univirsity, 1993.
- [45]Ibrahim WLF, El-Nahas HA and Abdel-Raouf HA. Comparative studies on the effectiveness of two synthetic pesticides and plant molluscicides on B. alexandrina and some larvae of S. mansoni. J Egypt Ger Soc Zool. 2007; 53 (D): 119-141.
- [46]Abdel Kader A, Ramzy F, Tantawy A. Evalution of the molluscicidal and in vitro schistosomicicidal activity of butanol extract of the plant Agave lophantha. Egypt J Biomed Sci. 2004; 16: 53–67.
- [47]Tantawy AA. Molluscicidal effect of fenitrothion and anilofos on L. natalensis and B. alexandrina snails and on the free larval stages of S. mansoni. J Egypt Soc Parasitol. 2006; 36 (2): 629-642.