Ramipril, an angiotensin-converting enzyme inhibitor ameliorates oxidative stress, inflammation, and hepatic fibrosis in alloxan-induced diabetic rats
Abstract
Angiotensin-II is considered as a peptide responsible for the vascular dysfunction and complications in various tissues including liver through inducing free radicle mediated oxidative stress. This study aimed to evaluate the effect of ramipril, an angiotensin-converting enzyme inhibitor (ACE inhibitor), on oxidative stress, inflammation, and fibrosis in the liver of alloxan-induced diabetic rats. In this investigation, rats were divided into four groups (six rats in each group): control, control +ramipril, alloxan, and alloxan+ ramipril. A single dose (90 mg/kg) of alloxan was given intra-peritoneally to induce type two diabetes. After the induction of diabetes, ramipril (10 mg/kg) was administered to each animal for 21 days. An oral glucose tolerance test (OGTT) was performed. All animals were sacrificed at the end of the study. Blood and liver tissues were collected from each animal and stored for further biochemical studies. Liver marker enzymes and oxidative stress parameters were also assayed followed by histological examination in the liver. Alloxan administration in rats showed oral glucose intolerance and increased fasting blood glucose levels. Ramipril (10 mg/kg) treatment in alloxan administered rats improved the OGTT and lowered fasting blood glucose level. This study also revealed the elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) enzymes activities in the alloxan administered rats which were attenuated by ramipril treatment. Oxidative stress parameters such as advanced protein oxidative products (APOP), nitric oxide (NO), and malondialdehyde (MDA) were also increased in alloxan administered rats which were diminished by the treatment of ramipril. Moreover, alloxan administration increased inflammation and fibrosis in the liver, which was further prevented by ramipril treatment. In conclusion, ramipril alleviated oxidative stress and fibrosis in the liver by suppressing oxidative stress. This investigation suggests that ACE inhibitors may be useful for treating diabetic complications and liver injury in alloxan-administered rats.
INTRODUCTION
World health organization (WHO) considers diabetes mellitus a major health concern. Approximately 382 million people were diagnosed with diabetes in 2013 worldwide, and it might be raised to 592 million diabetes cases by 2035 [1]. Diabetes can be characterized by persistent hyperglycemia, which develops due to relative or complete lack of insulin level in plasma or insulin resistance in various tissues [2]. Hyperglycemia in the course of diabetes usually leads to the development of end-organ complications where patients are more prone to suffer multiple diseases such as cerebrovascular disease, coronary artery disease, and dyslipidemia, steatohepatitis, and microvascular disease, including retinopathy and nephropathy [3, 4]. Type 1 diabetes is a consequence of selective destruction of the pancreatic beta cells, leading to absolute insulin deficiency in genetically susceptible individuals [5].
On the other hand, type 2 diabetes develops due to insulin resistance, characterized by reduced insulin action on the muscle, liver, and adipose tissue [3]. Recent evidence suggests that oxidative stress significantly contributes to the pathogenesis of both types I and II diabetes mellitus [6]. Free radicals are generated by glucose oxidation, nonenzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins in diabetes [7]. Diabetic patients lack antioxidant enzymes and tend to have more oxidative stress as a result of reactive oxygen species (ROS) generation in comparison to healthy subjects [8] [7]. The antioxidant enzymes are affected by hyperglycemia, which increases lipid peroxidation and insulin resistance [3]. Chronic oxidative stress is hazardous for beta cells because they have the lowest antioxidant enzyme expression levels and require high oxidative energy [9]. Furthermore, increased free radicals impair glucose-stimulated insulin secretion, reduce expression of the vital genes, and eventually induce beta cell death [10].
Angiotensin-II (Ang II) is an active peptide of the renin-angiotensin system (RAS), which is primarily involved in blood pressure regulation and fluid homeostasis [11]. However, recently, a novel role has been suggested in the pathophysiology of type 2 diabetes mellitus [12]. This hormone is formed in circulation and in some local tissues such as the pancreas, adipose, skeletal muscle, and liver [11]. Local RAS in these tissues are associated with events like inflammation, oxidative stress, endothelial dysfunction, tissue remodeling, thrombosis, proliferation, and fibrosis [13]. Previous report also showed that RAS is overactive in diabetes patients, which May be further reversed by the treatment with RAS inhibitors [14]. Other clinical studies also demonstrated that an angiotensin receptor blocker (ARB) or angiotensin-converting enzyme (ACE) inhibitor prevented Ang-II action and restored beta-cell function, and improved insulin sensitivity in diabetes patients [15]. Additionally, Ang-II can interfere with the insulin-stimulated up-regulation of phosphoinositide 3-kinases (PI3K) activity and thus results in insulin resistance [16].
ACE inhibitors are well known and widely prescribed in cardiovascular and renal diseases [17]. The ACE inhibitor ramipril was approved in the early 1990s in the United States and Canada for the treatment of hypertension [18]. Afterward, it is also approved for the preventive treatment of congestive heart failure and stroke among high-risk patients. The active metabolite, ramiprilat, reversibly and competitively inhibits ACE in the circulation and local tissues, thus, prevents the conversion of angiotensin I to angiotensin II [19, 20]. Considering the role of ACE in the development of oxidative stress related complications in diabetes, this study focused on whether ramipril treatment attenuates oxidative stress and hepatic fibrosis in alloxan induced diabetic rats.
MATERIALS AND METHODS
Chemicals and reagents
Alloxan, thiobarbituric acid (TBA) and 5,5-dithiobis-2-nitrobenzoic acid (DTNB) (Ellman’s reagent) were purchased from Sigma Chemical Company (USA). Active pharmaceutical ingredient of ramipril was obtained from Beximco Pharmaceutical Ltd, (Bangladesh). Reduced glutathione (GSH), trichloroacetic acid (TCA) were purchased from J.I. Baker (USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) was obtained from DCI diagnostics (Budapest, Hungary), and sodium hydroxide from Merck (Germany). All other chemicals and reagents used were of analytical grade.
Animals and treatment
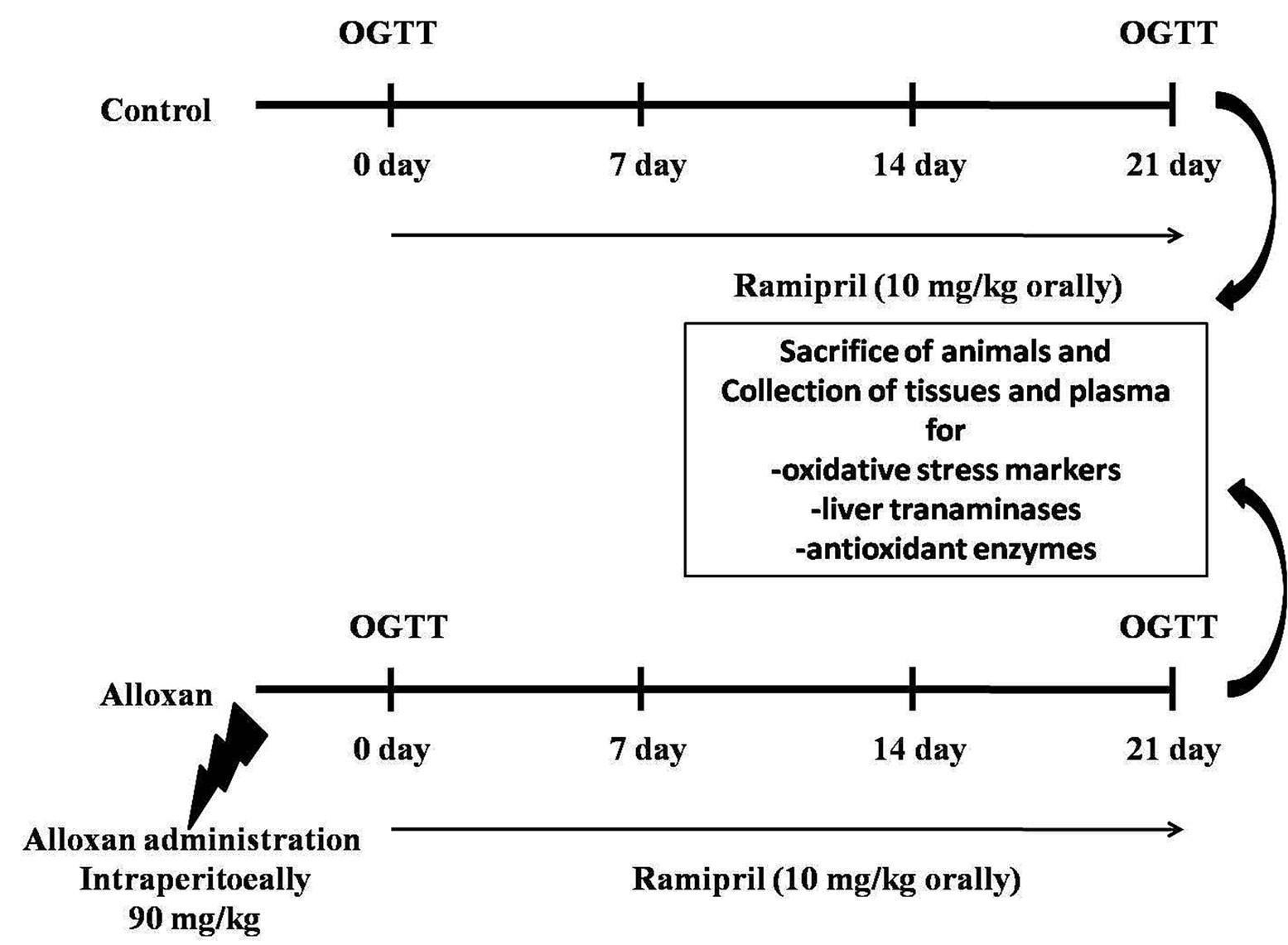
To evaluate the effect of ramipril on alloxan-induced diabetes, twenty-four Long Evans male rats (Ten to Twelve weeks old) were obtained from the Animal Breeding Unit of Animals House, Department of Pharmaceutical Sciences, North South University, Bangladesh. Individual cages were organized for every rat and were kept at room temperature (22 ± 2°C). Standard laboratory food and tap water were given to the animals. After developing diabetes, keeping six rats in each group, animals were divided into four groups presented in the schematic flow chart in Figure 1. The first group was a control group and given only normal food and water for three weeks. The second group was assigned normal food and water together with ramipril (10 mg/kg) for every day up to three weeks and termed as (control +ramipril) group. The third animal group (diabetic group) was administered with a single alloxan dose (90 mg/kg body weight) injected intra-peritoneally to induce type II diabetes. Animals are given glucose water after alloxan administration to prevent hypoglycemia. After alloxan administration, all animals were kept separately for a week to check for diabetes development. The fourth group of animals was treated with ramipril (10 mg/kg) orally for three weeks after the alloxan administration and diabetes induction. Water intake, food intake and body weight measurement of animals were noted on a daily basis. At the end of the study, blood samples and internal organs, including liver, pancreas, kidney, heart, and spleen, were collected after the sacrifice of all the animals. All organs were weighed and preserved in neutral buffered formalin (pH 7.4) for histological analysis. The tissue parts were also preserved in the refrigerator at −20°C for biochemical assays. To separate the plasma, collected blood samples were centrifuged at 8000 rotation per minute (rpm), and then the separated plasma samples were stored in the refrigerator at -20°C for further analysis. This experiment and the sacrifice procedure were conducted with the approval of the Committee of the Department of Pharmaceutical Science, North South University, Dhaka, Bangladesh (AEC-020-2017).
Hepatotoxicity assessment
Liver enzymes such as alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) activities in plasma were analyzed following the manufacturer’s protocol. The units are expressed as U/L.
Oxidative stress markers assessment
Preparation of tissue samples
Homogenization of hepatic tissue took place in 10 volumes of phosphate buffer saline (PBS, pH 7.4), and then the homogenized liver tissues were centrifuged at 8000 rpm for 30 min at 4°C. The supernatant was collected and used to determine enzymes and proteins as described below.
Lipid peroxidation estimation
The colorimetric method was used to measure thiobarbituric acid reactive substances (TBARS), which was described previously to estimate lipid peroxidation in the liver [21]. In brief, 0.1 ml of tissue homogenate (PBS, pH 7.4) was treated with 2 ml of HCl-TBA-TCA reagent (0.25 N HCl, 0.37% TBA and 15% TCA) with a ratio of (1:1:1) and placed in a water bath (hot) for 15 min and then cooled to ambient temperature. At 535 nm, against a reference blank, the absorbance of clear supernatant was measured.
Nitric oxide (NO) assay
A method which was described by Tracy et al. as nitrate was accorded to determine the nitric oxide (NO) [22]. To modify the Griess-Illosvoy reagent, in this study, naphthyl ethylene diamine dihydrochloride (0.1% w/v) was used instead of 1-naphthyl amine. The 3 ml reaction mixture contained 0.5 ml PBS, pH7.4 and 2 ml tissue homogenates. The reaction mixture was incubated for 150 minutes at a temperature of 25°C. The absorbance was taken at 540 nm against the corresponding blank solutions. The standard curve was used and, NO level was measured and expressed as nmol/g of tissue.
Advanced protein oxidation products (APOP) assay
The previously described method was adopted to determine the APOP levels [23, 24]. PBS was used to dilute 2 ml of plasma in a ratio of 1:5. A second reagent potassium iodide (0.1 ml of 1.16M) was then added to each tube. After 2 minutes, 0.2 ml acetic acid was incorporated into the reaction mixture. Placing 0.2 ml of acetic acid, 0.1 ml of KI, and 2 ml of PBS solution as a blank, the absorbance of the reaction mixture was immediately read at 340 nm. At 340 nm within the range of 0 to 100 nmol/ml, the chloramine-T absorbance was linear. nmol·ml−1 chloramine-T equivalents were used to express the APOP concentrations.
Assay for catalase (CAT) activity
To determine the CAT activities, a reaction mixture was formed containing 0.4 ml of 5.9 mmol hydrogen peroxide, 0.1ml of plasma or tissue, and 50 mmol phosphate buffer (pH 5.0) was used in the amount of 2.5 ml [25]. After waiting for one min, determination of the changes in absorbance of the reaction solution took place at 240 nm wavelength. A change in absorbance in 0.01 as units/min was defined as one unit of CAT activity.
Assay for reduced glutathione (GSH)
A previously described method was used to estimate the reduced glutathione level in plasma and tissues [26]. One ml of (4%) sulfosalicylic acid was used to precipitate 1.0 ml sample derived from 10% tissue homogenate. At the temperature of 4ºC, the samples were kept for 1 hour and then centrifugation was done for 20 min at 4ºC at 1200 rpm. Three ml assay mixture was composed of 0.1 ml aliquot sample (filtered), 0.2 ml DTNB (5,5-dithiobis-2-nitrobenzoic acid), (100 mM) and 2.7 ml phosphate buffer (0.1 M, pH 7.4). The reagent mixture developed a yellow color, and then, without any delay, absorbance reading was taken at 412 nm using Smart SpecTM plus Spectrophotometer and expressed as ng/mg protein.
Assay for superoxide dismutase (SOD) activity
Tissue homogenates and plasma were the sources for SOD assay, and the assay method was described previously [27]. The 3 ml reaction mixture consisted of PBS and enzyme preparation aliquot to make up the volume to 2.94 ml. The addition of 0.06 ml of 15 mM epinephrine was the first step to start the reaction. At the wavelength of 480 nm, absorbance change was recorded for one min with 15-sec interval. Except enzyme preparation keeping all the ingredients as control, was run simultaneously. It was defined that auto-oxidation of epinephrine present in the assay system was inhibited around 50% by per unit of enzyme activity.
Myeloperoxidase (MPO) activity estimation
A dianisidine-H2O2 method was used to determine MPO activity which was modified for 96-well plates [28]. In short, 10 μg protein was used as plasma samples, and those were added in triplicate to 50 mM potassium phosphate buffer (pH 6.0) in 0.15 mM H2O2 and 0.53 mM o-dianisidine dihydrochloride. The change in absorbance was recorded at 460 nm. Results were expressed as units of MPO/mg protein.
Histopathological analysis
To prepare the histological tissues, liver tissues were treated with neutral buffered formalin and then refined using graded ethanol and xylene, respectively. Then, these refined liver tissues were fixed in paraffin blocks and sectioned at 5 μm with a rotary microtome. These sections were stained with eosin/ hematoxylin to observe liver tissue structure and infiltration of inflammatory cells. Staining with Sirius red was also done in liver sections to determine collagen deposition and fibrosis. A light microscope (Zeiss Axio scope) was used to analyze the stained sections and photographed at 40X magnifications.
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). Evaluation of the results took place by using the Graph Pad Prism Software. One-way ANOVA followed by Newman-Keuls test was used as a posthoc test. Value of p < 0.05 was considered as statistical significance in all cases.
RESULTS
Effect on glucose level, body weight and organ wet weights
While experimenting, each rat’s body weight was logged daily, and calculation for the percentage changes were done for all four groups. An increase in body weight was found for all rat groups, except no noteworthy increase in body weights were found in alloxan administered rats compared to the control rats (Table 1).
Oral glucose tolerance test (OGTT) results of all experimental groups are shown in Figure 2. Fasting blood glucose level was shown to be increased significantly in alloxan treated diabetic rats compared to the control. Alloxan administered rats showed a rapid rise in glucose level after 30 minutes of glucose administration followed by a slow decline at 120 minutes compared to the control. Ramipril treatment significantly improved OGTT as marked in AUC determination compared to alloxan administered rats (Figure 2B).
No considerable change was found in the wet weight of liver in alloxan treated group while comparing with the control group. However, ramipril treatment in alloxan administered rats changed the wet liver weight. Another key finding in this study was a marked increase (p<0.05) in kidney’s wet weight in alloxan treated group. The same trend was also observed in wet pancreas weight in the alloxan-administered rats. Treating alloxan administered rats with ramipril normalized kidneys and pancreases weights (Table 1).
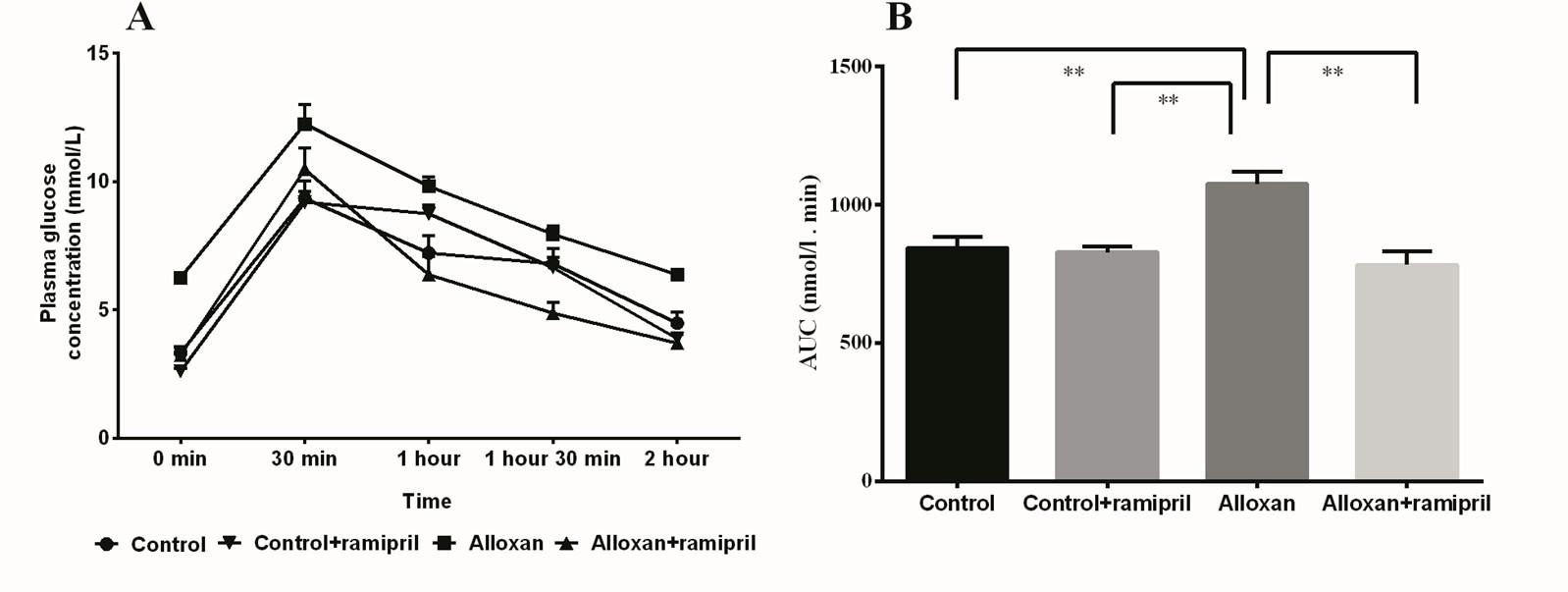
Table 1. Effect of ramipril treatment on body weight, food and water intake and organ weights in alloxan-induced diabetes rats.
Effect of ramipril on the biochemical parameter of liver functions in alloxan administered rats
Biochemical evaluation of liver function tests showed that alloxan administration increased plasma ALT, ALP, and AST activities compared to control rats (Table 2). Treatment with ramipril (10 mg/kg of body weight) in alloxan administered rats significantly normalized ALP, AST, and ALT activities in plasma. Moreover, MPO activity in alloxan-administered rats was also increased in the liver, which was normalized by ramipril treatment (Table 2).
Table 2. Effect of ramipril on biochemical parameters in plasma and liver in alloxan-induced diabetes rats.
Effect of ramipril on oxidative stress markers and antioxidant enzymes in alloxan administered rats
To determine oxidative stress, several oxidative stress markers, including MDA (malondialdehyde), NO (nitric oxide), and APOP (advanced protein oxidation products) levels in plasma and liver homogenates, were assayed. A higher concentration of the lipid peroxidation product (MDA) was present both in plasma and liver of alloxan-treated rats compared to control rats (Table 2). In this study, treatment with ramipril decreased MDA levels compared to the alloxan-administered rats.
Alloxan administration also significantly increased the APOP concentration in plasma and tissues (Table 2). Ramipril treatment significantly lowered the APOP level in plasma and liver of alloxan administered rats. Alloxan administered diabetic rats also showed a higher level of NO measured as nitrate both in plasma and liver compared to control rats (Table 2). Ramipril treatment significantly reduced nitric oxide levels in plasma and tissues (Table 2).
Furthermore, alloxan-induced a notable decrease in antioxidant enzyme activities such as catalase (CAT) and SOD and reduced glutathione (GSH) concentration compared to the control (Table 2). Ramipril treatment increased the GSH concentration, CAT, and SOD activities in the liver and plasma of alloxan administered rats (Table 2).
Effect of ramipril treatment on histological assessment of liver in alloxan-induced rats
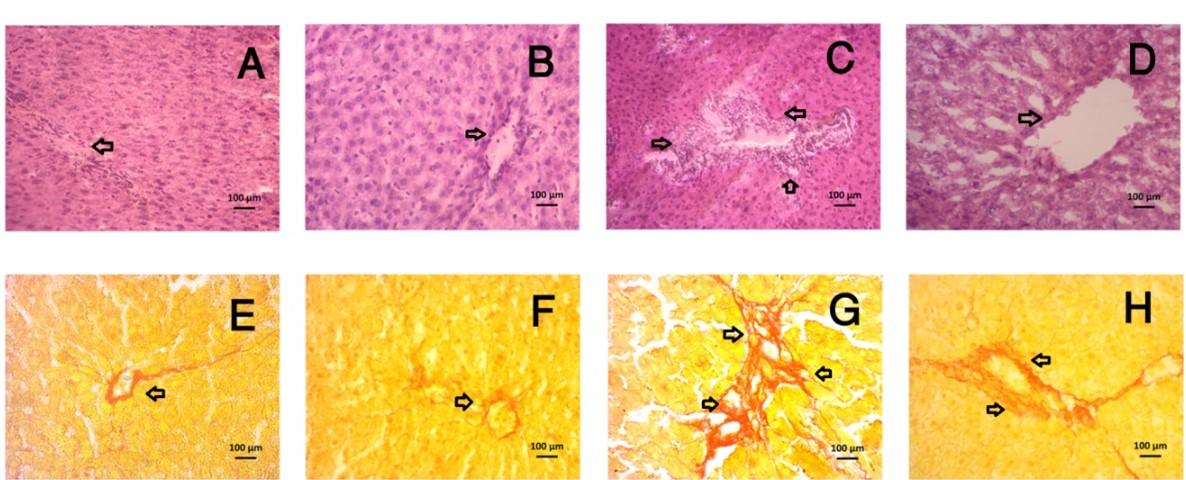
Alloxan-induced diabetic rats showed toxicity in the liver, which was observed at the morphological level by performing histological staining with H&E stain (Figure 3). Congestion was noted in central venules of hepatic tissue with the widening of sinusoids and inflammatory cells infiltration (Figure 3). Congestion was ameliorated in central venules by ramipril treatment followed by mild or no inflammatory cells infiltrations in the liver of alloxan administered rats. Necrotized scar tissue was also found in alloxan administered rat livers (Figure 3). Ramipril treatment significantly prevented the necrosis of the liver (Figure 3).
The Sirius red staining evaluated liver fibrosis by visualizing the red color of collagen fibers deposition (Figure 3). Collagen fibers were heavily deposited in alloxan-intoxicated rats, whereas ramipril treatment prevented the excess deposition of collagen and fibrosis in the liver of alloxan-administered rats (Figure 3).
DISCUSSION
Hyperglycemia is a common characteristic of all forms of diabetes either due to the autoimmune destruction of pancreatic beta cells (type-I) or insulin resistance as suppression or retard in metabolic responses of the muscle, liver, and adipose tissue to insulin action (type-II) [3]. Inhibition of angiotensin-II or angiotensin-converting enzymes is a new perspective for treating diabetes mellitus associated with oxidative stress [29]. Our study revealed that ramipril ameliorates oxidative stress, inflammatory cells infiltration, and hepatic fibrosis in alloxan-induced type-II diabetic rats. Alloxan enters the pancreatic beta-cell with the aid of glucose transporter-2 (GLUT-2) glucose transporter. Subsequently, it generates ROS in the presence of intracellular thiols, which eventually leads to necrosis of pancreatic beta cells, inflammatory cells infiltration, and fibrosis [30]. In type II diabetes, increased insulin resistance at the peripheral level promotes the oxidation of fatty acids at the adipose tissues [31]. Uncontrolled free fatty acid oxidation in the liver may produce ROS that initiates lipid peroxidation and destruction of hepatocytes [32]. During this study, alloxan administration resulted in the development of diabetes and eventual reduction of body weight as an outcome of impaired metabolism.
Earlier clinical research established that in type II diabetic patients, ALT, AST and ALP enzyme activities were increased than the normal level [33]. In our study, hepatic damage was evaluated by biochemical analysis of the plasma tests, including plasma ALT, AST, and ALP activities to demonstrate liver function [34]. This study showed that significant elevation of liver function marker enzymes such as AST, ALT, and ALP activities were observed in the alloxan treated rats, which were significantly lowered by ramipril treatment. The serum level of aminotransferases enzymes may appear in the circulation due to any hepatic injury [35]. Oxidative stress may provoke lipid peroxidation at the hepatocyte membrane and aid the release of these enzymes in the circulation. One of the essential criteria to study oxidative stress is to measure the lipid peroxidation of membrane lipids or fatty acids. Malondialdehyde (MDA) is considered as one of the foremost lipid peroxidation products, generated as a consequence of fatty acid auto-oxidation, commonly estimated by its reaction with thiobarbituric acid, which produces thiobarbituric acid reactive substances (TBARS) [36]. In this current investigation, alloxan administration resulted in increased lipid peroxidation in the liver and plasma, which were reduced to normal level by the treatment with ramipril.
Our study also revealed that alloxan administration in rats increased nitric oxide in the liver and plasma. NO is synthesized by NO synthase (NOS) from l-arginin and acts as a neurotransmitter or signaling molecule in various tissues. There are three types of NO synthases such as neuronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS) [37]. NO production through iNOS pathway is usually higher than other pathways [38]. Superoxide anions react rapidly with NO and produce peroxynitrites, which are more tissue-damaging by nitrosylation of cellular proteins and lipids [39]. Our study also showed that ramipril administration in rats prevented NO levels in the liver and plasma of alloxan-induced diabetic rats.
Previous studies have also shown that AOPP more accurately mark oxidative stress than markers of lipid peroxidation. [23]. A clinical trial on 52 type II diabetic patients revealed a significant elevation in the APOP as well as advanced glycation end-products (AGEs) [40]. Our study found that alloxan-administered diabetic rats showed an increased levels of AOPP in tissues and plasma, which are alleviated by ramipril treatment. Moreover, preclinical research showed that untreated diabetic rats generate lipid hydroperoxide radicals at a higher level with reduced antioxidant enzymes, including CAT and GSH [41]. In our study, liver antioxidant enzyme activity was assayed by measuring SOD and CAT activities, and reduced glutathione (GSH) concentration. Alloxan administration resulted in a notable reduction in these antioxidant enzymes activities, which were considerably restored by ramipril treatment.
Finally, the extent of inflammation and fibrosis were investigated in the liver of alloxan-administered rats. The histological assessment of liver sections using various staining also supports the biochemical data. A substantial outburst of inflammatory cells was observed in the centrilobular part of the liver section, which is supported by a previous study [42]. A previous study suggests that the inflammatory cells are generally mononuclear cells and neutrophils [43]. Necrotized tissue scars were also found in the liver of alloxan-administered rats.
Moreover, alloxan administration induced severe extracellular matrix (ECM) deposition and fibrosis in the liver. Liver fibrosis is considered as the end-stage liver dysfunction in all chronic liver diseases. Oxidative stress-mediated tissue damage in the liver attracts more inflammatory cells infiltration and activates local inflammatory cells such as Kuffer cells [44, 45]. Kuffer cells activation is reported in various experimental liver injuries [46, 47]. It has been suggested that Kupffer cells produce ROS and secrete proinflammatory cytokines [48]. Kupffer cells further stimulate hepatic stellate cells (HSCs) in the liver, leading to ECM synthesis through TGF-beta mediated pathway [49]. Recent reports also suggest that Ang-II may participate in hepatic fibrosis in various experimental models [50, 51]. Previous studies showed that AT receptor blockers prevented hepatic injury and fibrosis in carbon tetrachloride-administered rats [52]. Our investigation also found that treatment with ramipril satisfactorily revived the inflammatory and fibrotic conditions in the liver of alloxan-administered rats.
Diabetes mellitus (DM) is the most devastating, chronic, common non-communicable disease (NCD) and has become a global health problem. Considering the present study, we propose that ACE inhibitors can be an alternative treatment for diabetic complications. Our current investigation found that ACE inhibitor, ramipril, alleviated oxidative stress and fibrosis in the liver probably by suppressing the angiotensin-converting enzyme activity. Still, it needs more focused research to develop an exact mechanism for the treatment of diabetes.
ACKNOWLEDGEMENTS
The research was conducted in the Department of Pharmaceutical Sciences, North South University, Bangladesh. The authors gratefully acknowledge the logistic support provided by the Department of Pharmaceutical Sciences, North South University Bangladesh. This research work was not funded from any government, non-government, profit, or non-profit organizations. However, all logistic supports and laboratory facilities were provided by the Department of Pharmaceutical Sciences, North South University.
AUTHORS CONTRIBUTIONS
SS, AU and BS conducted the animal handling, feeding and weighing throughout the study. AU, FAS and BS performed the animal sacrifice and collected the plasma samples and tissues for further analysis. SS, NA and MNI performed the biochemical assays. AU, FAS and SS performed the histological staining of tissues. NAS, MNI and MAA performed data analysis and drafted the manuscript. MNI and MAA designed the experiment, supervised, and trained SS, AU, BS and FAS for animal study and the biochemical assays. All authors also took part in manuscript preparation and finalization of the content of this manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103:137-49.
- [2]Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107:1058-70.
- [3]Maria-Luisa L-d-l-V-M, Cristina F-M. Oxidative stress in diabetes mellitus and the role of vitamins with antioxidant actions. In (Ed.), Oxidative Stress and Chronic Degenerative Diseases – A Role for Antioxidants. 2013, IntechOpen.
- [4]Kangralkar VA, Patil SD, Bandivadekar RM. Oxidative stress and diabetes: a review. International Journal of Pharmaceutical Applications. 2010, 1(1):38-45.
- [5]Morran MP, Vonberg A, Khadra A, Pietropaolo M. Immunogenetics of type 1 diabetes mellitus. Mol Aspects Med. 2015;42:42-60.
- [6]Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107:1058-70.
- [7]Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology. 2003;17:24-38.
- [8]Ceriello A, Mercuri F, Quagliaro L, Assaloni R, Motz E, Tonutti L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834-8.
- [9]Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radical Biology & Medicine. 2011;50:567-75.
- [10]Park K, Gross M, Lee D-H, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32:1302-7.
- [11]Chu KY, Leung PS. Angiotensin II in type 2 diabetes mellitus. Current Protein & Peptide Science. 2009;10:75-84.
- [12]Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin–angiotensin–aldosterone system, glucose metabolism and diabetes. Trends in Endocrinology & Metabolism. 2005;16:120-6.
- [13]Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovascular Research. 2007;75:29-39.
- [14]Siragy H. Evidence for benefits of angiotensin receptor blockade beyond blood pressure control. Current Science Inc. 2008;10:261-7.
- [15]Ribeiro-Oliveira A, Nogueira AI, Pereira RM, Boas WWV, dos Santos RAS, e Silva ACS. The renin–angiotensin system and diabetes: An update. Vascular Health and Risk Management. 2008;4:787-803.
- [16]Sowers JR. Insulin resistance and hypertension. American Journal of Physiology – Heart and Circulatory Physiology. 2004;286:H1597-H602.
- [17]Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104:1985-91.
- [18]Yeung E, Wong FS, Wanless IR, Shiota K, Guindi M, Joshi S, et al. Ramipril-associated hepatotoxicity. Archives of Pathology & Laboratory Medicine. 2003;127:1493-7.
- [19]Dzau VJ. Mechanism of action of angiotensin-converting enzyme (ACE) inhibitors in hypertension and heart failure. Role of plasma versus tissue ACE. Drugs. 1990;39 Suppl 2:11-6.
- [20]Lonn E, Gerstein H, Smieja M, Mann J, Yusuf S. Mechanisms of cardiovascular risk reduction with ramipril: insights from HOPE and HOPE substudies. European heart journal supplements. 2003;5:A43-A8.
- [21]Niehaus WG SB. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry. 1968;6.
- [22]Tracey WR, Tse J, Carter G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. Journal of Pharmacology and Experimental Therapeutics. 1995;272:1011-5.
- [23]Witko-Sarsat V FM, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen A, et al. . Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int (1996) 49: 1304-13.
- [24]Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacology. 2014;2014:7.
- [25]Khan R. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-induced oxidative stress in the thyroid tissue of rats. BMC Complementary and Alternative Medicine. 2012;12:181.
- [26]Jollow D, Mitchell J, Zampaglione N, Gillete J. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacol. 1974;11:151.
- [27]Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-5.
- [28]Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. The Journal of Investigative Dermatology. 1982;78:206-9.
- [29]Lupi R, Del Guerra S, Bugliani M, Boggi U, Mosca F, Torri S, et al. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. European Journal of Endocrinology. 2006;154:355-61.
- [30]Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216-26.
- [31]Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clinical Biochemistry. 2005;38:203-17.
- [32]Haque M, Sanyal AJ. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best Practice & Research Clinical Gastroenterology. 2002;16:709-31.
- [33]Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care. 2002;25:815-21.
- [34]Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scandinavian Journal of Gastroenterology. 1986;21:109-13.
- [35]Mohd Azam Hyder MH, and Abdelmarouf Hassan Mohielde. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. European Journal of Experimental Biology. 2013; 3(2):280-4
- [36]Finaud J, Lac G Fau – Filaire E, Filaire E. Oxidative stress: relationship with exercise and training, Sports Med. 2006;36(4):327-58.
- [37]Channon KM, Qian H, George SE. Nitric oxide synthase in atherosclerosis and vascular injury: insights from experimental gene therapy. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1873-81.
- [38]Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sciences. 2004;75:639-53.
- [39]Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chemical Research in Toxicology. 1997;10:485-94.
- [40]Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiological Research 2002;51:597-604.
- [41]Lucchesi AN, Freitas NT, Cassettari LL, Marques SF, Spadella CT. Diabetes mellitus triggers oxidative stress in the liver of alloxan-treated rats: a mechanism for diabetic chronic liver disease. Acta Cirurgica Brasileira 2013;28:502-8.
- [42]Lucchesi AN, #xe1, lia, Cassettari LL, Spadella C, et al. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. Journal of Diabetes Research. 2015:11.
- [43]Nolasco EL, Zanoni FL, Nunes FPB, Ferreira SS, Freitas LA, Silva MCF, et al. Insulin modulates liver function in a type I diabetes rat model. Cellular Physiology and Biochemistry. 2015;36:1467-79.
- [44]Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. Journal of Biological Chemistry. 2000;275:2247-50.
- [45]Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. The Journal of Clinical Investigation. 2007;117:539-48.
- [46]Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. American Journal of Physiology – Gastrointestinal and Liver Physiology. 1991;260:G355-G62.
- [47]Stachlewitz RF, Seabra V, Bradford B, Bradham CA, Rusyn I, Germolec D, et al. Glycine and uridine prevent d-galactosamine hepatotoxicity in the rat: Role of kupffer cells. Hepatology. 1999;29:737-45.
- [48]Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediators of Inflammation. 2012;2012:21.
- [49]Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28-36.
- [50]Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046-55.
- [51]Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334-48.
- [52]Wei Y, Jun L, Qiang C. Effect of Losartan, an Angiotensin II Antagonist, on hepatic fibrosis induced by CCl4 in rats. Digestive Diseases and Sciences. 2004;49:1589-94.