Protective effect of L-carnitine nanoparticles Vs carnitine on lead acetate-induced toxicity in male rats
Abstract
Chitosan nanoparticles are important materials that are widely used in many biological, engineering and food industries and are also used as plant growth stimulants as well as use as vectors for drug delivery to target cells. Whereas L-carnitine (LC) is a water-soluble compound that contributes to the transport of long-chain fatty acids across the mitochondrial membranes and the oxidation of β-lipids. 60 male rats (Rattus Rattus) were divided into six equal groups. The first group (control group) received orally distilled water. The second group received 1ml lead acetate orally at a dose of 30 mg/kg of body weight daily for 30 days. Third group received 1ml lead acetate (30mg/kg B.W) + L-carnitine (100mg/ kg B.W. /daily). The fourth group received 1ml lead acetate (30mg/kg B.W.) + Nano L-carnitine (100mg/ kg B.W./ daily). The fifth group received 1ml of L-Carnitine orally at a dose of 100mg/ kg B.W /daily. The sixth group received 1ml of L-Carnitine-NPs orally at 100mg/ kg B.W /daily. Our findings demonstrated that exposure to lead acetate caused a significant increase in liver enzymes aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) and renal function (creatinine and urea) in the lead acetate group. Whereas lead treatment increased oxidative stress and reactive oxygen species (ROS). Histopathological study showed significant changes in the brain (cerebellum) that disrupted the normal arrangement of the three layers, with large distances between the Purkinje cell layer and the molecular or granular layer. According to the study, we can conclude that the Nano L-Carnitine had a greater role in protecting against the effect of lead at the hematological parameters and a clear role in the protection against histopathology change of lead poisoning. L-Carnitine and Nano L-Carnitine had an active role in protecting against lead acetate toxicity.
INTRODUCTION
In recent years, tremendous progress has been made in nanotechnology, particularly in the fields of material science and medicine. Medical applications of nanotechnology are often referred to as “nanomedicine.” This has provided a crucial impetus for creating several types of drug-loaded nanocarriers ranging in size from 1 to 1000 nm. In the biomedical field, a wide range of nanocarriers or nanoparticulate systems composed of various materials such as lipids, polymers, and inorganic materials have been proposed. That results in delivery systems depending on their physicochemical properties, which will be suitable for various applications [1,2]. Chitosan is a linear polysaccharide formed from chitin, an abundant natural polymer found in crustaceans, insects, arthropods, and fungal cell walls. Most commercially available chitosan is generated from marine chitin collected from shrimp, lobster, and crab shells. Chitosan is the N-deacetylate form of chitin composed of D glucosamine and N-acetyl glucosamine monomers [3,4].
Chitosan advantages have shown that it may be used as a drug delivery system for the controlled release of antibiotics, anticancer medications, antihypertensive agents, proteins, peptide pharmaceuticals, and vaccines [5]. Chitosan has also been used to increase the solubility of insoluble drugs in water, tissue engineering, ocular bandage lenses, gene delivery, and other uses are also possible [6,7]. L-carnitine is a natural substance that prevents long-chain fatty acid accumulation by transporting them into the mitochondria and oxidizing them to produce adenosine triphosphate [8-10]. L-carnitine suppresses both oxidative stress-induced mitochondrial damage and mitochondria-dependent apoptosis in various cell types [11].
According to recent research, L-carnitine may play a significant role in oxidative/antioxidative equilibrium and has an anti-peroxidative impact on various tissues [12,13]. Lead (Pb) is one of the most dangerous heavy metals found in the environment [14-16]. Because lead has no good properties, its presence in the body in high quantities affects all animal organs, resulting in hazardous consequences [17]. Lead exposure in the workplace and environment has grown many-fold, resulting in a wide range of uses in industries, cosmetics, folk cures, pharmaceuticals, and medicine. [18,19]. Lead acetate is a bio-toxic contaminant that accumulates in all bodily tissues, including the liver, lungs, bones, reproductive systems, brain, and immune system. This hazardous lead’s physiological, biochemical, and behavioral consequences in animals have been recorded.
High in vivo stability, long-term payload capacity release and passage via small capillaries and cellular compartments are only a few of the various advantages of employing nanoparticle delivery systems. Nanoparticles may also increase the therapeutic index of the treatment, control its pharmacokinetics and biodistribution, and aid in the formation of long-term drug reservoirs [7]. Additional characteristics include smaller nanoparticles (50-200 nm) with high loading capacity, delayed complex dissociation in vivo, and target optimization to the targeted location with limited absorption by neighboring tissues. Effective delivery methods need the development of formulations that include these features while being cost and complexity-effective [17,20].
MATERIALS AND METHODS
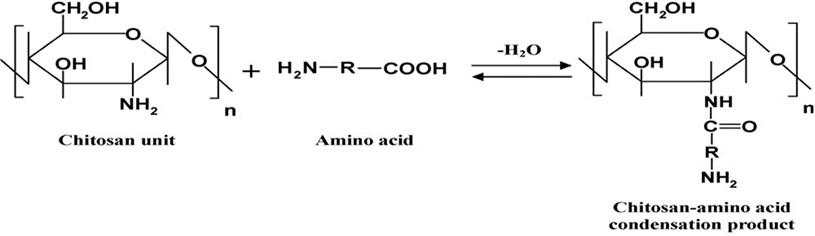
Chitosan-carnitine acid adduct (Cs-Ca) synthesis
According to references, these steps were used to make chitosan-l-carnitine adduct [21, 22, 23]. 1% of l-carnitine and chitosan were combined in equal amounts using Dean-Stark (Clevenger) apparatus. The condensation process was carried out in the presence of xylene until the water had been separated. An electric oven was used to dry and weigh the chitosan amide product after separating and washing it with three different solvents (methanol, hot distilled water, and ethanol) (Figure 1).
Nanoparticles of chitosan and carnitine synthesis
Synthesis of Cs-Ca NPs, by ionic gelation method, was used using TPP and Cs-Ca adduct. Cs-Ca (1 mg/ml) and TPP solution were mixed for six hours at ambient temperature with constant stirring at a ratio of 1: 2.5 (w/w %) in an acetic acid solution (1% w/v). TPP triggered ionic gelation of Cs-Ca/TPP nanoparticles. This was the first step in the process. Separation, washing, and drying of these nanoparticles resulted in the precipitate being re-suspended in water and drying [22, 23].
Characterization of L-carnitine-chitosan nanoparticles
These characterization tests were done in the material research laboratories at the ministry of sciences and technology environment and water research and technology director (EWRTD). Scanning Electron Microscopy (SEM) Observation–the surface morphology of N.P.s was studied using a scanning electron microscope (SEM). The Ministry of Science and Technology conducted this test as well as all other characterizations of biogenic Nano L-Carnitine. The electron microscopy-based approach’s direct observation of the nanoparticles identifies their size, shape, and surface morphology.
Animal of the study
The present study was conducted in the college of Veterinary medicine at the University of Basrah, in the animal house of the department of physiology. Sixty male adult albino rats (Rattus rattus) were used in the current study. With an average weight of 200±20g and the ages of the animals ranging from 8 to 10 weeks. They were housed for two weeks for an adaptation before the experiment. Every ten animals were housed in an individual plastic cage measured as 15x35x50cm. They were fed ad libitum with the meal of standard pellet of diet supplied by IPA (Institute for Public Accuracy). They had free access to drinking water and were kept under the exact condition of temperature (22-25) °C and light, the regime of 14 hours of light and 10 hours of darkness. The author has signed an animal welfare statement. The project was approved by the local ethical committee in the University of Al-Ameed register in number 113 on 21/2/2022.
Experimental design
Animals in the study were divided into six groups. Each group consists of 10 male rats used for the design of experiments as the following:
Group-1 (control group): Animals received orally distilled water. Group -2 (lead acetate group): Animals received lead acetate orally at a dose of 30 mg/kg B.W. daily for 30 days. Depended on LD50, group-3 animals received lead acetate (30mg/kg B.W.) for 30 days + L-carnitine (100mg/ kg B.W) daily for 2 months. Group-4 received lead acetate (30mg/kg B.W.) for 30 days + Nano L-carnitine (100mg/ kg B.W) daily for two months. Group-5 (L-carnitine): Animals received L.C. orally at a dose of 100mg/kg B.W daily for two months. Group-6 (nano L-carnitine): Animals received LC-NPs orally at a dose of 100mg/ kg B.W daily for two months. All the experiments proceed for two months.
Sample collection
At the end of the treatment period, rats were dissected for sample collection. The blood sample was collected via cardiac puncture after anesthetizing the animal, according to [24]. Samples were collected by using a 5ml disposable syringe. About 5ml of the collected blood was put in a non-heparinized plane tube for centrifugation at 3000 rpm for 15 minutes to obtain the serum. Then the serum was transferred to Eppendorf tubes and stored at (-4°C) until analyzed for the measurement of liver enzymes (AST, ALP, and ALT) and kidney function (creatinine and urea) to evaluate oxidative stress (MDA and GPX). Also, the brain was collected for microscopic evaluation.
Scanning electron microscopy (SEM) analysis
The surface morphology of N.P.s was studied using a scanning electron microscope (SEM). The Ministry of Science and Technology conducted this test as well as all other characterizations of biogenic Nano L-Carnitine. The electron microscopy-based approach’s direct observation of the nanoparticles identifies their size, shape, and surface morphology.
Biochemical parameters
An aspartate aminotransferase (AST) test kit is used to measure AST activity in the serum (Agape diagnostic, India code 683-562) (Clin. Chem, Acta 1976) and Dtsch. Med Wschr 1974. An alanine aminotransferase (ALT) test kit is used to measure in serum (Agappe diagnostic, India.). An alkaline phosphatase (ALP) test kit is used to measure in serum (Agappe diagnostic, India).
Researchers used a specialized kit (Agappe diagnostics, India) to detect the blood creatine levels. When creatinine combines with picrate in an alkaline media, a colorful complex form. A spectrophotometer that measures the absorbance at 500 nm may be used to estimate the quantity of creatinine in a specimen [13]. The following reaction is used to determine urea through enzymatic means. Urea was determined according to the kit protocol of the agape diagnostic, India.
Serum malondialdehyde measurement (MDA)
The ability to accurately measure lipid peroxidation in disease states necessitates this method of assessing oxidative stress. MDA and 4-hydroxynonenal (4-HNE) are the natural bi-products of lipid peroxidation. One of the most commonly acknowledged methods to evaluate oxidative damage is measuring lipid peroxidation products. It is easy to use the MDA microplate assay kit to detect MDA in various samples. Thiobarbituric acid (TBA) reacts with MDA in the sample to form the MDA-TBA adduct. MDA-TBA adduct can be readily measured using a colorimeter (λ= 532 nm) [20].
Measurement of serum glutathione (GSH)
This kit utilizes the Sandwich-ELISA technique. The micro-ELISA plate that comes with this kit has been pre-coated with an anti-rat GPX1 antibody. Antibodies are pre-incubated in micro-ELISA plate wells before addition, and then samples or standards are added to the plate. Antibodies specific to rat GPX1 and avidin-horseradish peroxidase (HRP) conjugate are added to each micro plate well and incubated. Everything that isn’t necessary is swept away. Each well receives a substrate solution. The wells containing rat GPX1 biotinylated detection antibody and avidin-HRP conjugate will show blue.
Histological study
The brain was removed from each animal and fixed in 10% formalin for the preparation of a slide to investigate the histopathological changes in both the control and treated groups according to the Mescher method [21] with the aid of the light microscope.
Statistical analysis
The Statistical Package for Social Scientists (SPSS version 18.0) and Microsoft Office Excel 2016 were used to examine the data. A one-way ANOVA with LSD post hoc test for significance was used to assess if there was a significant difference between the groups’ means. The paired t-test was used for mean comparisons, where p<0.05 values were considered significant [25].
RESULTS
Carnitine-loaded chitosan nanoparticles (LC-NPs)
The nanoparticles were produced by interactions between the positively charged chitosan and the negatively charged phosphate groups of TPP in the ionic gelation technique.
Characterization of L-carnitine nanoparticles (LC-NPs)
Different spectrophotometric techniques like SEM analysis investigated the morphology, elemental composition, crystalline nature, and stability of synthesized LC-NPs.
SEM (scanning electron microscope)
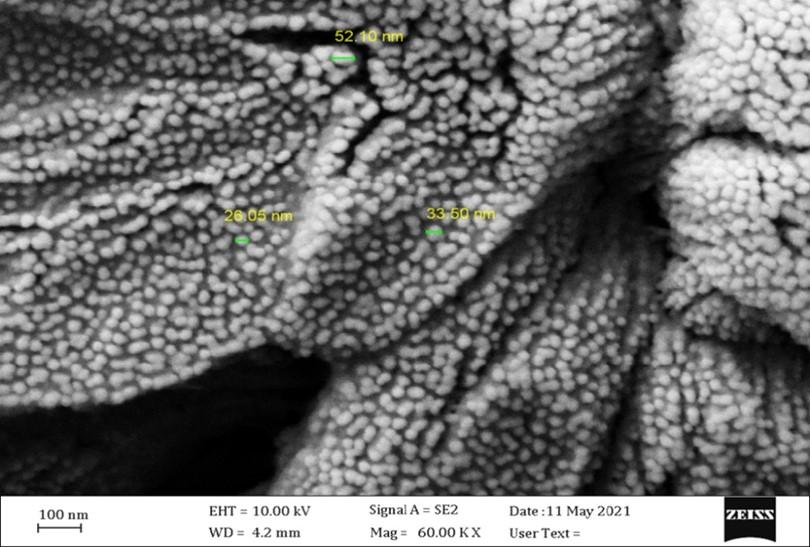
Scanning electron microscope analysis of prepared chitosan nanoparticles to compare this with L-Carnitine nanoparticles (LC-NP) with different magnification pictures shows the distribution and nanoparticles size as shown in Figure 2. The results of SEM images show that synthesized LC-NPs were smooth, spherical particles, singular or in aggregates with particle sizes in the range of (26.05), (33.50), and (52.10 nm).
Liver functions
Table 1 shows the enzyme activity of AST, ALT, and ALP (alkaline phosphatase) to be significantly increased (P<0.05). While the enzyme activity of the liver (AST, ALT, and ALP) decreased significantly (P<0.05) in the nano L-carnitine and (L-carnitine) groups. Also exhibited a significant (P<0.05) decrease in lead + carnitine and lead + nano carnitine groups when compared with the lead acetate group. This indicates that the nano carnitine has more ameliorative effects on lead toxicity in animals.
Table 2 depicts the distinguishing aspects of kidney function (creatinine and urea). When lead acetate was given, serum creatinine and urea levels rose considerably (P<0.05) compared to the other experimental groups. Nano L carnitine with lead acetate resulted in significant (P<0.05) ameliorative effects on the creatinine and urea levels in the rats. In contrast, the impact on the L-carnitine and nano L-carnitine group alone was lower than that in the control group.
The results in Table 3 revealed a significant (P<0.05) increase in serum MDA level in the lead acetate group (8.2 ± 0.47 nmol/ml) compared with control (5.9 ± 0.36 nmol/ml) and other treated groups. In contrast, no significant (P<0.05) difference was recorded in MDA level in L-carnitine and nano L-carnitine treated group compared with the control group. The MDA level was significantly decreased in lead acetate + nano L-carnitine treated groups (6.9 0.56) compared to the lead acetate group (8.2 0.47). This revealed a significant (P<0.05) decrease in serum GSH level in the lead acetate group (83.9 5.29) compared to the control (119.5 6.81) and other groups. In contrast, there was a substantial (P<0.05) rise in blood GSH levels in the lead acetate + nano L-carnitine, nano L-carnitine, and L-carnitine groups as compared to the lead acetate group (83.9 5.29).
Table 1. Effects of different treatments on liver enzyme activity in male rats.
Table 2. Effects of different treatments on kidney enzyme activity in male rats.
Table 3. Effects of different treatments on oxidative activity in male rats.
Histological examination of the brain (cerebellum cortex)
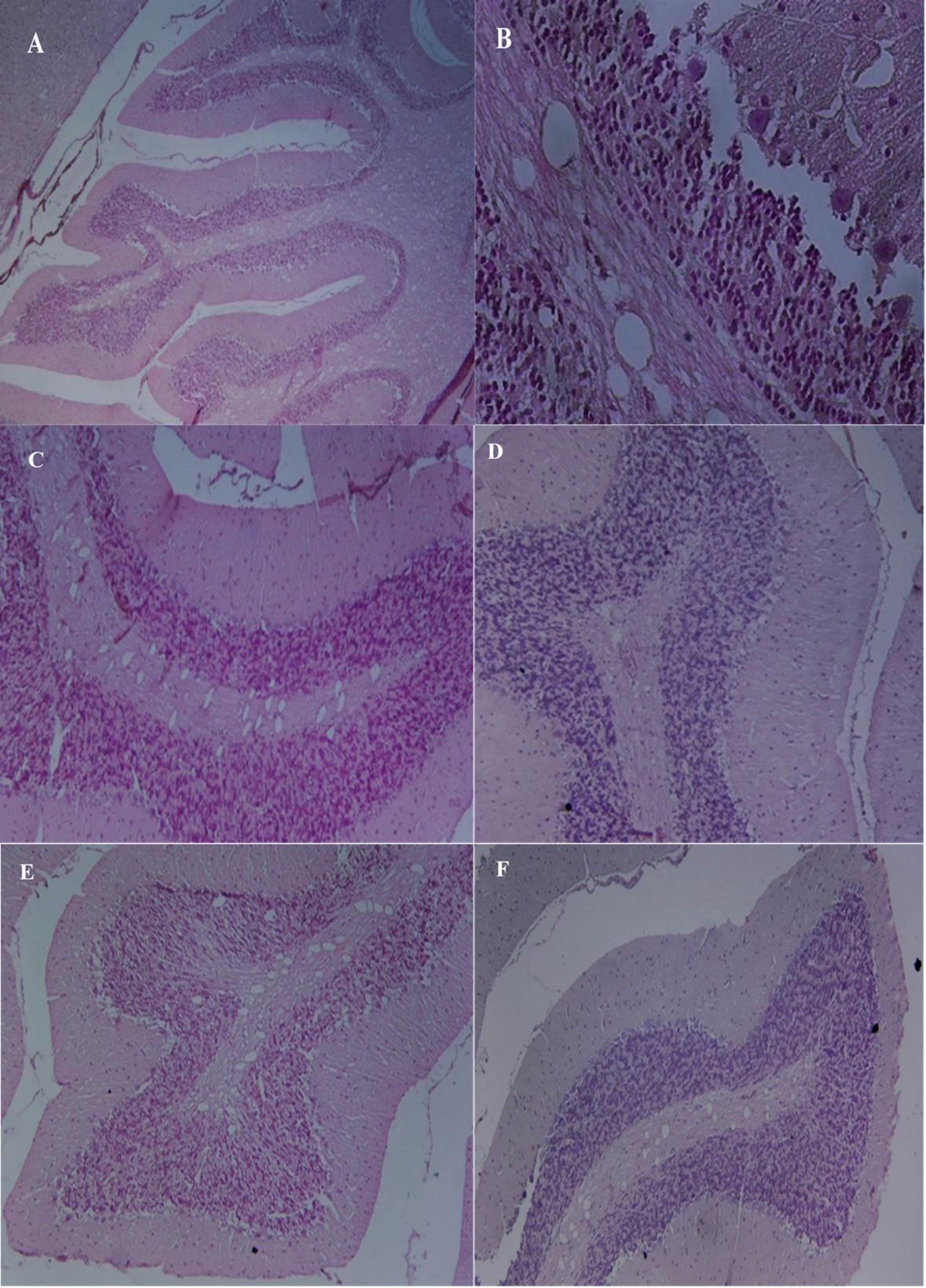
Histological examination of cerebellum sections of Control animals was found to have a normal structure, with an outside molecular layer, an inner granular layer, and a single layer of Purkinje cells in between (Figure 3A and 3B). The treated rats in the lead acetate group exhibited signs of disrupting the three-layer structure. Degeneration, necrosis, and a reduction in the number of Purkinje cells and molecular cells were seen in the interval between Purkinje cells and a molecular granular layer (Figure 3A and 3B). The antioxidative effect of L-carnitine was identified in the groups where the rats were administrated lead acetate with a combination of L- carnitine or nano L- carnitine. L-carnitine reduced the dangerous effect of lead, which showed normal Purkinje cells and normal arrangement of cerebellum layers (Figure 3C and Figure 3D). When rats were given L-carnitine and nano L-carnitine, they protected and developed brain tissue. There was no histological change in the cerebellar cortex, which revealed normal architecture and different layers of the cerebellum after treatment with l-carnitine and nano L-carnitine (Figure 3E and Figure 3F).
DISCUSSION
Ionic gelation was used to produce nano chitosan utilizing two forms of chitosan, soluble chitosan (low molecular weight) and insoluble (high molecular weight) chitosan (high molecular weight as well as the diacylation percent) [26]. Chitosan nanoparticles may be synthesized via the gelation method based on the cross-linking of anionic molecules (TPP) [27]. The scanning electron microscope technique was employed to visualize the size and shape of compounds. The SEM characterizations of the synthesized chitosan nanoparticles are shown in Figure 2. The image of SEM exhibited relatively smooth nano-spherical particles, singular or in aggregates with particle sizes (26.05), (33.50) and (52.10 nm). The above results agree with the authors [28]. This observation of SEM images of optimized formulation (O.P.) showed that the chitosan nanoparticles were spherical. In the optimal formulation, the lecithin/chitosan nanoparticles are spherical in shape, uniform, and polydisperse, with sizes ranging from 200 to 350nm with an average size of 273nm in diameter. Saglam et al. [29] mentioned that the chitosan nanoparticles loading oxaliplatin mean size and their homogeneous size distribution, without forming aggregated, besides showing they are spherical [30]. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate. SEM characterized their physical and structural morphology, and spherical N.P.s were successfully synthesized with a mean diameter of 174–898 nm [31]. SEM characterized the morphological properties of the nanoparticles. Chitosan nanoparticles were found to have a smooth surface morphology [32]. Similar results reported that SEM profiles of smooth and spherically shaped GNPs synthesized of L-asparaginase showed little homogeneity and clumps of spherical structures [33].
In Table 1 ALT, ALP, and AST enzymes are highly concentrated in the liver. Because of liver development and repair, these enzymes are generally detected in small amounts in the bloodstream. When administrated lead acetate and compared to a control, blood liver enzymes (AST, ALT, and ALP) increased considerably; the liver was involved in detoxifying hazardous compounds such as lead acetate, which are removed by the liver following metabolism and breakdown. This process may cause cell membrane rupture, increasing serum liver enzymes. Also, rats treated with lead developed liver dysfunction and damage and histological changes demonstrated by higher ALT, AST, and ALP blood levels. L-carnitine and nano L-carnitine, when given together with lead, reduced liver damage significantly and decreased the consequences of acute toxic liver damage, according to plasma biochemical markers [34].
This is due to the role of the L-carnitine in increasing the production of amino acids. Especially methionine, which turns into cysteine, which is an antioxidant. It has a role in directly removing oxidative compounds or being one of the components of glutathione as an antioxidant. In addition, it protects cells from toxic substances and pollutants [35]. The blood urea and creatinine levels of the control and both the nano and L-carnitine treated groups were considerably lower than the lead-induced toxicity group, as shown in Table 2. Protein metabolism’s main nitrogen-containing metabolic product is urea. Urea and creatinine levels in the blood are used as markers of renal function [36]. Furthermore, a prior investigation employed elevated blood creatinine levels as a diagnostic marker of renal failure [37]. The administration of both L-carnitine and Nano L-carnitine in combination with lead showed a significant reduction in blood urea and creatinine in the L-carnitine treated groups suggesting that it protects renal function against lead toxicity. Renal damage may take two forms: reversible and irreversible interstitial nephropathy. Reversible interstitial nephropathy is more frequent in long-term occupational lead exposure petering [38,39].
The results of oxidative stress biomarkers revealed a significant decrease in antioxidant enzyme activities (glutathione peroxidase), and an increase in the level of lipid peroxidation products (MDA) in rats given lead acetate compared to the control group (Table 3). Current results indicate a change in oxidant/antioxidant status of serum and the PbAc treated group. Moreover, the results have been attributed to oxidative stress induced by PbAc, causing ROS formation, including different pathways including hydroperoxides, singlet oxygen, and hydrogen peroxides, resulting in cellular dysfunction. These results agree with [40, 41]. The extensive lipid peroxidation caused by lead exposure releases lipid hydroperoxides into the circulation, causing significant tissue oxidative damage [42,43,44]. The impairment of mitochondrial oxidative phosphorylation, the collapse of mitochondrial membrane potential, the inhibition of mitochondrial respiratory enzyme activities, ATP depletion, and energy crises are only a few of the adverse outcomes seen in many models of Pb-induced toxicity [45,46]. To assess lead-induced oxidative damage, researchers examined a variety of antioxidant enzymes and compounds. The most widely utilized ones are reduced, such as GSH, glutathione disulfide, GPX, superoxide dismutase (SOD), and catalase (CAT) activity [47].
Cellular molecules are protected from free radical damage by antioxidant enzymes such as CAT, SOD, and GPX [48,49]. The activity of these enzymes was decreased significantly for the administration of lead acetate with L-carnitine. In contrast, these effects declined to non-significant levels compared to the control group in groups treated with nano L-carnitine and lead acetate. L-carnitine acts as an antioxidant by reducing metabolic stress [50].
Alzheimer’s disease and geriatric depression have lately been linked to the use of L-carnitine in treating Parkinson’s disease [51,52]. L-carnitine decreases exercise-induced oxidative stress, enhances antioxidant levels, and improves performance in individuals with end-stage renal failure [53,54]. L-carnitine exhibited antioxidative effects by lowering MDA, hydrogen peroxide cytotoxicity, and enhancing CAT and SOD activity in hepatocytes [55]. In addition to activating antioxidant enzymes like GSH, L-carnitine may also protect mitochondrial electron transport chain competence and scavenge free radical activity [56,57]. Lead acetate-treated rats showed the harmful effect of lead on the brain, especially the cerebellum. The BBB is permeable to lead acetate, as evidenced by the brain accumulating lead acetate when blood Pb levels increase [58]. Overwhelming epidemiological evidence shows that low-level Pb exposure causes developmental neurotoxicity [59].
This corresponds to the study [60] on how it was assumed that lead-induced brain toxicity is primarily due to oxidative stress. There was support for this work from other researchers [61]. Reduced intracellular antioxidant enzymes and lipid peroxidation products are signs of oxidative stress in the plasma and brain tissue of rats exposed to lead. Total carnitine levels in old rats were increased by giving them a daily dosage of L.C. in drinking water of 100 mg/kg of body weight [62]. L-carnitine for neuroprotection in several disorders, including hypoxia-ischemia and traumatic brain injury, Alzheimer’s disease, and conditions leading to central or peripheral nervous system injury and improve energy status, decrease oxidative stress and prevent cell death in models of adult, neonatal, and pediatric brain injury [63]. L-carnitine has a strong neuroprotective impact when given immediately after reperfusion from acute global cerebral ischemia [64]. Carnitine is a potent free radical scavenger, which means it may protect tissues from oxidative damage by neutralizing free radicals. It also may have a role in decreasing oxidative damage reported in several hereditary neuro-metabolic illnesses. According to new studies, decreased L-carnitine concentrations have been seen with specific disorders, in part because of the compound’s interaction with accumulating toxic metabolites, particularly organic acids, or because of protein restriction [64]. Because of the increased formation of reactive species in these disorders, L-carnitine supplementation may help prevent tissue shortage in this element and prevent oxidative damage. L- carnitine’s capacity to pass the blood-brain barrier suggests that it may be useful in reducing neurological damage caused by oxidative toxicity [65].
As L.C. may also help the body with energy metabolism and protect cells from damage, multiple studies have shown this chemical to have protective, modulatory, and trophic effects on the brain. Under metabolically challenged situations, free acetyl-CoA and ketosis may become critical for brain functioning despite the brain’s low level of -oxidation [66]. L-Carnitine increased metabolic stress caused by mitochondrial dysfunction and reactive oxygen species (ROS) generation may defend cells from oxidative damage in major neurodegenerative disorders [67,68]. These enzymatic antioxidants are crucial because they detoxify H2O2 in the water. After chronic hypoperfusion in rats, L.C. reduced white matter damage and offered neuroprotection in old mice by enhancing the brain’s antioxidant activity [69].
CONCLUSIONS
The conjugated L-carnitine on chitosan nanoparticles was successfully synthesized based on the reaction between dissolving CS+LC in an aqueous acidic solution to obtain cationic chitosan+ L-carnitine and have successfully incorporated the powerful antioxidant. Biogenic L-carnitine nanoparticles (LC-NPs) alleviate the harmful effects of lead further than/ or equal to ordinary L-carnitine with a less administrated dose than L-carnitine. LC-NPs improve the liver enzyme, kidney function, antioxidant enzyme activity, and histopathological changes in the brain cerebellum returned to normal levels.
ACKNOWLEDGEMENT
We would like to express our gratitude and appreciation to Dr. Ali Majeed Emara, who is invaluable in helping to do statistics, and to Dr. Labib for helping with nanomaterial work and characterization.
AUTHOR CONTRIBUTIONS
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Conception and design of the study: Hayder Talib Mahdi. Drafting the manuscript: Rashad Fadhil Ghadhban. Revising the manuscript: Hayder Talib Mahdi and Rashad Fadhil Ghadhban. Analysis and/or interpretation of data: Hayder Talib Mahdi and Rashad Fadhil Ghadhban.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Abdulidha NA, Jaccob AA, AL-Moziel MS. Protective effects of Co-Q10, Ginkgo biloba, l-carnitine on brain, kidney, liver, and endocrine system against sub-acute heavy metals toxicity in male rats. Toxicology and Environmental Health Sciences. 2020; 12(4): 331-341. DOI: 10.1007/s13530-020-00061-7.
- [2]Alwan S, Al-Saeed M, Abid H. Safety assessment and biochemical evaluation of biogenic silver nanoparticles (using bark extract of C. zeylanicum) in Rattus norvegicus rats. Baghdad Journal of Biochemistry and Applied Biological Sciences. 2021; 2(03): 138-150. DOI: org/10.47419/bjbabs.v2i03.67.
- [3]Alwan SH, Al-Saeed MH. Biosynthesized silver nanoparticles (using Cinnamomum zeylanicum bark extract) improve the fertility status of rats with polycystic ovarian syndrome. Biocatalysis and Agricultural Biotechnology. 2021; 38(3) DOI: org/10.1016/j.bcab.2021.102217.
- [4]Nam SM, Seo JS, Nah, SS, Chang, BJ. Effects of ascorbic acid on osteopontin expression and axonal myelination in the developing cerebellum of lead-exposed rat pups. International journal of environmental research and public health. 2019; 16(6): 983. DOI: org/10.3390/ijerph16060983.
- [5]Zaboon MH, Saleh AA, Al-Lami HS. Synthesis, characterization and cytotoxicity investigation of chitosan-amino acid derivatives nanoparticles in human breast cancer cell lines. Journal of the Mexican Chemical Society. 2021; 65(2): 178-188. DOI: org/10.29356/jmcs.v65i2.1265.
- [6]Abdul HM, Butterfield DA. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and alpha-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Free Radical Biology and Medicine. 2007; 42(3): 371–384. DOI: 10.1016/j. freeradbiomed.2006.11.006.
- [7]Ahmed HA, Ali HA, Mutar TF. Protective effects of olive leaf extract against reproductive toxicity of the lead acetate in rats. Environmental Science and Pollution Research. 2021; 28(44): 63102-63110. DOI: 10.1007/s11356-021-15240-3.
- [8]Ahmed TA, Aljaeid BM. Preparation, characterisation, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug design, development and therapy. 2016; 10(483): DOI: 10.2147/DDDT.S99651.
- [9]Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of animal science and biotechnology. 2016; 7(1): 1-14. DOI: org/10.1186/s40104-016-0097-5.
- [10]Al-Dulimi AG, Al-Saffar AZ, Sulaiman GM, Khalil KA, Khashan KS, Al-Shmgani HS, et al. Immobilisation of l-asparaginase on gold nanoparticles for novel drug delivery approach as anticancer agent against human breast carcinoma cells. Journal of Materials Research and Technology. 2020; 9(6): 15394-15411. DOI: org/10.1016/j.jmrt.2020.10.021.
- [11]Al-Fartosy AJM, Awad NA, Alsalimi SA. Insulin resistance and specific biomarkers in blood and urine of type 2 diabetic patients with or without nephropathy in Basrah, Iraq. African Journal of Biochemistry Research. 2020; 14(4):125-134. DOI: 10.5897// AJBR2020.1101.
- [12]Alharthi WA, Hamza RZ, Elmahdi MM, Abuelzahab HS, Saleh H. Selenium and L-carnitine ameliorate reproductive toxicity induced by cadmium in male mice. Biological Trace Element Research. 2020; 197(2): 619-627. DOI: 10.1007/s12011-019-02016-7.
- [13]Ali R, Hou GJ, Zhu ZG, Yan QB, Zheng QR, Su G. Stable mixed group II (Ca, Sr) and XIV (Ge, Sn) lead-free perovskite solar cells. Journal of Materials Chemistry A. 2018; 6(19): 9220-9227. DOI: org/10.1039/C8TA01490F.
- [14]Al-Okaily BN, Murad HF. Role of alpha lipoic acid in protecting testes of adult rats from lead toxicity. Iraqi Journal of Veterinary Sciences. 2021; 35(2): 305-312. DOI: 10.33899/ijvs.2020.126814.1386.
- [15]Anjum MR, Reddy PS. Recovery of lead‐induced suppressed reproduction in male rats by testosterone. Andrologia. 2015; 47(5): 560-567. DOI: 10.1111/and.12303.
- [16]Assi MA, Hezmee MN, Sabri MY, Rajion MA. The detrimental effects of lead on human and animal health. Veterinary world. 2016; 9(6): 660-671. DOI: 10.14202/vetworld. 2016.660-671.
- [17]Al-Megrin WA, Alkhuriji AF, Yousef AO, Habotta OA, Kassab RB, Metwally D, et al. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants. 2019; 9(1): 10. DOI: 10.3390/antiox9010010.
- [18]Basiouny FES, Mohamed FB, Ehab T. Ameliorating role of L-carnitine and Ginkgo biloba extract on pentylenetetrazole induced bone marrow injury in epileptic seizures disease in the rat. GSC Advanced Research and Reviews. 2020; 5(2): 001-011. DOI: 10.30574/gscarr.2020.5.2.0092.
- [19]Caito SW, Aschner M. Mitochondrial redox dysfunction and environmental exposures. Antioxidants & redox signaling. 2015; 23(6): 578-595. DOI: 10.1089/ars.2015.6289.
- [20]Debnath SK, Saisivam S, Debanth M, Omri A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PloS one. 2018; 13(1): e0190976. DOI: 10.1371/journal. pone.0190976.
- [21]Dkhil MA, Moneim AEA, Al-Quraishy S. Indigofera oblongifolia ameliorates lead acetate-induced testicular oxidative damage and apoptosis in a rat model. Biological trace element research. 2016; 173(2): 354-361. DOI: 10.1007/s12011-016-0689-0.
- [22]Dugard P. Randomisation tests: A new gold standard. Journal of Contextual Behavioral Science. 2014; 3(1): 65-68. DOI: org/10.1016/j.jcbs.2013.10.001.
- [23]El‐Ghaffar MA, Hashem MS. Immobilisation of α‐amylase onto chitosan and its amino acid condensation adducts. Journal of applied polymer science. 2009; 112(2): 805-814. DOI: org/10.1002/app.29292.
- [24]El‐Ghaffar MA, Atia KS, Hashem MS. Synthesis and characterisation of binary copolymers of methyl methacrylate with glycidyl methacrylate and 2‐hydroxy ethyl methacrylate as carriers for cellulase. Journal of Applied Polymer Science. 2010; 117(2): 629-638. DOI: org/10.1002/app.30063.
- [25]Essawy AE, El-Sayed SA, Tousson E, El-gawad A, Horeya S, Alhasani RH, et al. Anti-kindling effect of Ginkgo biloba leaf extract and L-carnitine in the pentylenetetrazol model of epilepsy. Environmental Science and Pollution Research. 2022; 1-15. DOI: 10.1007/s11356-022-19251-6.
- [26]Fajardo AR, Pereira AG, Martins AF, Paulino AT, Muniz EC, Hsieh YL. Chitin and chitosan-based (NANO) composites. John Wiley & Sons Inc. 2017; 671-700: DOI: 10.1002/9781119441632 . ch147.
- [27]Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, Chatzinikolaou,A, et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med Sci Sports Exerc. 2010; 42(10): 1809-18. DOI: 10.1249/MSS.0b013e3181dbacab.
- [28]Ferreira GC, McKenna MC. L-Carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochemical research. 2017; 42(6): 1661-1675. DOI: 10.1007/s11064-017-2288-7.
- [29]Glicklich D, Frishman WH. The Case for Cadmium and Lead Heavy Metal Screening. The American journal of the medical sciences. 2021; 362(4): 344-354. DOI: 10.1016/j.amjms.2021.05.019.
- [30]Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. Journal of veterinary internal medicine. 2014; 28(6): 1676-1683. DOI: 10.1111/jvim.12445.
- [31]Hammed MS. Evaluation of Performance of Date Palm Pollen on Urea and Creati-nine Levels in Adult Female Rats Exposed to Lead Acetate Intoxication. International Journal of Biomedicine and Advanced Researches. 2015; 6(1) 20: DOI: org/10.7439/ijbar.v6i1.1565.
- [32]Hinerfeld D, Traini MD, Weinberger RP. Cochran B, Doctrow SR, Harry J, et al. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase two null mice. Journal of Neurochemical. 2004; 88(3): 657–667. DOI: 10.1046/j.1471-4159.2003.02195.x.
- [33]Hou P, Wang F, Luo B, Li A, Wang C, Shabala L, et al. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants. 2021; 10(3): 436. DOI: 10.3390/plants10030436.
- [34]Hudson SA, Tabet N. Acetyl‐l‐carnitine for dementia. Cochrane Database of Systematic Reviews. 2003; (2): DOI: 10.1002/14651858.CD003158.
- [35]Ilk S, Sağlam N, Özgen M, Korkusuz F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. International journal of biological macromolecules. 2017; 94: 653-662. DOI: 10.1016/j.ijbiomac.2016.10.068.
- [36]ackie T, Haleagrahara N, Chakravarthi S. Antioxidant effects of Etlingera elatior flower extract against lead acetate-induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats. BMC research notes. 2011; 4(1): 1-8. DOI: 10.1186/1756-0500-4-67.
- [37]Juliet PA, Joyee AG, Jayaraman G, Mohankumar MN, Panneerselvam C. Effect of L-carnitine on nucleic acid status of aged rat brain. Experimental neurology. 2005; 191(1): 33-40. DOI: 10.1016/j.expneurol. 2004.09.009.
- [38]Khatab LA, Abdel-Raheem IT, Ghoneim AI. Protective effects of melatonin and l-carnitine against methotrexate-induced toxicity in isolated rat hepatocytes. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2022; 395(1): 87-97. DOI: 10.1007/s00210-021-02176-1.
- [39]Kumar A, Kumar A, MMS CP, Chaturvedi AK, Shabnam AA, Subrahmanyam G, et al. Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. International journal of environmental research and public health. 2020; 17(7): 2179. DOI: 10.3390/ijerph17072179.
- [40]Kumar SD and Kumar SS. Effect of heat treatment conditions on ballistic behaviour of various zones of friction stir welded magnesium alloy joints. Transactions of Nonferrous Metals Society of China. 2021; 31(1): 156-166. DOI: org/10.1016/S1003-6326(20)65484-X.
- [41]Li JL, Wang QY, Luan HY, Kang ZC, Wang CB. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. Journal of biomedical science. 2012; 19(1): 1-9. DOI: org/10.1186/1423-0127-19-32.
- [42]Lopes ACBA, Peixe TS, Mesas AE, Paoliello M. Lead exposure and oxidative stress: a systematic review. Reviews of Environmental Contamination and Toxicology. 2016; 236: 193-238. DOI: 10.1007/978-3-319-20013-2_3.
- [43]Matos BN, Pereira MN, Bravo MDO, Cunha-Filho M, Saldanha-Araújo F, Gratieri T, et al. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. International journal of biological macromolecules. 2020; 154: 1265-1275. DOI: 10.1016/j.ijbiomac.2019.11.001.
- [44]Morris GA, Kök SM, Harding SE, Adams GG. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnology and genetic engineering reviews. 2010; 27(1): 257-284. DOI: 10.1080/02648725.2010.10648153.
- [45]Munir M, Habib M, Khan SA, Kim MH, Lee S, Song TK, et al. Energy storage and piezoelectric properties of lead‐free SrTiO3-modified 0.965 Bi0. 5Na0. 5TiO3–0.035 BaTiO3 ceramics. Journal of Materials Science: Materials in Electronics. 2021; 32(8): 10712-10725. DOI: org/10.1007/s10854-021-05728-6.
- [46]Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. International Journal of Biological Macromolecules. 2020; 164: 2726-2744. DOI: org/10.1016/j.ijbiomac.2020.08.153.
- [47]Ommati MM, Jamshidzadeh A, Heidari R, Sun Z, Zamiri MJ, Khodaei F, et al. Carnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanisms. Biological trace element research. 2019; 187(1): 151-162. DOI: 10.1007/s12011-018-1358-2.
- [48]Owen L, Sunram-Lea SI. Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients. 2011; 3(8): 735-755. DOI: 10.3390/nu3080735.
- [49]Ozsoy SY, Ozsoy B, Ozyildiz Z, Aytekin I. Protective effect of L-carnitine on experimental lead toxicity in rats: a clinical, histopathological and immunohistochemical study. Biotechnic & Histochemistry. 2011; 86(6): 436-443. DOI: 10.3109/10520295.2010.529825.
- [50]Patwa J, Sharma A, Flora SJ. Arsenic, cadmium, and lead. In Reproductive and Developmental Toxicology. 2022; (pp. 547-571). DOI: 10.1016/B978-0-12-804239-7.00031-7.
- [51]Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Molecular psychiatry. 2000; 5(6): 616-632. DOI: 10.1038/sj.mp.4000805.
- [52]Qian Y, Wang L, Evelyn T. Tiffany-Castiglioni. Lead-Modulated Gene Expression in the Central Nervous System. Reference Module in Biomedical Sciences. 2014; DOI: 10.1016/B978-0-12-801238-3.01926-7.
- [53]Qiao K, Xu L, Tang J, Wang Q, Lim KS, Hooper G, et al. The advances in nanomedicine for bone and cartilage repair. Journal of Nanobiotechnology. 2022; 20(1): 1-42. DOI: org/10.1186/s12951-022-01342-8.
- [54]Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environmental monitoring and assessment. 2019; 191(7): 1-21. DOI: 10.1007/s10661-019-7528-7.
- [55]Rathbone MJ, Hadgraft J, Roberts MS. Modified-release drug delivery technology. Drugs and the pharmaceutical sciences. 2003; 126: 101-114. DOI: org/10.1201/9780203910337.
- [56]Renugadevi J, Prabu SM. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Experimental and Toxicologic Pathology. 2010; 62(5): 471-481. DOI: 10.1016/j.etp.2009.06.006.
- [57]Ribas GS, Vargas CR, Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014; 533(2): 469-476. DOI: 10.1016/j.gene.2013.10.017.
- [58]Rosenthal RE, Williams R, Bogaert YE, et al. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-L-carnitine. Stroke. 1992; 23(9): 1312-1317. DOI: 10.1161/01.str.23.9.1312.
- [59]Al-Mosawi RAS, Al-Fartosi KG. Oxidant and Antioxidant Status of patients with Sickle cell–ß Thalassemia in Thi-Qar Province, Iraq. Research Journal of Pharmacy and Technology. 2020; 13(10): 4796-4800. DOI: 10.5958/0974-360X.2020.00843.4.
- [60]Salama A, Elgohary R. L-carnitine and Co Q10 ameliorate potassium dichromate-induced acute brain injury in rats targeting AMPK/AKT/NF-κβ. International Immunopharmacology. 2021; 101: 107867. DOI: 10.1016/j.intimp. 2021.107867.
- [61]Sawicka AK, Renzi G, Olek RA. The bright and the dark sides of L-carnitine supplementation: a systematic review. Journal of the International Society of Sports Nutrition. 2020; 17(1): 1-10. DOI: 10.1186/s12970-020-00377-2.
- [62]Shaban NZ, El-Kader A, Sara E, Mogahed FA, El-Kersh, MA, Habashy NH. Synergistic protective effect of Beta vulgaris with meso-2, 3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Scientific Reports. 2021; 11(1): 1-18. DOI: org/10.1038/s41598-020-80669-4.
- [63]Soltanzadeh M, Peighambardoust SH, Ghanbarzadeh B, et al. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials. 2021; 11(6): 1439. DOI: org/10.3390/nano11061439.
- [64]Sudjarwo SA, Giftania Wardani K. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Research in pharmaceutical sciences. 2017; 12(5): 381. DOI: 10.4103/1735-5362.213983.
- [65]Surai PF. Carnitine Enigma: From Antioxidant Action to Vitagene Regulation Part 2. Transcription Factors and Practical Applications. J Veter Sci Med. 2015; 3(2): 17. DOI: 10.13188/2325-4645.1000017.
- [66]anaka Y, Sasaki R, Fukui F, Waki H, Kawabata T, Okazaki M, et al. Acetyl-L-carnitine supplementation restores decreased tissue carnitine levels and impaired lipid metabolism in aged rats. Journal of Lipid Research. 2004; 45: 729–735. DOI: 10.1194/jlr.M300425-JLR200.
- [67]Tang ZX, Qian JQ, Shi LE. Preparation of chitosan nanoparticles as carrier for immobilised enzyme. Applied Biochemistry and Biotechnology. 2007; 136(1): 77-96. DOI: 10.1007/BF02685940.
- [68]Taskin T, Dogan M, Arabaci T. Bioassay-guided isolation and antiproliferative efficacy of extract loaded in chitosan nanoparticles and LC-QTOF-MS/MS analysis of Achillea magnifica. South African Journal of Botany. 2020; 133: 236-244. DOI: org/10.1016/j.sajb.2020.08.002.
- [69]Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterisation of extracellular vesicles. American Journal of Reproductive Immunology. 2021; 85(2): e13367. DOI: 10.1111/aji.13367.
- [70]Rogers WA. The tangled web of medical and commercial interests. Health Expectations: an International Journal of Public Participation in Health Care and Health Policy. 2007; 10(1): 1. DOI: 10.1111/j.1369-7625.2007.00432.x.
- [71]Ueno Y, Saito A, Nakata J, Kamagata K, Taniguchi D, Motoi Y, et al. Possible Neuroprotective Effects of l-Carnitine on White-Matter Microstructural Damage and Cognitive Decline in Hemodialysis Patients. Nutrients. 2021; 13(4): 1292. DOI: 10.3390/nu13041292.
- [72]Uwikor FK, Nwachuku EO, Igwe F, Echonwere B, Bartimaeus ES. Evaluation of Haematological Changes In Lead-Acetate-Induced Albino Rats Treated With Aqueous Extract Of Hypoestes Rosea Leaf. European Journal of Biomedical. 2020; 7(2): 509-517. DOI: 10.9734/jocamr/2020/ v9i130135.
- [73]Virgolini MB and Aschner M. Molecular mechanisms of lead neurotoxicity. In Advances in neurotoxicology. 2021; 5: 159-213 Academic Press. DOI: 10.1016/bs.ant.2020.11.002.
- [74]Virmani A and Binienda Z. Role of carnitine esters in brain neuropathology. Mol. Aspects Med. 2004; 25(6): 533–549. DOI: org/10.1016/j.mam.2004.06.003.
- [75]Virmani MA and Maria C. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. International Journal of Molecular Sciences. 2022; 23(5): 2717. DOI: 10.3390/ijms23052717.
- [76]Yang L, Li X, Jiang A, Li X, Chang W, Chen J, et al. Metformin alleviates lead-induced mitochondrial fragmentation via AMPK/Nrf2 activation in SH-SY5Y cells. Redox biology. 2020; 36: 101626. DOI: org/10.1016/j.redox.2020.101626.