Investigation of the relationship between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 with SARS CoV-2 infections
Abstract
SARS-CoV-2 stands for severe acute respiratory syndrome coronavirus 2. Matrix metalloproteinases- 9 (MMP-9) performs a crucial physiological role. In addition to its role in the molecular basis of lung fibrosis, this enzyme may also play a part in the “cytokine storm,” which may represent one of the potential scenarios during coronavirus infection. Tissue inhibitors of metalloproteinase (TIMPs) are well-known for their ability to regulate MMP activity during remodeling of the extracellular matrix. As cytokines, they are also thought of as signaling molecules that impact on a wide range of biological processes. This study aimed to investigate the link between each of MMP-9 and TIMP-1, and COVID19 disease. A total of 58 COVID-19 patients and 30 apparently healthy adults enrolled in this study. The ORF1ab, E and N genes of SARS-CoV-2 were detected using multiplex real-time PCR, while the ELISA technique was used to estimate the level of serum MMP-9, TIMP-1, and C-reactive protein (CRP). The study results demonstrated higher concentrations of MMP-9 in COVID-19 patients (2810 ± 1160 pg/ml) compared to controls (2110 ± 850 pg/ml), with non-significant differences (p=0.002). Unlike, TIMP-1, showed considerably higher levels in the patient’s group (541.53 ± 201.42 pg/ml) than in controls (276.33 ± 67.26 pg/ml) with high significant differences (p ≤ 0.001). Considering this study, TIMP-1 in COVID patients most likely play an important role in inflammatory response. Its clinical utility as a biomarker may be insufficient, but it provides a useful data in the diagnosis of COVID‐19.
INTRODUCTION
An infectious disease emerged in March 2020 as a pandemic, triggers acute respiratory tract diseases in humans, later identified as SARS disease corona virus-2 and is abbreviated by SARS-CoV-2 [1]. This virus, along with Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV), can lead to aggressive respiratory consequences [2]. The viral nucleic acid test is the most used diagnostic test for SARS-CoV-2. The structural proteins of SARS are spike (S), envelope (E), nucleocapsid (N) proteins, and membrane glycoprotein (M). The SARS-CoV-2 spike protein attaches to the receptor angiotensin-converting enzyme 2 (ACE2), allowing the virus to enter the host cell [3]. Mutations may alter this adhesion ability that the (M) protein and (S) protein undergo, as evidence suggests that some proteinases (especially MMPs) control those processes [4]. MMP-9 is a member of MMPs, a large family of 23 calcium-dependent, zinc-containing endoproteinases. These endoproteinases also degrade and remodel the extracellular matrix [5] and participate in several physiological functions, including wound healing, embryogenesis, reproduction, and tissue remodeling [6]. This enzyme may play a role in the “cytokine storm” that is one of the coronavirus infections features and in lung fibrosis-associated molecular mechanisms [7]. MMP9 is not expressed in healthy lung tissue but is released in case of inflammation like infections and neoplastic diseases; neutrophils, lymphocytes, macrophages, and mast cells are the common producing cells [8]. Since overexpression may be part of the pathology, which can lead to tissue damage and a set of pulmonary diseases, it is crucial to keep the balance between MMPs and TIMPs. TIMPs can down-regulate MMPs activities by covalently binding to the pre-or active forms of MMPs at molar equivalence [9]. They have both MMP-dependent and independent biological functions [10]. TIMPs can be indirectly regulate MMP-mediated processes such as cytokines processing, degradation of growth factor binding proteins, as well as the liberation of ECM-bound growth factors [11]. This regulation plays a vital role in balancing MMPs and TIMPs. Several pathologies may occur in case of any disturbance in their ratio [12]. TIMP-1 is one of the four investigated mammalian TIMPs, considered a soluble protein that can be secreted and vastly expressed upon inflammatory stimuli [13]. Through studying TIMP-1’s cytokine-like function, several studies have recognized complex regulatory networks that include a specific surface receptor and successive signaling pathways which eventually control the cell’s fate and behavior [14]. Recently, minimal data was provided about the diagnostic/therapeutic role of MMPs/ TIMPs in COVID-19 [15]. The objective of the present study was to evaluate the association between MMP-9, tissue inhibitor of metalloproteinase (TIMP-1), and COVID-19, as well as the diagnostic potential of these markers.
METHODS AND MATERIALS
Study subjects
SARS CoV-2 infected patients participated in this study. Blood samples were collected from patients admitted to several hospitals in Baghdad city. The Bioethics Committee approved the study (University of Baghdad- College of science- dept. of Biology Ref. no. CSEC/0222/0007). Fifty-eight COVID-19 patients who had a positive nasopharyngeal swab were selected. The control group included 30 healthy people whose nasopharyngeal was negative and matched in age and gender. Exclusion criteria included any healthy person who had a history of autoimmune disease, allergic reactions or was a pregnant woman.
Specimen collection and processing
The oropharyngeal swabs were taken and placed in a disposable viral sample tube with virus preservation solution. Specimens can be frozen and thawed up to five times for 24 hours at 2°C-8°C or three months at -70°C.
For virus inactivation, the water bath was preheated to 56°C, and the sealed package containing the specimen was sprayed with 75% ethanol in a biosafety cabinet for 30 minutes prior to placing it in the water bath. The specimen was gently mixed for 10 minutes to keep the collecting tube from floating.
An assay is carried out in the following manner. The qRT-PCR mix was made according to the instructions in Table 1. Two liters of internal control per test were transported to the negative control, positive control, and specimen for nucleic acid extraction.
Table 1. qRT-PCR mix preparation.
SARS-CoV-2 detection using multiplex PCR
SARS-CoV-2 is detected using a multiplex real-time polymerase chain reaction (RT-PCR) method that targets the virus’s ORF1ab, E, and N genes using particular primers and fluorescent probes. Fluorescence intensity is used to detect virus nucleic acid in real-time. Internal controls are implemented to ensure no PCR inhibitors are present in the samples, preventing false-negative results (Chengdu, China-based Maccura Biotechnology Co., Ltd.).
PCR analysis of the specimens
In a PCR reaction tube containing qRT-PCR mix, 40 L of RNA templates (nucleic acid isolated from the negative control, positive control, and specimen) were added. The plate’s lid was promptly fastened to avoid contamination.
Amplification through PCR (polymerase chain reaction): The negative control, positive control, and test specimen were all placed in the correct order, and the PCR reaction tube was inserted into the machine.
Deciding on a fluorescence route: The detection channels are FAM (Reporter: FAM, Quencher, None) and ROX (Reporter: ROX, Quencher, None); the internal control channels are HEX or VIC (Reporter, HEX\VIC, Quencher: None).
The reaction volume is 40 μl. Table 2 shows the cycling protocol. Once the first run was completed, the findings were kept. Set the baseline threshold level, start value, and end value for each channel manually: Set the start and end values to 3-15 and 5-20, respectively. The negative control (NG) should be higher than the fluorescence background. The NC’s NG, FAM, ROX, and CY5 amplification curves should be horizontal or lower than the threshold level, depending on the fluorescence background. The “analyze” button was pressed, and the results were displayed on the report screen (Maccura Biotechnology Co., Ltd.).
Table 2. PCR amplification protocol.
Detection of MMP-3, TIMP-2, and CRP using ELISA
Serum levels of MMP-9 (Catalogue No. SL 1157Hu), TIMP-1 (Catalogue No. SL 1711Hu), and CRP (Catalogue No. SL0535Hu) were determined using the Enzyme-linked immunosorbent assay (ELISA), this ELISA kit uses Sandwich-ELISA as the method (SunLong Biotech Co., LTD).
Hematological and biochemical analysis
Fresh whole blood samples were used for WBC count. Hemoglobin concentration and platelets counting was performed using the D-Cell 60 DIGON Ltd (Europe/Hungary) as a fully automated haematology analyzing device.
Erythrocyte sedimentation rate (ESR): 1.5 mL of blood preserved in an EDTA vial was added to 0.5 mL of sodium citrate solution, and the diluted blood was mixed well and then carefully drawn into a Westergren tube, and then the tube was placed in a Westergren rack in a vertical position for 1 h. Afterward, the distance in millimeters from the bottom of the surface meniscus to the top of the sedimented red blood cells was read. The result was read in millimeters per h.
Serum specimens were used for glucose levels measurement using Glucose – Cobas c501/ Roche/Hitachi Cobas c systems as a fully automated device. It is an UV test using enzymatic reference method with hexokinase. Hexokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate by ATP. Glucose-6-phosphate dehydrogenase oxidizes glucose-6-phosphate in the presence of NADP to gluconate-6-phosphate. No other carbohydrate is oxidized. The rate of NADPH formation during the reaction is directly proportional to the glucose concentration and is measured photometrically.
Body mass index (BMI) is a calculation of the relationship between weight and height, measuring the weight in kilograms divided by the height square in meters (kg/m). BMI is an indicator of body fatness. Studies show a link among body fat, BMI, and the health risk features; thus it is important detecting in BMI for evaluating the health risks and obesity [16].
Statistical analysis
SPSS version 24.0 software was used for the statistical analysis. Normality tests were conducted for data measurement where normal data was represented as mean and standard deviation [16], while a t-test was used for comparison between two groups to compare the differences in serum MMP-9 and TIMP-1 levels between the two groups. Correlations between MMP-9 and TIMP-1 were estimated by Pearson correlation analysis. A two-sided p < 0.05 are considered statistically significant.
RESULTS
Level of MMP-9 in SARS-CoV-2 infection
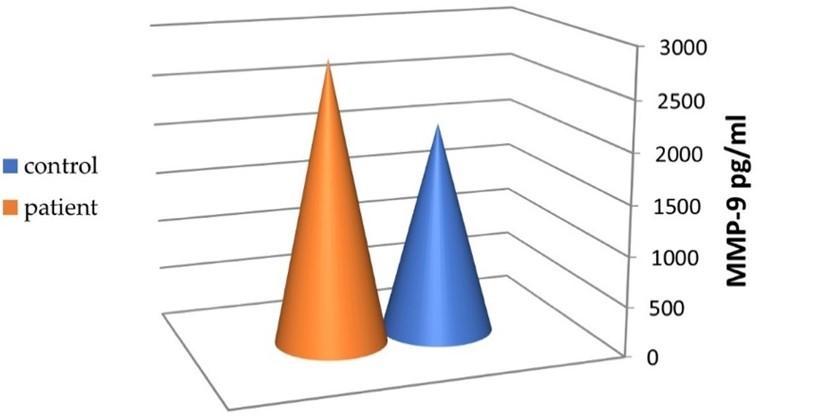
The MMP-9 serum levels in the patients and control group are shown in Figure 1. The mean level of MMP-9 was 2810±1160 pg/ml in the patient group and 2110 ± 850 pg/ml in the control group, with non-significant differences between the two groups (p=0.002).
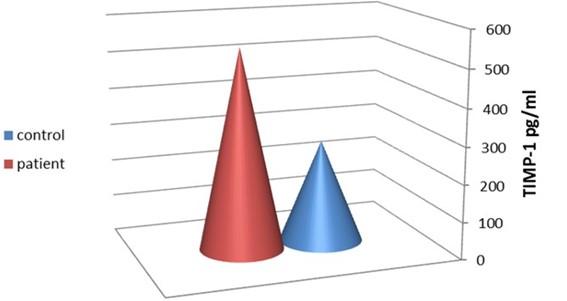
Level of TIMP-1 in SARS-CoV-2 infection
The mean level of TIMP-1 was 541.53 ± 201.42 pg/ml and 276.33 ± 67.26 pg/ml in the patients and controls, respectively. The variation was highly significant between these two groups (p ≤ 0.001), as shown in Figure 2.
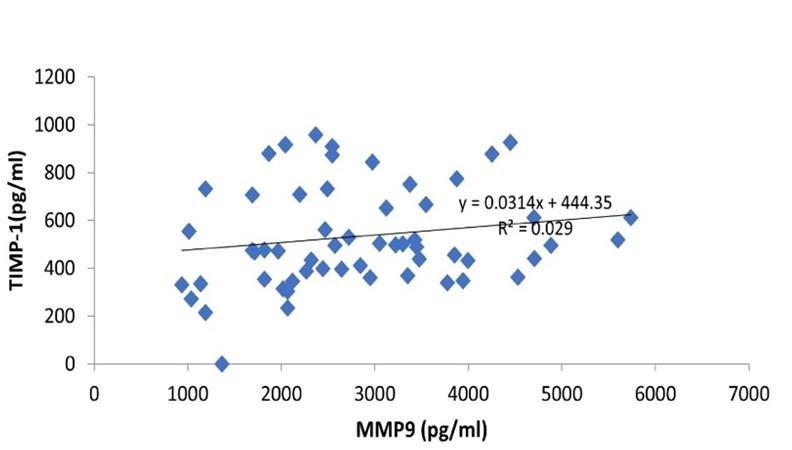
Correlation of MMP-9 and TIMP-1 in SARS-CoV-2 infection
Pearson correlation analysis demonstrated that serum levels of MMP-9 were significantly (p = 0.001) and positively correlated with serum TIMP-1 (Figure 3).
Hematological and biochemical parameters in SARS-CoV-2 infection
Regarding CRP, the results showed a notable elevation in patients than the controls, with highly significant differences between the two groups (p < 0.001) (45.12 ± 10.11 pg/ ml and 2.5 ± 0.59 pg/ ml, respectively) as shown in Figure 4.
Table 3 illustrates the hematological biochemical parameters examined in the laboratory. COVID-19 patients had a higher WBC count (12.11 ± 5.14) than the control group (5.75±1.67). The random blood sugar (RBS) and erythrocyte sedimentation rate (ESR) values of patients were significantly higher than the controls. In contrast, no significant differences in hemoglobin (Hb), platelet number, or body mass index (BMI) were detected between the two groups.
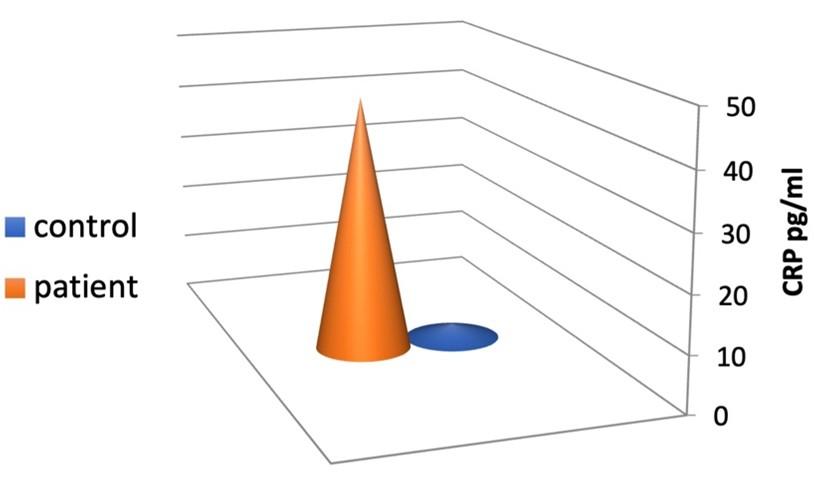
Table 3. Hematological and biochemical parameters of COVID-19 patients and healthy groups.
DISCUSSION
The present study investigated MMP-9 and TIMP-1 levels in serum during SARS-CoV-2 infection. Our study enrolled 58 adult hospitalized patients. Although the MMP-9 level was higher in patients, it did not change remarkably compared to the control group (p = 0.002). The concentration of MMP-9 in normal lung tissue is at the minimum level and starts to increase with various lung illnesses such as fibrosis, chronic obstructive pulmonary disease (COPD), and asthma. Yet, the role of MMP-9 during lung inflammation is not well understood [17]. In addition, the MMP-9 gene is up-regulated, and the protein contributes to cytokine recruitment in lung tissue of COVID-19 patients [18]. Moreover, it is induced by WBCs like neutrophils and monocytes, which may be parallel to our findings of a notable increase in total patient’s WBC count. MMP-9 production may be disease-stage related, as a recent study found that the release of MMP-9 during COVID-19 is not an early event in 18 patients of the WHO 3 subgroup compared to controls. All WHO subgroups had higher MMP-9 serum levels after a week of hospitalization compared to admission. Moreover, increased levels of MMP-9 were recorded after one month of admission in most of the patients [19]. MMP-9 levels may affect the progression of recovery from a disease. This study has some limitations. All the studied parameters were tested without making follow-up after patient admission, so no correlation between serum levels of studied biomarkers and the disease stage was assessed. Similarly, in 39 COVID-19 patients, an early increase of plasma MMP-9 was recorded, which also did not evaluate the correlation with the disease [20]. Another study on 175 patients reported a gradual increase of serum MMP-9 with the severity of COVID-19 [21]. These controversial levels of MMP-9s might depend on the sample type, whether serum or plasma. Therefore, higher levels of MMP-9 have been noted in sera samples as it is released during blood coagulation. Further studies with larger sample sizes and categorizing according to disease severity are required.
So far, the recognized four TIMP molecules (TIMP-1, -2, -3, and -4) were found to inhibit all known MMPs. TIMPs bind reversibly in a 1:1 stoichiometric ratio to the catalytic subunit of MMPs, consequently inhibiting their function. A member of the same β-coronavirus genus as SARS-CoV-2, murine hepatitis virus (MHV; m-β-CoV) was used in a recent study. It was demonstrated that during acute infection following MHV infection, TIMP-1 induction could serve as an antiviral host response to modulate MMP activities [22]. While TIMP-1 mRNA expression has been linked to viral virulence and load in earlier investigations [23]. In accordance with our study results, TIMP-1 is significantly increased in COVID-19 patients; this may add further information in understanding the role of TIMP-1 as its elevation could be part of a fundamental host defense mechanism against virus-induced metalloproteases [24]. MMPs substrate has expanded over time to include growth factors, chemokines, and hormones [25]. MMPs work on cytokines or chemokines that have been immobilized on ECM or cell surface. It was found that MMPs can release soluble effector molecules for successful inflammatory signaling like tumor necrosis factor (TNF) and interleukine-1 (IL-1). In pulmonary infection, MMPs induce the various cytokine and chemokine signals by host immune cells [26-29].
Changes in other blood parameters were noticed as COVID-19 individuals showed an increased level of CRP, which is considered a critical measure that turns obviously during infection [30]. Moreover, an elevated count of white blood cells was recorded [31], which was consistent with the current findings. A recent study found patients with increased WBC levels have a higher risk of death [32]. Furthermore, the SARS coronavirus infiltrates pancreatic islets and kills them, resulting in acute hyperglycemia [33].
CONCLUSION
The elevation tendency of TIMP-1 in COVID‐19 patients may refer to its importance as an advantage in disease identification. Consequently, this may pave the way for adopting TIMP-1 as a useful biological marker in diagnosing COVID‐19.
ACKNOWLEDGEMENTS
None
AUTHOR CONTRIBUTIONS
Conceptualization: MKI; Investigation: MKI and LMR; Resources: YBQ; Writing Original Draft Preparation: MKI, LMR and YBQ; Data analysis: YBQ. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ben Moftah M, Eswayah A. Intricate relationship between SARS-CoV-2–induced shedding and cytokine storm generation: A signaling inflammatory pathway augmenting COVID-19. Health Sciences Review. 2022; 100011.
- [2]Ismael MK. Article Review: Toll-like Receptors and COVID-19. Inter J Res Appl Sci Biotech. 2022; 9(2):78-95.
- [3]Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367: 1260–1263.
- [4]Hardy E, Patron CF. Targeting MMP-regulation of inflammation to increase metabolic tolerance to COVID-19 pathologies. A Hypothesis Biomol. 2021; 11:390.
- [5]Patron CF, Kassiri Z, Leung D. Modulation of systemic metabolism by MMP-2: From MMP-2 deficiency in mice to MMP-2 deficiency in patients. Compr. Physiol. 2016; 6: 1935–1949.
- [6]Hao W, Li M, Zhang Y, Zhang C, Xue Y. Expressions of MMP-12, TIMP-4, and neutrophil elastase in PBMCs and exhaled breath condensate in patients with COPD and their relationships with disease Severity and acute exacerbations. J Immunol Res. 2019; 2019: 1-10.
- [7]Charzewski L, Krzyśko KA, Lesyng B. Structural characterization of inhibitory and non‑inhibitory MMP‑9–TIMP‑1 complexes and implications for regulatory mechanisms of MMP‑9. Scientific Reports. 2021;11:13376.
- [8]Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJG. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLOS Pathogens. 2012; 8(4): e1002641.
- [9]Esa, SA, Rawy AM, El-Behissy MM, El-Bastawisy M. Study the level of sputum matrix metalloproteinase-9 and tissue inhibitor metaloprotienase-1 in patients with interstitial lung diseases. Egypt J Chest Dis Tuberc. 2016; 65: 303–309.
- [10]Allen JR, Ge1 L, Huang Y, Brauer R, Parimon T, Cassel S, et al. TIMP-1 promotes the immune response in influenza induced acute lung injury. Lung. 2018;196(6): 737–743.
- [11]Rossano R, Larocca M, Macellaro M, Bilancia D, Riccio, P. Unveiling a hidden biomarker of inflammation and tumor progression: The 65 kDa isoform of MMP-9 new horizons for therapy. Curr. Issues Mol. Biol. 2022; 44: 105–116.
- [12]Silva AM, Silva CI, Clemente M, Figueiredo JP, Pereira T, Gabriel A, et al. Evaluation of MMP-10 and TIMP-1 levels associated with Resveratrol supplementation. European Journal of Public Health. 2020; 30: 2.
- [13]Savarin C, Bergmann CC, Hinton DR, Stohlman SA. MMP independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis. ASN Neuro. 2013; 5:5.
- [14]Ries C. Cytokine functions of TIMP-1. Cell and Mol Life Sci. 2014; 71: 659–672.
- [15]Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov. 2020; 7:100052.
- [16]WHO 2021. Obesity and overweight. Accessed on July 28, 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- [17]Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell. Mol. Biol. 2003; 28: 12–24.
- [18]Hazra S, Chaudhuri AG, Tiwary BK, Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis. Life Sci. 2020; 257: 118096.
- [19]Gelzo M, Cacciapuoti S, Pinchera B, Rosa AD, Cernera G, Scialò F, et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID‑19 patients. Scientific Reports. 2022; 12:1212.
- [20]Ueland T. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J. Infect 2020; 81:e41–e43.
- [21]Abers MS, Delmonte OM, Ricotta EE, Fintzi J, Fink DL, Almeida de Jesus AA,et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021; 6: e144455.
- [22]Sengupta S, Addya S, Biswas D, Banerjee P, Sarma JD. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in murine β-coronavirus-induced neuroinflammation. Virology. 2022; 566: 122–135.
- [23]Zhou J, Stohlman SA, Atkinson R, Hinton DR, Marten NW. Matrix metalloproteinase expression correlates with virulence following neurotropic mouse hepatitis virus infection. Virol. 2002; 76 (15): 7374–7384.
- [24]Cui N, Hu M, Khalil, RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017; 147:1-73.
- [25]Young D, Das N, Anowai A, Dufour A. Matrix metalloproteases as influencers of the cells’ social media. Int. J. Mol. Sci. 2019; 16: 20. 6720954.
- [26]Hussein SA, Ismael MK, Abdulmajeed NG. Antineutrophil cytoplasmic antibodies in patients with tuberculosis. Iraqi J Sci. 2014; 55:360-366.
- [27]Nasser HA, Ismael MK, Al. Halbosiy MF. The relationship between Chlamydia pneumoniae infection and TNF-α in cardiovascular disease patients. Iraqi J Sci. 2018; 59: 1836-1842.
- [28]Al. Halbosiy MF, Ismael MK, Nasser HA. Correlation between Chlamydia pneumoniae infection and lipid profile in patients with cardiovascular diseases. Iraqi J Sci. 2019; 60(10): 2129-2135.
- [29]Hafid ES, Ismael MK. Detection of serum levels of IL-17 and CCL-5 in a sample of Iraqi pulmonary tuberculosis patients. Iraqi J Sci. 2021; 62(9): 2887-2893.
- [30]Ali N. Elevated level of C‐reactive protein may be an early marker to predict risk for severity of COVID‐19. letter to the editor. J Med Virol. 2020; 1–3.
- [31]Husain-Syed F, Wilhelm J, Kassoumeh S, Birk HW, Herold S, Vada´sz I, et al. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol Dial Transplant. 2020; 35: 1271–1274.
- [32]Zhu B, Feng X, Jiang C, Mi S, Yang L, Zhao Z, et al. Correlation between white blood cell count at admission and mortality in COVID-19 patients: a retrospective study. BMC Infect Dis. 2021; 21(574):1-5.
- [33]Thomas S. the structure of the membrane protein of SARS-CoV-2 resembles the sugar transporter semi SWEET. Pathog Immun. 2020; 5(1): 342-363.