Detection and biological control measures of anthracnose causing fungus isolated from Citrus limon (L.)
Abstract
Anthracnose is one of the most devastating fungi causing twig dieback and postharvest fruit decay. Present study was aimed to identification of anthracnose fungus. Infected leaves of Citrus limon were collected and cultured on potato dextrose agar (PDA) media for pathogenic fungus isolation. The isolated fungal pure culture was characterized by physiological and morphological characterization methods. Biological control measures of the fungus were evaluated by disc diffusion methods. The highest growth and development of isolated fungus was detected in PDA media at pH 7 in fructose as the best carbon source and 0.05gm NaCl concentrations at 37°C. Pathogenicity potency of isolate was performed on lemon, orange and malta, and showed typical anthracnose symptoms after incubation at 25°C for 5 days. For antifungal activity, 200µgm/disc methanolic extract of Psidium guajava showed 14.33±0.66 mm inhibition zone against the isolated fungus. From the present investigations, identified anthracnose causing fungus and it’s controlling techniques may help for further research for the isolation of drugs related compound for controlling this disease.
INTRODUCTION
Citrus limon is an important fleshy, juicy, and edible fruits (Rutaceae family). Lemon is originated in Asia and now growing universally in hot, sub-tropical, warm temperate countries and the Mediterranean region [1]. Lemon fruits are enriched with vitamin C, containing 64% of dietary value in 100 gm of consumption and contain numerous phytochemical and tannins [2]. Lemon juice contains about 47 gm/l citric acid [3]. Lemon fruits are the major source of citric acid in industries [4].
Anthracnose is one of the most devastating diseases of citrus causing twig dieback, premature leaf drop, dark staining on fruit and postharvest fruit decay, commonly caused by Colletotrichum gloeosporioide. Colletotrichum pathogens infect plants and develop dark, water-soaked lesions on stems, leaves, or fruits [5]. Anthracnose can rot a harvested lemon in just a few days. The fungal disease overwinters in fruits and on seeds. Presence of adequate moisture influence fungus germination, the development and infection. It is spread by wind, rain, insects and garden tools [6] during developmental and post-harvest stages [7]. Usually, phytopathogenic fungi are controlled by artificial fungicides. However, these fungicides are harmful to human health and the environment [8]. Moreover, there is no sufficient report on in vitro and in vivo controlling measures of the pathogenic fungus, studied in the present research.
Hence, we aimed to identify anthracnose-causing fungus on C. limon. Moreover, to evaluate the biological control measures of the isolated fungus to provide supportive data for establishment of a proper biological control system was aimed.
MATERIALS AND METHODS
Plant materials
Anthracnose disease-infected leaves of C. limon were used as plant materials for this present research. Anthracnose disease-infected leaves of C. limon were collected from the local market of Rajshahi, Bangladesh. Experiments were conducted at Microbiology and Biotechnology Lab., Department of Genetic Engineering and Biotechnology, University of Rajshahi, Rajshahi-6205, Bangladesh.
Isolation of pathogen
Leaves were surface disinfected by washing them with 1% sodium hypochlorite (HIMEDIA Laboratories Pvt. Ltd., India) for 1 min, and then washed extensively with sterile distilled water. Diseased parts were cut into small pieces and transferred to potato dextrose agar, sabouraud dextrose agar and nutrient agar media (HIMEDIA Laboratories Pvt. Ltd., India) using sterile forceps. Cultured plates were incubated in a dark place at room temperature.
Culture of isolates
PDA, SDA, and NA media (HIMEDIA Laboratories Pvt. Ltd., India) were used to culture of isolate. For media preparation, powdered was mixed with water according to the kit’s instructions. Ingredients of the media were suspended in 1000 ml distilled water and the pH was adjusted. Sterilization was done by autoclaving at 15 lbs pressure (121°C) for 15 minutes. The media were cooled to around 50°C and poured into Petri dishes which were covered immediately.
Lactophenol cotton blue staining
Isolated fungal conidia were transferred into a clean glass slide and 2-3 drops of Lactophenol Cotton blue (HIMEDIA Laboratories Pvt. Ltd., India) stain were added. A coverslip was carefully applied on the sample. After 5 minutes of allowing the stain to penetrate through the transferred colony and images were taken under a light microscope (BioBlue, Euromex, Netharlands).
Determination of temperature, pH, carbohydrates, and NaCl
Growth profiling of isolate was studied at 4-5°C, 21-25°C, and 37°C. Isolated pathogen was studied at different pH levels such as 3.0, 5.0, 7.0 and 9.0 which were adjusted before autoclaving for maintaining the pH constant. PDA media having 5% of Fructose, glucose, lactose, and maltose (HIMEDIA Laboratories Pvt. Ltd., India) were individually used. At first, PDA media was prepared in culture vessels with 5% of glucose, lactose, maltose, and fructose. After that, cultured vessels were incubated in room temperature. Five different concentrations of NaCl (FUJIFILM Wako Pure Chemical Corp. Japan) such as 0.01, 0.05, 1, 1.5 and 2 g were fixed for the experiment and added each to the five different culture vessels. Then the cultures were kept at room temperature for 7 days. The growth characters and dry mycelia weights were recorded after 7 days of incubation through using an electrical balance (BioBlue, Euromex, Netharlands).
Pathogenicity test
For the pathogenicity test, healthy green lemon, orange, and malta were surface sterilized with 70% ethanol (FUJIFILM Wako Pure Chemical Corp. Japan), and wounds were made in each of the fruits using sterilized wooden rod. In each fruit, each wound was inoculated with mycelial plugs (3 mm) from 7 days old culture of each isolate, and one was treated with non-culture pure PDA as a control. Samples were covered by poly bags and placed at room temperature for 7 days. Fungal strains were isolated from artificially infected fruits and data were analyzed for identification.
Antifungal activity test
The antifungal activity of seven plant extracts was tested by disc diffusion method [9]. Different plant extract was applied against the isolate at the concentrations of 200 μgm/disc for each. Different parts of seven plants viz., Psidium guajava, Azadirachta indica, Coccinia grandis, Hibiscus rosa-sinensis, Spondias mombin and Cassia alata were collected from different place of the Rajshahi University Campus area. They were dried under shade, crushed by mortar and pestle, and extracted with methanol (FUJIFILM Wako Pure Chemical Corp. Japan). Total 50 mg of each plant extracts were dissolved in 1 ml of solvent. Disc with methanol solvent was impregnated as negative control. The inhibitory activities of plant extracts were measured after 72 h of incubation using a millimeter (mm) scale.
Antagonistic activity test
Antagonistic activity of two soil borne bacterial strains against isolated fungi was determined by disc diffusion method [9]. Rhizobium leguminosarum and Rhizobium phaseoli were used for antagonistic test. For antagonistic efficiency test, previously collected soil bacteria (20 µl) were cultured and isolated fungus was applied on paper disc.
Statistical analysis
All the above investigations of the present study were conducted in triplicate and repeated thrice for consistency of results and statistical purpose. The data were expressed as mean and standard error (Mean ± SE) by using Microsoft Excel 2013 version and DMRT (Duncan Multiple Range Test) at 5% significant level was performed by SPSS-2001 software.
RESULTS
Characteristics of isolated fungus
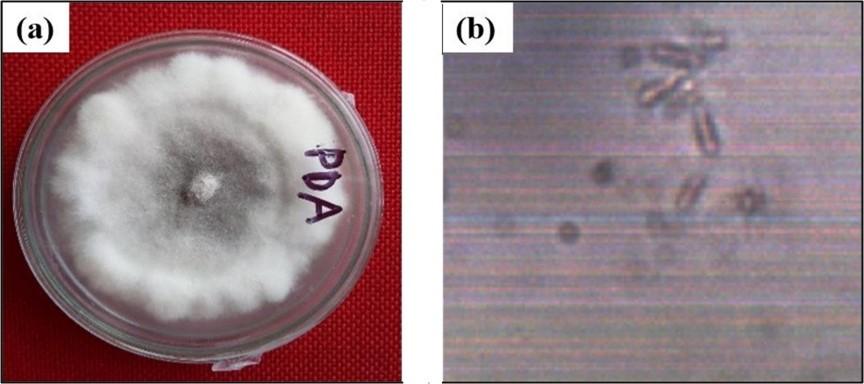
Using cotton blue staining, it is found that the isolated fungus produced cottony white fluffy growth and were spread on the culture plate within 5 to 7 days (Figure 1a). Mycelium produced pycnidia, branched mycelia and the conidia were cylindrical (Figure 1b).
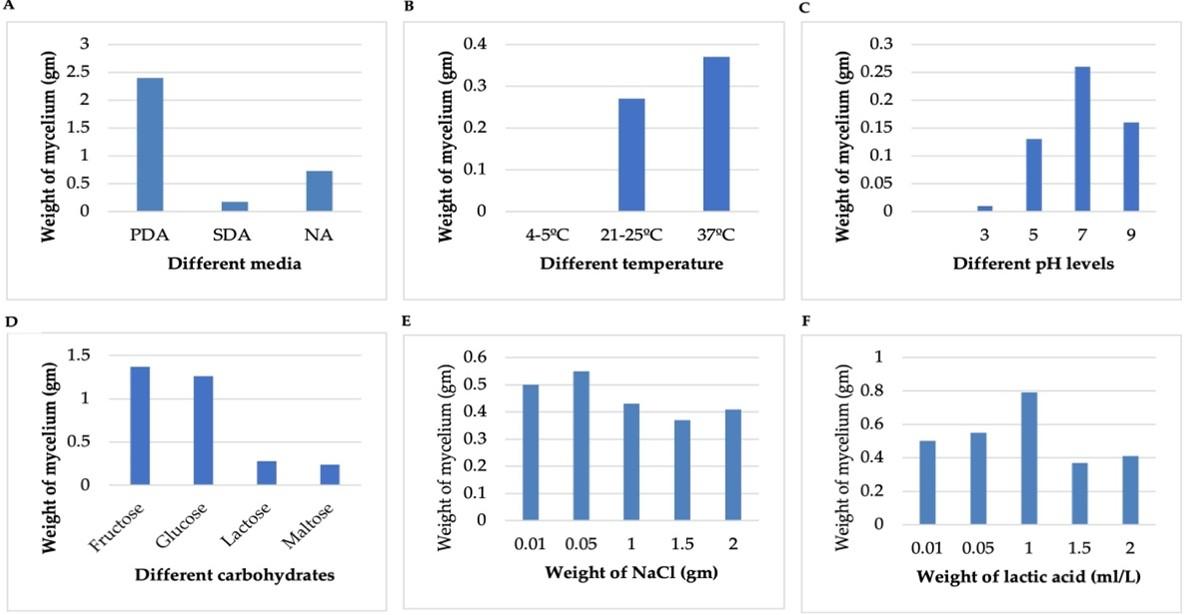
Effect of different growth factors on isolated fungus
PDA showed the highest 2.4 gm weight of mycelium, while SDA showed the lowest 0.17 gm of dry weight (Figure 2a). The optimum temperature for mycelia growth of isolated fungus was 30°C while at 37°C the maximum weight (0.37 gm) was observed (Figure 2b). The optimum level of pH 5-7 was best for mycelia growth of Glomerella cingulata while pH was 7.0- 9.0, the growth was decreased gradually. Effects of pH are given in Figure 2c. The growth of anthracnose was estimated 1.37 gm as the highest response in fructose followed by 1.26 gm in glucose and comparatively lower at 0.28 gm in lactose (Figure 2d). The highest 0.55 gm weight of mycelium was found at 0.05 gm concentration of NaCl, and the lowest weight was 0.37 gm at 1.5 gm of NaCl. PDA media having 1 ml/L lactic acid was best for mycelia growth. Data are presented in Figure 2A-F.
Effect of fungus on pathogenicity against the fruits
Inoculated lemon, orange and malta fruits showed typical anthracnose symptoms after incubation at 25°C for 5 days, which were sunken, circular, necrotic, and white lesions. Later, darkish mycelia developed on the lesions with subsequent dark colored conidial structures. Re-isolation of pathogen from the diseased fruits was done. Morphological as well as cultural characters of re-isolated fungi were compared with those of previously isolated fungus. Data are presented in Figure 3A-D.

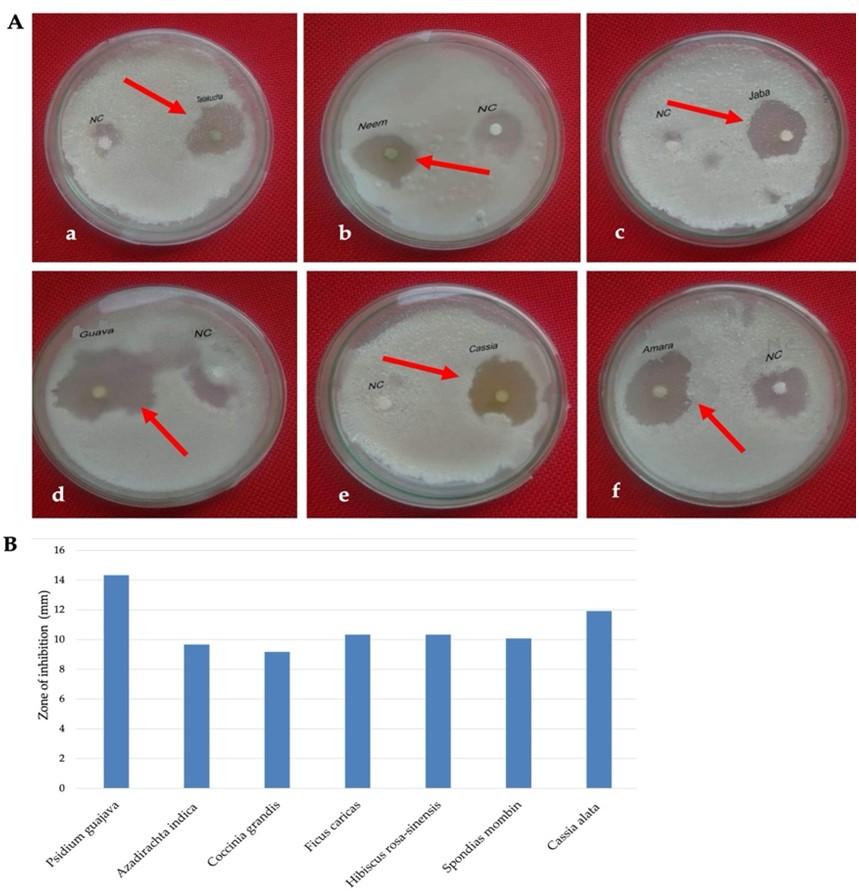
Effect plant extracts on fungal activity
Methanol extract of Psidium guajava leaves showed the highest diameter of 14.33±0.66 mm of zone of inhibition followed by 11.92±0.72 mm showed by Cassia alata leaves at the concentration of 200 µgm/disc (Figure 4A and B). On the other hands, the lowest inhibition zone was 9.17±0.82 mm showed by Coccinia grandis leaves extract at the same concentration. Negative control did not show any zone of inhibition (Figure 4A and B).
Effect on antagonistic activity
Rhizobium leguminosarum and R. phaseoli were studied against the isolated fungus at the concentration of 20 µl/disc (108 cells/ml). Both R. leguminosarum and R. phaseoli did not show any inhibition zone against the isolated fungus (Figure 5).

DISCUSSION
The occurrence of Colletotrichum gloeosporioides and its teleomorph Glomerella cingulata on numerous host plants species in various climatic zones and its high pathogenicity have led to the identification of this fungus as one of the most devastating pathogenic species causing plants anthracnose [10,11,12]. In our study, the fungus was identified as G. cingulata based on colony and conidia morphology as described previously [13].
The pathogen was isolated from the symptomatic infected leaves of C. limon on PDA under 25±2°C was similar with the study of Saroj et al. [14]. The isolated fungus can grow in different culture media. Among these medium used, good to moderate colony diameter and mycelia weights was observed on PDA medium, evidence similar to the work [15; 16]. Maheswari et al. [17] also stated PDA to be the best media for mycelial growth which was consistent with our present findings. Temperature from 20°C to 28°C and high humidity facilitate fungus sporulation in infected parts of plants, conidia germination and widespread secondary infections [18]. The highest growth of the fungus was found by Davis et al. [19] and the range between 20-30°C. Mycelial growth was not much inhibited even at 35°C. In our study, the optimum temperature for mycelia growth of isolated fungus G. cingulata was 30°C while at 37°C the growth was higher which concludes that mycelial growth of the Colletotrichum species is good at high temperatures similar to the previous results [20,21].
The acid/alkaline criteria for growth of fungi have been identified in earlier studies [22]. In the present study, highest mycelia growth of G. cingulata was found at pH 7.0 to 9.0 was higher. According to Sharma and Kulshrestha [16] the growth of this pathogen found highest at pH 5.8 to 6.8 which states that the foremost acidic and alkaline pH is not best suitable for the growth of the pathogen in our study.
The growth of C. gloeosporioides showed significant variations in manitol, followed by fructose and sucrose [23]. Some previous reports showed best growth effect by sucrose on Colletotrichum [24,25,26]. Glucose was reported as carbon source for the growth of C. gloeosporioides [27,28]. Hasan et al. [29] observed best response of fungal stain in PDA medium having fructose as the best carbon source which supports our present investigation.
NaCl was suggested as the best salt for the growth of Aspergillus [30,31]. Highest 0.55 gm/dish weight of mycelium was found at 0.05 gm concentration of NaCl and the lowest weight was 0.37 gm at 1.5 gm of NaCl in the present study concluding that the growth of anthracnose is higher in low NaCl condition while the growth is lower in high NaCl condition, which was similar with the previous results [32].
Pathogenicity tests with Colletotrichum species on healthy green lemon, orange, malta, apple and pomegranate fruits showed virulence on the susceptible with similar morphological characteristics of anthracnose symptoms. In the present study, tested fruits were susceptible on isolated fungus.
In the present study, different controlling techniques were evaluated against the C. gloeosporioides fungus. Various strains of Rhizobium spp. and Trichoderma spp. have been described as effective biological control agents antagonistic to many plant pathogens [33,34]. A variety of traits are considered responsible for the action of Rhizobium strains as biological control agents articulated synchronously or in a controlled sequence in antagonistic studies [35,36]. The antagonistic assay was determined using R. phaseoli and R. leguminosarum. Thus, the fungus is proved to be fully resistant against these bacteria. Also, the antibiotic effects were proved to be resistant showing no zone of inhibition while evaluated with six different antibiotics in our study.
Hafeez et al. [37] and Aqil et al. [38] reported some medicinal plant extracts as effective antifungal agents. Methanol extracts of Psidium guajava leaves showed the highest 14.33±0.66 mm diameter of zone of inhibition against the isolated fungus at the concentration of 200 µgm/disc. This concentration could be used as a natural controlling agent.
CONCLUSION
The present investigation showed evidence of characterization and biological control technique of anthracnose disease on C. limon. Different biological control techniques were studied against the isolated fungal stain. P. guajava leaves extract showed efficient inhibition of G. cingulata. Thus, this study provided information regarding the prevalence and the alternative for chemical fungicide for the isolate from anthracnose disease of C. limon. The present data may help to provide information on appropriate natural fungicide to controlling the devastating anthracnose disease of citrus fruits in agriculture sector.
ACKNOWLEDGEMENTS
All authors are grateful to the Faculty of Biological Sciences, University of Rajshahi, Bangladesh (Grant no.101/5/52/RU/life16/20-21) for providing financial support during this research work.
AUTHOR CONTRIBUTIONS
MFH; Conceptualization, methodology, writing-review and editing: JF; Investigations and writing-original draft preparation, AKD, FBM, SK; Data analysis, article writing and reviewing: BS; Project administration, funding acquisition and supervision.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Paliyath G, Murr DP. Common fruits, vegetables, flowers, and their quality characteristics. Postharvest Biology and Technology of Fruits, Vegetables, and Flowers. Wiley-Blackwell, 2008, 8:498.
- [2]Rauf A, Uddin G, Ali J. Phytochemical analysis and radical scavenging profile of juices of Citrus sinensis, Citrus anrantifolia, and Citrus limonum. Organic and Medicinal Chemistry Letters, 2014, 4(1):1–3.
- [3]Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially available fruit juice products. J Endourol 2008, 22(3):567–570.
- [4]Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T. New and classic families of secreted fungal heme peroxidases. Applied Microbiology and Biotechnology, 2010, 87(3): 871–897.
- [5]Kumar AG. Colletotrichum gloeosporioides: biology, pathogenicity and management in India. J Plant Physiol Pathol 2014, 2(2):2–11.
- [6]Sarkar AK. Anthracnose diseases of some common medicinally important fruit plants. J Med Plants Stud 2016, 4(3):233–236.
- [7]Ribera AE, Zuñiga G. Induced plant secondary metabolites for phytopatogenic fungi control: a review. J Soil Sci and Plant Nutrit 2012, 12(4): 893–911.
- [8]Harris CA, Renfrew MJ, Woolridge MW. Assessing the risks of pesticide residues to consumers: recent and future developments. Food Additives & Contaminants, 2001, 18(12):1124–1129.
- [9]Hasan MF, Sikdar B. Screening of antimicrobial, cytotoxic and pesticidal activities of Coccinia grandis (L.) Voigt. J Microbiol Biotech Food Sci 2016, 5(6):584-588.
- [10]Lenné JM. Colletotrichum diseases of legumes. CAB International Wallingford UK, 1992, 134–166.
- [11]Farr DF, Aime MC, Rossman AY, Palm ME. Species of Colletotrichum on agavaceae. Mycological Research 2006, 110(12):1395–1408.
- [12]Medeiros LV, Maciel DB, Medeiros VV, Kido LMH, Oliveira NT. pelB gene in isolates of Colletotrichum gloeosporioides from several hosts. Genetics and Molecular Research 2010, 9(2):661–673.
- [13]Punithalingam E, Booth C, Waller JM. CMI descriptions of pathogenic fungi and bacteria. Commonwealth Mycological Institute Kew UK, 1981, 71:701-710.
- [14]Saroj A, Kumar A, Qamar N, Alam M, Singh HN, Khaliq A. First report of wet rot of Withania somnifera caused by Choanephora cucurbitarum in India. Plant Disease 2012, 96(2):293.
- [15]Sharma G. Influence of culture media on growth, colony character and sporulation of fungi isolated from decaying vegetable wastes. J Yeast and Fungal Research 2010, 1(8):157–164.
- [16]Sharma M, Kulshrestha S. Colletotrichum gloeosporioides: an anthracnose causing pathogen of fruits and vegetables. Biosciences Biotechnology Research Asia 2015, 12(2):1233–1246.
- [17]Maheswari SK, Singh DV, Sahu AK. Effect of several nutrient media on the growth and sporulation of Alternaria alternata. J Mycopathol Res 1999, 37(1):21–24.
- [18]Frencel IM. Report on first detection of anthracnose (Colletotrichum gloeosporioides) on lupins in Poland. Plant Disease 1998, 82(3): 350.
- [19]Davis RD, Irwin JAG, Cameron DF, Shepherd RK. Epidemiological studies on the anthracnose diseases of Stylosanthes spp. caused by Colletotrichum gloeosporioides in North Queensland and pathogenic specialization within the natural fungal populations. Australian Journal of Agricultural Research 1987, 38(6):1019–1032.
- [20]Chakravarty T. Anthracnose of banana (Gloeosporium musarum Cke. & Massee) with special reference to latent infection in storage. Transactions of the British Mycological Society 1957, 40(3):337.
- [21]Stover RH, Simmonds NW. Bananas. Longman Scientific & Technical. New York: Wiley, 1987, 3.
- [22]Pardo E, Marin S, Ramos AJ, Sanchis V. Ecophysiology of ochratoxigenic Aspergillus ochraceus and Penicillium verrucosum isolates. Predictive models for fungal spoilage prevention–a review. Food Additives and Contaminants 2006, 23(4):398–410.
- [23]Sangeetha CG, Rawal RD. Nutritional Studies of Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. The Incitant of Mango Anthracnose. World Journal of Agricultural Sciences 2008, 4(6):717-720.
- [24]Durairaj V. Growth of Colletotrichum capsici in pure culture. J Indian Bot Soc 1956, 35:409–413.
- [25]Verma ML. Effect of sucrose concentration on growth and sporulation of three species of Colletotrichum pathogenic on chillies. Indian Journal of Mycology and Plant Pathology 1979, 9(1):130-131.
- [26]Babureddy PN. Studies on morphological, cultural and pathogenic variations among the isolates of colletotrichum gloeosporioides (penz.) penz. and sacc. of some sub-tropical fruits. University of Agricultural Science, M.Sc. Thesis. Submitted to University of Agricultural Sciences, Bangalore, 2000, Pp: 69.
- [27]Chandra S, Tandon RN. The Effect of Sorbose on the Utilization of Different Monosaccharides by Certain Fungi Causing “Leaf‐Spot” Diseases. J Phytopathol 1962, 45(2):130–137.
- [28]Jeffries P, Dodd JC, Jeger MJ, Plumbley RA. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathology 1990, 39(3):343–366.
- [29]Hasan MF, Islam MA, Sikdar B. Characterization and Antagonistic Control Measures of Pichia kudriavzevii Yeast like Fungus in Brown Rot Disease of Citrus sinensis (L.) Osbeck. Indian Journal of Applied Microbiology 2019, 22(1):31-48.
- [30]Spielvogel A, Findon H, Arst Jr HN, Araújo-Bazán L, Hernández-Ortíz P, Stahl et al. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochemical Journal 2008, 414(3):419–429.
- [31]Shantappa S, Dhingra S, Hernández-Ortiz P, Espeso EA, Calvo AM. Role of the zinc finger transcription factor SltA in morphogenesis and sterigmatocystin biosynthesis in the fungus Aspergillus nidulans. PLoS One 2013, 8(7): e68492.
- [32]Dubey AK, Barad S, Luria N, Kumar D, Espeso EA, Prusky DB. Cation-stress-responsive transcription factors SltA and CrzA regulate morphogenetic processes and pathogenicity of Colletotrichum gloeosporioides. PLoS One 2016, 11(12): e0168561.
- [33]Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR. Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochemical Engineering J, 2007, 37(1):1–20.
- [34]Arfaoui A, Sifi B, Boudabous A, Hadrami IE, Cherif M. Identification of Rhizobium isolates possessing antagonistic activity against Fusarium oxysporum f. sp. ciceris, the causal agent of Fusarium wilt of chickpea. J Plant Pathology 2006, 67–75.
- [35]Nirmala J, Gaur YD. Detection of bacteriocinogenic strains of Cicer-Rhizobium by modified simultaneous antagonism method. Current Science 2000, 79(3):286–287.
- [36]Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J Ethnopharmacol, 2009, 123(3):494–498.
- [37]Hafeez FY, Naeem FI, Naeem R, Zaidi AH, Malik KA. Symbiotic effectiveness and bacteriocin production by Rhizobium leguminosarum bv. viciae isolated from agriculture soils in Faisalabad. Environmental and Experimental Botany 2005, 54(2):142–147.
- [38]Aqil F, Zahin M, Ahmad I, Owais M, Khan MSA, Bansal SS, Farooq S. Antifungal activity of medicinal plant extracts and phytocompounds: a review. Combating Fungal Infections, 2010, 449–484.