Evaluation of phytochemical profile and antimicrobial activity of Tragia brevipes extracts against pathogenic bacteria
Abstract
Infectious diseases remain a global health challenge as result of antimicrobial resistance. The use of natural products has revealed a potential source of alternative antimicrobial agents. The aim of this research was to evaluate the antibacterial activities and the phytoconstituents of Tragia brevipes against pathogenic bacteria. The crude extracts of leaves, roots and stems were obtained using polar and non-polar solvents and the antibacterial assay was performed using agar well diffusion and disc diffusion methods. The results showed that in the leaves and roots, flavonoids, saponins, glycosides, phenols, tannins, and resins were present whereas in the stem, flavonoids, glycosides, and resins were present. Only S. aureus ATCC43300, E. coli ATCC25922 did not show inhibition zone to all plant parts. P. aeruginosa ATTCC27853 showed the highest inhibition zone compared to other bacteria. It was observed that methanolic extract has a great potential antimicrobial activity, followed by distilled water extracts. The combination of plant extracts showed a marked synergic effect. The two-way ANOVA showed a statistical difference in the mean zone of inhibition obtained among bacteria and leaves (f = 2.3478, p < 0.05), roots (f = 2.3478, p < 0.05) whereas stem extracts difference was between solvents and tested bacteria (f = 3.4923, p < 0.05). T. brevipes could be a potential candidate in the treatment of bacterial infection as a source of novel antimicrobial agents. Further studies are recommended to isolate the active ingredients and investigate pharmacological properties of T. brevipes.
INTRODUCTION
Traditional medicine began long ago with the use of natural products and have displayed high efficacy due to a wide spectrum of pharmacological compounds [1]. According to World Health Organization, most of populations in developing countries consult traditional healers for the treatment of infectious diseases where medicinal plants are used [2].
It is known that antimicrobial resistance is associated with high morbidity and mortality and remains one of the global health concerns of this century. Multidrug resistance patterns in Gram-positive and Gram-negative bacteria have been reported and have presented difficulties to treat with conventional antibiotics. By 2050, the antimicrobial-resistant infections will lead to millions of deaths per year with subsequent socio-economic impacts if no appropriate actions at different levels [3]. Therefore, there is a need to promote the development of alternative antimicrobial agents considering the current emergency of new strains, ineffectiveness of the existing therapies, ineffective awareness and prevention measures [4].
Tragia brevipes is a species of shrub in Euphorbiaceae or Spurge family and is one of the hundreds Tragia species. Studies have reported the antimicrobial activity of T. brevipes by inhibiting the growth of bacteria including E. coli, Salmonella species, Enterobacter aerogenes, and Proteus vulgaris [5]. In addition, the qualitative analysis of the plant has revealed the presence of bioactive compounds such as flavonoids, phenols, terpenoids, tannins, glycosides, saponins but lack alkaloids and steroids [6].
Several studies have reported considerable activity against bacteria by various extracts of medicinal plant [7]. Medicinal plants have been used in Rwanda for many years and remain the main therapeutic option for both rural and urban populations. However, the lack of experimental scientific studies confirming the possible antimicrobial properties from Rwandan plants is still a gap. Among the high number of medicinal plant species, only few have been studied and underwent pharmacological investigations. It has been reported that plant bioactive compounds could display antimicrobial activities against multidrug-resistant microbial pathogens by targeting specific sites other than those used by the current antibiotics [8]. Thus, efficacy of many of the plant drugs including Tragia brevipes (vernacularly known as isusa) used by local traditional healers as effective anti-bacterial agent is still not well documented.
The present study was initiated to evaluate the potential antimicrobial activity and screen bioactive compound of T. brevipes parts against selected bacteria as potential alternative antibacterial compounds.
MATERIALS AND METHODS
Plant sampling method
Parts of the plant, T. brevipes (leaves, stem and roots) were collected in natural forest in Musanze district, Rwanda.
Sample processing
Each part of the plant was separated from the whole herb which resulted in 3 groups (leaves, roots and stem). The plant was washed twice using distilled water. Stem and roots were cut into pieces for the quick air dry. Each sample was thoroughly mixed and spread to dry at room temperature (23.5 oC) in the laboratory under sterile conditions in shade place.
Kinetic maceration technique
Dried parts of T. brevipes were again cut into too small pieces and passed in the oven for 15 min at 75oC. It was later ground into the fine powder using sample mill machine (electronical powder grinder, DE-200 g). Total powder of leaves, stem and roots parts was weighed. From each group, 25 grams of the powdered group part was dissolved in 3 different solvents, 250 ml of 99.8% methanol, 250 ml distilled water and 250 ml of 90% petroleum ether. The mixture was placed on stopper container and stand on frequent agitation by the help of Orbital shaker / OS-34oC at 60 rpm for 5 days. Later the mixture was filtered using a gauze and remained with a homogenous solution.
Extract preparation
Crude plant extract was prepared by Hydro-distillation method, rotary vacuum evaporator (R-11) with 120 rpm and a water bath at low temperatures 45 oC was used to evaporate the solvent. The obtained extract was put in sterilized labelled tubes with a proper labeling for future use and stored at 4 oC for further antimicrobial studies [9].
Screening of phytochemicals in the plant
The standard methods provided by Harborne [10] were used to screen the presence of different bioactive secondary metabolites in the plant. Resins, tannins, phenols, alkaloids, glycosides, saponins and flavonoids were screened in this study.
Microorganisms
The study used eleven strains of bacteria obtained from the National Reference Laboratory (Rwanda): S. typhi ATCCB69, S. typhi ATCC B71, S. aureus ATCC29213, S. aureus ATCC43300, E. coli ATCC35218, E. coli ATCC29922, P. mirabilis ATCC126, S. pneumonia ATTCC49619, S. pyogene ATTCC 12344, K. oxytoca ATTCC700524 and P. aeruginosa ATTCC27853.
All bacteria were sub-cultured on their selective media and incubated at 37 ℃ for 24 h. Colony characterization was recorded, and Gram staining was performed followed by biochemical tests for a better identification.
2-5 similar colonies were lifted with a sterile wire loop and transferred into a 4 ml of normal saline contained in a sterilized tube. The bacterial suspensions were standardized to 108 CFU/ml. To adjust the turbidity of the suspension, 0.5 McFarland standard solution was used as reference.
Assay of antibacterial activity
Under aseptic conditions, a sterilized cotton swab was dipped (50 μl) in a prepared bacteria suspension and uniformly distributed on the surface of the Mueller Hinton agar (MHA) using streaking method. For agar well diffusion method, wells of 5 mm were punched in which the extract was added and for disc diffusion method, blank discs were dipped in the extract. The plates were incubated at 37 0C for 24 h. The zones of inhibition were measured in millimeter and the antimicrobial assays were interpreted according to Kirby Bauer technique [11]. Each solvent was used as a negative control and duplicate assay was done on each antimicrobial test.
Antimicrobial study of combined parts of Tragia brevipes
The triple (leaves, roots and stem) combinations of plant extracts parts were prepared by mixing equal quantities of each extract respective to the solvent. Each of the obtained mixture extracts was tested for antibacterial activity under sterile conditions. Duplicate assay was done on each antimicrobial test.
Statistical analysis
The results were analyzed depending on effect and control used. For data entry and analysis, all results were statistically analyzed using Statistical Package for the Social Sciences version 22. To compare the means mentioned in this study objective. Two-way ANOVA was useful and to find a difference between two groups, a post hoc test (Bonferroni) was run to indicate a marked difference.
RESULTS
Phytochemical compounds
The phytochemical qualitative study revealed the presence of some major secondary metabolites whereas other were absent in the plant extracts (Table 1).
Table 1. Phytochemical screening of the Tragia brevipes.
Antimicrobial activity of T. brevipes against selected pathogenic bacteria
Results obtained from the work of testing T. brevipes antimicrobial activity indicated that the most non inhibited strains to all plant extracts were E. coli ATCC25922 and S. aureus ATCC43300, while other tested strains were potent to the plant extracts as shown in Figure 1. It was observed that depending on the solvents used, the zones of inhibition (ZI) vary and the methanolic extract was more potent against tested bacteria compared to others.
T. brevipes stem was weak to inhibit the bacteria, leaves responded highly with methanol and petroleum ether solvents whereas roots responded highly with the extraction done by distilled water. On the other hand, the tested bacteria showed a variation in their susceptibility to the extracts as shown below in Figure 1. The highest inhibition was generated against the growth of P. aeroginosa ATTCC27853 (ZI: 17 mm) and the lowest against S. pneumonia ATTCC49619 (ZI: 8 mm). Both disc and agar well diffusion methods were conducted, the obtained results were almost the same as shown in the Figure 1.
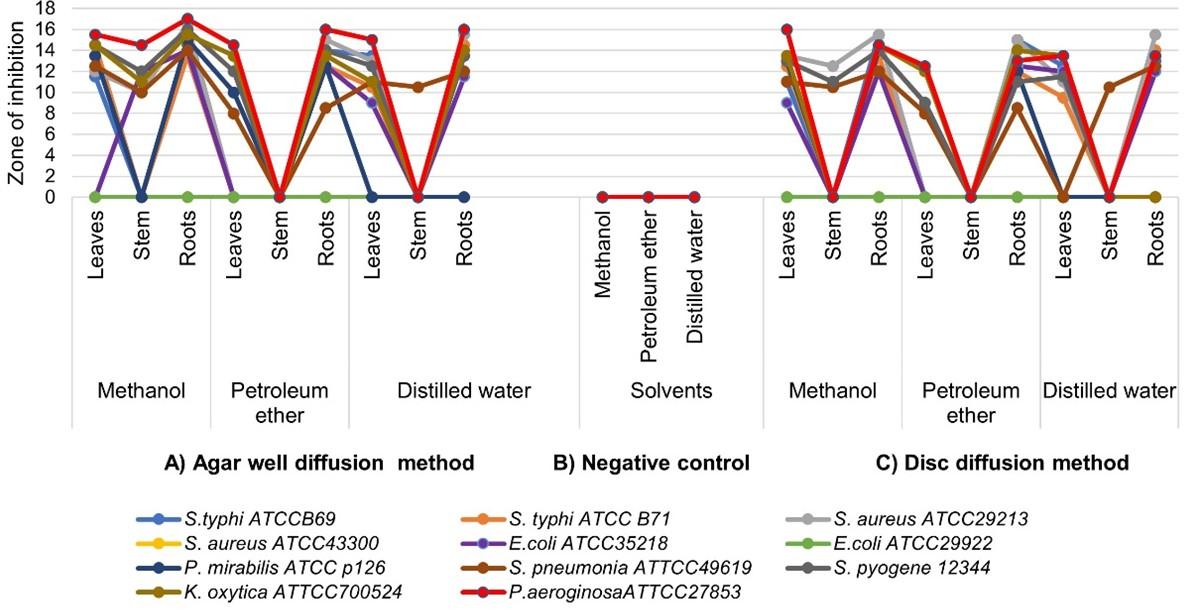
Variance of antimicrobial activity of T. brevipes
The analysis of variance indicated that there was a significant difference on the mean zones of inhibition obtained from both leaves(f = 2.3478, p < 0.05) and roots (f = 2.3478, p < 0.05) extracts against bacteria (Table 2). Therefore, Bonferroni correction was runned and revealed a significant difference in some bacteria as showed in Table 3. A post hoc analysis (Bonferroni correction) showed a marked difference between bacteria and the used P value was 0.004545 (Table 3). However, for the stem extracts, a significant difference was observed among solvents (f = 3.4923, p < 0.05) (Table 2). The bonferroni correction showed a clear difference between methanol vs. petroleum ether solvent used in stem extracts (p = 0.0028, p = 0.016667) (Table 3).
Table 2. Variance of antimicrobial activity of Tragia brevipes.
Table 3. Marked difference with post hoc test.
Effects of combined parts of T. brevipes on selected pathogenic bacteria
The triple combinations of plant extracts parts resulted to potent activity (Figure 2). Combined extract of methanol showed a marked increased activity compared with other solvents. Obtained zone of inhibition ranged between 10 to 18 mm. Generally, bacteria strains were inhibited by methanol and distilled water extracts. Petroleum ether only inhibited S. aureus strains. In addition, the combination showed an increase in zone of inhibition against all bacteria, highly on Gram positive bacteria compared to Gram negative bacteria.
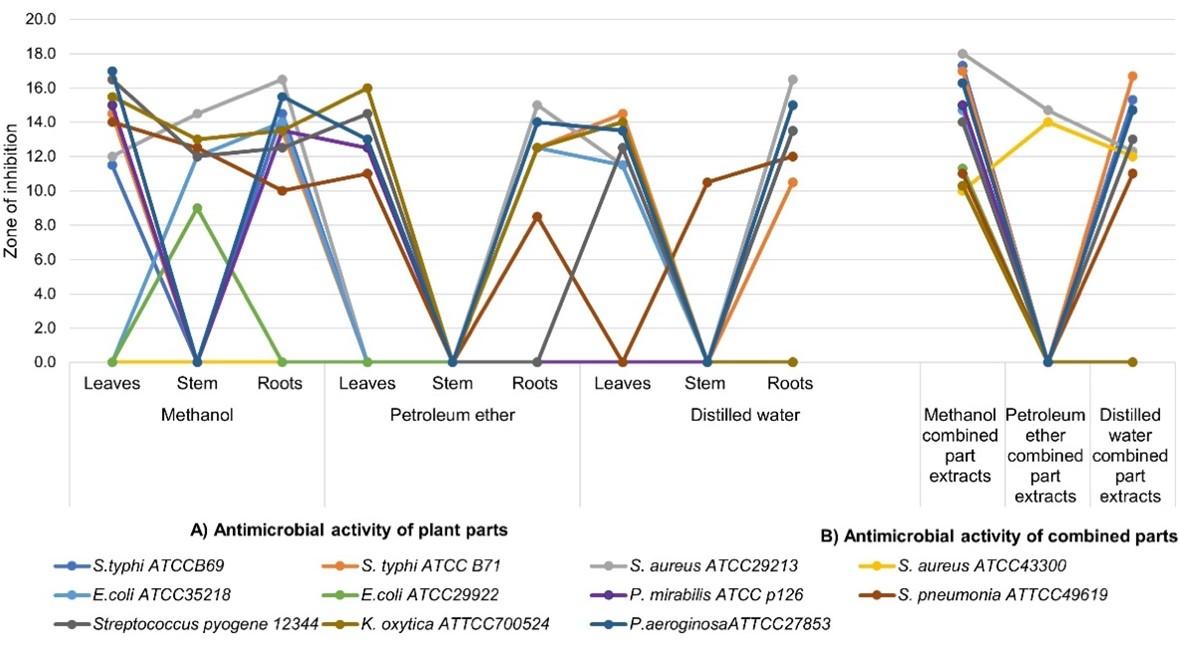
Variance of antimicrobial effects of combined parts of T. brevipes
Three different extracts of T. brevipes were tested for antimicrobial activity using agar well diffusion method. The presence of zone of inhibition proved that plant was able to inhibit the growth of bacteria but at a different extent depending to the solvents (Table 4). The analysis of variance of two factors showed that there was significant difference on the mean zones of inhibition obtained against bacteria through different solvents (f = 3.492828, p < 0.05) (Table 4). A post hoc test using Bonferroni correction indicated that a significant difference was between methanol vs. petroleum ether solvent (Table 5).
Table 4. Comparison of the activity produced by combined parts crude extracts.
Table 5. Marked difference observed between solvents.
DISCUSSION
Worldwide a large number of medicinal plants are harvested from the natural environment for traditional medicine [12]. Thus, the natural area is very important for local populations to satisfy their primary healthcare needs. Testing the antimicrobial activity of various medicinal plants require complex methods such as sampling, air drying, grinding, maceration, extraction, and antimicrobial testing although extraction is key step in this experiment. Different solvents were used while macerating and thus different extracts were obtained. Maceration is a convenient and less costly method for small and medium research projects compared to other modern extraction methods [13]. This technique softens and breaks the plant’s cell wall or other various substances to release the soluble phytochemicals as reported previously [14].
Three solvents were used to evaluate the plant activity based on the polarity of solvents from low to high where the petroleum ether is a nonpolar solvent while methanol are distilled water polar solvents [15]. Bioactive compounds represent the phytoconstituents which are synthesized as secondary metabolites in all plant cells with different concentrations depending on the plant part [16]. In the present study, resins, tannins, phenols, glycosides, saponins and flavonoids were present in the leaves and roots while flavonoids, glycosides and resins were present in stem. Similar findings have been reported by Swamy et al. [6] where the leaves contained all the components were present but they lacked alkaloids and steroids. Each compound has a specific target [17] and the presence of the same phytochemicals in the roots and leaves could be an indication of the same potential antibacterial activity. Maobe et al. [18] revealed that the active compounds interfere with the microorganism metabolic processes or may change their gene expression. Phenolic structures are highly active against the microorganisms and tannins have been reported as inhibitors to the growth of many fungi, yeast, bacteria and other viruses [19].
From this study, the plant extracts were found to have antibacterial activity against nine of the eleven bacteria tested and the observed antibacterial activity was closely associated with the polarity of solvents [20]. The experiment showed a future hope of having novel antibacterial agent as the potential antibacterial activity of T. brevipes recorded in this study was also confirmed by other studies. Swamy et al. reported that T. brevipes was found to inhibit the growth of E. coli, Salmonella sp., E. aerogenes, B. cereus, S. liquefaciens and P. vulgaris [5].
In this study, a very high zone of inhibition was observed on P. aeruginosa. It has been reported that P. aeruginosa is highly inhibited by the plant extracts with phenolic content [21]. Both S. aureus ATCC43300 and E. coli ATCC25992 were not inhibited by the plant extracts. Those bacteria have been reported among the most resistant bacteria [22]. The variations between different studied strains of S. aureus and E. coli could be attributed to different factors including the isolation site and the type of prophylactic antibiotics [23].
The combination of the plant extracts exhibited diverse antibacterial activity against the tested microorganisms with reference to plant parts alone. Interestingly, both S. aureus ATCC43300 and E. coli ATCC25992 (which were not inhibited in the first experiments) were inhibited by the combined parts. It has been observed that the increased activity was found more on Gram positive bacteria. The sensitivity of microbes to the extracts may be due by the cell wall structure of the bacteria as reported by Rachuonyo et al. [24]. Gram positive bacteria contain layers of peptidoglycan which make them more permeable to many molecules than the cell wall of Gram negative bacteria with the outer membrane and lipid bilayer which make them less sensitive to many extracts [25].
Both ANOVA and ad hock test indicated a significant difference between bacteria and within solvents. This could be explained by the fact that Gram positive respond differently to extracts compared to Gram negative and non-polar solvents react differently to polar solvents [26]. To exclude the action of solvents on bacterial growth, the antibacterial activity was screened using only solvents as negative control. Interestingly, the solvents alone didn’t exhibit any activity.
In additional to the antimicrobial activity studies, T. brevipes was found to be an anti-proliferative activity on cancer cells, relief stomach pain, purgative and in treatment of rheumatism [27, 28]. It was reported that its leaves and roots are used to treat peptic ulcers, diabetes, local anesthetic agent and antivenom [29–31].
CONCLUSION
This study has revealed that the antibacterial activity of T. brevipes is attributed to the phytochemicals present in the plant as the result showed that leaves and roots act as best antibacterial agents than stem extract which lack many bioactive compounds. Therefore, data obtained in this study could be used as a scientific report for plants traditional use and T. brevipes could be a potential good candidate in bacterial infection treatment and management as a source of novel antimicrobial agents. However, antibiotics that appear potent in vitro are not necessarily more beneficial in vivo when treating or preventing disease. Therefore, further studies are needed for better understanding the plant toxicity and side effects. In addition, it is recommended to evaluate the activity against other microorganisms with several other solvents, isolate the active ingredients, and investigate the mechanisms of action.
ACKNOWLEDGMENTS
The National Council for Science and Technology of Rwanda supported the work under the Excellent Research Grants theme (NCST/ERC1/03). The authors gratefully acknowledge the support of staff members of Microbiology and Chemistry laboratories of INES-Ruhengeri.
AUTHOR CONTRIBUTIONS
TH and FNN conceived, designed and supervised the study. HM collected samples. HM, and EM performed the experiments. HM, CI, CY, FNN and TH analyzed the data. HM,TH, and CI prepared the manuscript. HM, EM, CI, CY, FNN and TH revised the manuscript. All authors approved the final version.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules; 21. Epub ahead of print 2016.
- [2]Oyebode O, Kandala NB, Chilton PJ, Lilford RJ. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan 2016; 31: 984–991.
- [3]Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis 2019; 11: 36–42.
- [4]Frieri M, Kumar K, Boutin A. Antibiotic resistance. J Infect Public Health 2017; 10: 369–378.
- [5]Swamy A, Ngule MC, Jackie OK. In Vitro Antibacterial activity of Methanolic-aqua extract of Tragia brevipes Leaves. Int J Pharm Life Sci 2014; 5: 3289–3294
- [6]Swamy A, Ngule MC, Ngule ME, Francis R. Qualitative Analysis of Phytoconstituents in Tragia Brevipes Plant. Int J Pharm Res Anal 2013; 3: 93–98.
- [7]Marasini BP, Baral P, Aryal P, Ghimire KR, Neupane S, Dahal N, et al. Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Biomed Res Int; 2015. Epub ahead of print 2015.
- [8]Li S, Han Q, Qiao C, Song J, Cheng CL, Xu H. Chemical markers for the quality control of herbal medicines: An overview. Chin Med 2008; 3: 1–16.
- [9]Janvier H, Sabine I, Colores U, Bernard NJ. Antimicrobial and Antifungal Activity of Three Selected Homegrown Vegetables Consumed in Rwanda. European J Med Plants 2018; 23: 1–8.
- [10]Harborne JB. Phytochemical methods. a guide to modern tehniques of plant analysis. 1985. Epub ahead of print 1985.
- [11]Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 1998; 60: 1–8.
- [12]Abyot E, Zewdu B, Tefera A, Mohammedberhan AW, Mulugeta F. Capacity Buildings of Traditional Medicine Practitioners? As a Primary Health Care Workers in Gondar Town, Northwest Ethiopia. J Homeopath Ayurvedic Med; 03. Epub ahead of print 2014.
- [13]Tambun R, Alexander V, Ginting Y. Performance comparison of maceration method, soxhletation method, and microwave-assisted extraction in extracting active compounds from soursop leaves (Annona muricata): A review. IOP Conf Ser Mater Sci Eng 2021; 1122: 012095.
- [14]Azwanida N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med Aromat Plants 2015; 04: 3–8.
- [15]Abubakar AR, Haque M. Methodology Used in the Study. Asian J Pharm Clin Res 2017; 7: 1–5.
- [16]Stéphane FFY, Jules BKJ, Batiha GE, Ali I, Bruno LN. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. Nat Med Plants. Epub ahead of print 2022..
- [17]Ali S, Khan MR, Irfanullah, Sajid M, Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med 2018; 18: 1–15.
- [18]Maobe MAG, Gitu L, Gatebe E, Rotich H. Antifungal activity of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii Region, Southwest Kenya. World J Med Sci 2013; 8: 74–78.
- [19]Sharma K, Kumar V, Kaur J, Tanwar B, Goyal A, Sharma R, et al. Health effects, sources, utilization and safety of tannins: a critical review. Toxin Rev 2021; 40: 432–444.
- [20]Barchan A, Bakkali M, Arakrak A, Pagan R, Laglaoui A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int J Curr Microbiol Appl Sci 2014; 3: 399–412.
- [21]Ahmed D, Khan MM, Saeed R. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants 2015; 4: 394–409.
- [22]Boss R, Overesch G, Baumgartner A. Antimicrobial resistance of Escherichia coli, enterococci, Pseudomonas aeruginosa, and Staphylococcus aureus from raw fish and seafood imported into Switzerland. J Food Prot 2016; 79: 1240–1246.
- [23]Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance : a rundown of a global crisis. Infect Drug Resist 2018; 11: 1645–1658.
- [24]Rachuonyo HO, Ogola PE, Arika WM, Wambani JR, Gatheri GW, Nyamache AK. Combined Effect of Crude Leaf Extracts of Selected Medicinal Plants against Selected Enteric Bacterial Pathogens and Candida albicans. J Antimicrob Agents 2016; 02: 1–5.
- [25]Silhavy TJ, Kahne D, Walker S. The Bacterial Cell Envelope. Cold Spring Harb Perspect Biol 2010; 2: 1–16.
- [26]Bakht J, Khan S, Shafi M. Antimicrobial potentials of fresh Allium cepa against gram positive and gram negative bacteria and fungi. Pakistan J Bot 2013; 45: 1–6.
- [27]Irakiza R, Vedaste M, Elias B, Nyirambangutse B, Serge NJ, Marc N. Assessment of traditional ecological knowledge and beliefs in the utilisation of important plant species: The case of Buhanga sacred forest, Rwanda. Koedoe; 58. Epub ahead of print 2016.
- [28]Chepng’etich J, Ngule C, Jepkorir M, Mwangangi R, Njuguna DK, Ndung’u JW, et al. Total Phenolic Content and in vitro Antiproliferative Activity of Tragia brevipes (Pax) and Tetradenia riparia (Hochst) Leaves Extract. European J Med Plants 2018; 22: 1–10.
- [29]Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed; 10. Epub ahead of print 2014.
- [30]Kigen G, Kipkore W, Wanjohi B, Haruki B, Kemboi J. Medicinal Plants Used by Traditional Healers in Sangurur, Elgeyo Marakwet County, Kenya. Pharmacognosy Res 2020; 10: 24–30.
- [31]Omara T. Plants Used in Antivenom Therapy in Rural Kenya: Ethnobotany and Future Perspectives. J Toxicol; 2020. Epub ahead of print 2020.