Anti-inflammatory, anti-oxidative, and anti-microbial activities of the phytochemicals isolated from various parts of broccoli wastes
Abstract
Broccoli is an excellent source of vitamins, minerals, phytochemicals, and nutraceuticals. Phytochemicals, also known as phytonutrients, are chemical compounds that are present in fruits, vegetables, and other plants and can be broadly classified into carbohydrates, terpenoids, phenolics, lipids, alkaloids, and other nitrogen-containing compounds. Waste generation is a global problem and vegetable wastes that contain the same or higher amount of phytonutrients as the vegetable itself are discarded on daily basis leading to additional waste biomass. The unused portion of broccoli is considered to be waste which includes the stalk and the leaves, rotten and scraped portions of the vegetable. Only the fragile stems closest to the florets are eaten, while the lignified bottom stem of the vegetable is discarded. Approximately 30% of vegetable loss is generated at retail and consumer levels including post- harvest and processing. These wastes possess various nutrients and multiple bioactive compounds such as phytochemicals (phenolics, glycosylates, carotenoids, and flavonoids). Broccoli waste has an enormous amount of these nutrients and nutraceuticals which have a wider range of applications in food supplements, pharmaceuticals, and cosmetic industries. The phytochemicals from the leaf and stalk were extracted using the maceration process, and the stalk extract had stronger antioxidant and anti-inflammatory activity at a concentration of 500 g/ml. It also showed good anti-microbial activity at 300 μg/ml proving it to be a potential source of important bioactive compounds and implying the presence of anti-aging and anti-acne activity.
INTRODUCTION
Vegetables possess multiple nutrients that prove to be greatly beneficial to humankind. But so do their peels, seeds, leaves, stems, and stalks that are discarded every day from households as well as industries in large quantities. Extraction of nutritional chemical compounds from the edible parts of the vegetable, only leads to lesser available quantity to consumers and leads to more food wastage. Broccoli (Brassica oleracea var. italica) is a largely unused vegetable that contains numerous beneficial compounds. Phenolic compounds (flavonoids and hydroxycinnamic acids), glucosinolates, carotenoids, vitamin C, sterols, mineral elements, and fiber, all of which are incredibly important for good health, are among the key components of interest in broccoli by-products [1-4]. Broccoli plant by-products are generated in large quantities since leaves and stems account for more than 95 percent of the harvested material [5]. The majority of people only eat the florets portion, which contributes to about 30% of the vegetable’s biomass, resulting in a large quantity of waste, including leaves, stems, and stalks [6]. During the boiling and processing of vegetable florets, the leaves and stem are eliminated, resulting in a massive loss of potential nutrients. The utilization of by-products as a possible source of important bioactive chemicals with applications for the treatment and prevention of human illnesses has been demonstrated in recent years with the aid of several research [7]. In comparison to other macronutrients, these bioactive chemicals are products of the plant’s secondary metabolism and are generated in modest quantities. These rich plant components have been ascribed with a variety of helpful activities, including acting as defensive mechanisms against environmental challenges and protecting the plants, as well as biological features that have remarkable health-promoting benefits for consumers [8,9].
Broccoli by-products are a useful source of a variety of health-promoting compounds that have a wide range of uses in the beauty sector, owing to their antioxidant properties [10,11]. These by-products can be employed as supplementary additives in animal feed or as a source of nutraceuticals, reducing environmental effect while increasing economic value [12]. The interest to find or develop different food resources that are not only rich in critical nutrients but also have favorable impacts on the health of the humans has encouraged the search for novel food sources as public knowledge of the healthy lifestyle paradigm has grown [13].
Functional foods and nutraceuticals are gaining traction as a result of these developments, since these foods have the potential to lower the risk of chronic illnesses while also improving basic nutrition, resulting in total well-being [14,15]. Furthermore, numerous scientific initiatives and research organizations are concentrating on recovering the potential food wastes and reinforcing them into high-value byproducts that may be used as functional components in new product creation [16-19].
There are several studies on the compounds present in leaf and florets, but no overview of the beneficial properties associated with the largely discarded stalk The crude extract was obtained from using three solvents of different polarities: hexane, chloroform, and water. This paper presents the antioxidant, anti-inflammatory, anti-aging and anti-microbial properties of the Leaf and stalk in comparison, to understand the presence of beneficial compounds that could be utilized rather than extracting compounds from edible parts and generating more food waste.
Broccoli by-products, the leaf and the stalk extract contain beneficial bioactive compounds and has potential antioxidant, anti-inflammatory and antimicrobial activity implying anti-aging and anti-acne properties of the vegetable waste. The vegetable waste can thus be used to retrieve functional compounds to produce value added products, reducing the amount of waste generated and recycling for production of functional foods or cosmetics.
The aims of the current study are a) extraction of phytochemicals from the different parts of broccoli waste, b) qualitative analysis of bioactivities of the phytochemicals extracted from broccoli waste, and c) to prepare a value-added product.
MATERIALS AND METHODS
Chemicals and reagents
Analytical grade chloroform, n-Hexane, milli-Q water, Dimethyl sulfoxide (DMSO), acetone, perchloric acid, normal saline, Bovine serum albumin (BSA), acetylsalicylic acid, trypsin, Tris-HCL, Casein ,2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), Ascorbic acid, FRAP reagent, Mueller Hinton (MH) agar, Azithromycin.
Test-strains
Staphylococcus aureus (MTCC 96) was employed as the test organism for in vitro antibacterial activity. These type strains were obtained from the IMTECH, Chandigarh’s Microbial Type Culture Collection and GenBank.
Sample preparation
Broccoli leaves and stalks were procured from the local market, Chennai. The by- products were surface sterilized using 70% ethanol for about 10 seconds. The extraction of plant material was performed following the method of [20] with required modifications. Maceration technique was performed using three different solvents: hexane, chloroform, and water. The leaves were cut for about 1 inch and the stalks were grinded in pulsed mode. About 10 grams of the leaf and stalk were added to 150 ml of each of the solvent and placed in the orbital shaker for 48 hours. The extract was concentrated using a rotary evaporator (Z566861, IKA RV 10 rotary evaporator, basic) at a temperature below the boiling point of the different solvents after 48 hours. After that, the extracts were filtered using Whatman filter paper. A hot plate was used to remove the presence of any residual solvent and the samples obtained were stored in dry conditions and used for the further analysis.
All the experiments that were performed included positive control and negative control groups to compare with the treatment group. The positive control group contained all the elements of the treatment group except for the specific waste extract, and it contained the standard drugs. Negative control group did not contain the plant extract.
Yield percentage and estimation of bioactive compounds
Yield percentage of the leaf and stalk samples, obtained through the three solvents was calculated. The method of [21] was followed with modifications for estimation of Total Carotenoids, Chlorophyll A and Chlorophyll B. 1 ml of DMSO was added to the leaf and stalk samples of the different solvent extracts weighing 5 milligrams each. The samples were centrifuged for 10 minutes at the rate of 10,000 rpm followed by resuspending the pellet in 80% acetone, incubating for period of 24 hours, stored at 4ºC. The test samples were again centrifuged for 10 minutes at the rate of 10,000 rpm and the absorbance values for all the supernatants were read at 470, 645 and 663nm and the estimated according to [22].
GCMS analysis
The chloroform leaf and hexane stalk sample were diluted in their respective HPLC- grade solvents (chloroform and hexane). 1ml of sample was given for GCMS-analysis. The HP-5 MS (30mx250x0.25m) column was used with a GC-MS (7890B GC-5977A MSD) fitted with the HP-5 MS (30mx250x0.25m) column (Agilent, Santa Clara, Calif., USA). The flow rate of the helium carrier gas was 1.0 mL/minute, and 1µL of sample was injected. The injector temperature was set to 250ºC, and the detector temperature was set at 280ºC. The oven temperature was designed to rise from 50ºC (held for 3 minutes) to 280ºC (held for 20 minutes) at a rate of 10 C/minute. At 70 eV, mass spectra were collected. All the parameters were established according to [23], and the scan interval being 0.5 sec and 50 to 700 (m/z) was the mass spectral scan range. For both the samples, 44 minutes was the total running. The peaks were identified by comparing them to the National Institute of Standards and Technology’s standard library (NIST).
In vitro anti-inflammatory activity
Inhibition of albumin denaturation
Inhibition of albumin denaturation technique was performed to study the presence of anti-inflammatory property in the Broccoli leaf and stalk following methods of [24] and [25] with few modifications. 1 ml of test samples (100-500 μg/ml concentrations) and 1 ml of 1% aqueous solution of BSA was the composition of the experiment mixture. Required amount of 1N HCl was mixed to attain the required pH for expected reaction. The experiment mixture was incubated for 20 minutes at room temperature and then heated to 55ºC for 20 minutes, followed by measuring the turbidity of the samples at 660 nm after cooling it down using Multiskan GO Microplate Spectrophotometer (Thermo Scientific,Finland). Experiment was conducted in duplicate.
% Inhibition of protein denaturation = [(A0-AS)/A0] x100
A0 – absorbance value of the control; AS – absorbance value of the test sample.
Antiproteinase action
The experiment was carried out as per the method described in [25] and [26] with necessary modifications. 1 ml trypsin, 0.5 ml of test sample of varying concentrations (100 – 500 μg/ml) and 1 ml of 20 mM Tris HCl buffer maintained at the pH of 7.4 were included in the experiment mixture. Post 5 minutes of incubation at room temperature, 1 ml of casein [0.8% (w/v)] was added to the mixture and incubated for another 20 minutes. To stop the reaction, 0.5 mL of 70% perchloric acid was added. The samples were centrifuged, and the absorbance values of the clear solution was measured at 210 nm. The study was carried out three times.
% Inhibition of proteinase inhibitory activity = [(A0-AS)/A0] x100
A0 – absorbance value of the control; AS – absorbance value of the test sample.
Membrane stabilization
Blood was drawn from a healthy volunteer who had not taken any NSAIDs for two weeks before the experiment. The blood sample was centrifuged for 10 minutes at 3000 rpm followed by washing it three times with an equivalent volume of normal saline. The pellet was measured and reconstituted with normal saline in a 10% v/v solution [25, 27].
The reaction mixture contained 1 ml test sample of various concentrations (100-500 μg/ml) and 1 ml of 10% RBC suspension, whereas the control mixture contained 1 ml saline and 1 ml of 10% RBC suspension. For 20 minutes, all centrifuge tubes holding reaction mixture were placed in a water bath at 50°C. The tubes were cooled after the incubation period, and the reaction mixture was centrifuged for 5 minutes at 2500 rpm and the absorbance of the supernatants was measured at 560 nm. For the sample extracts, the test was done in triplicate [25, 28].
% Inhibition of haemolysis = [(A0-AS)/A0] x100
A0 – absorbance value of the control; AS – absorbance value of the test sample.
Antioxidant activity
Antioxidant activity was evaluated using two methods: DPPH scavenging activity and the ferric reducing antioxidant power (FRAP) assays, which were modified according to [29].
DPPH assay
The experiment was carried out by mixing 2.5 ml of 0.1 mM DPPH solution with 1 ml of leaf and stalk extracts ranging in concentrations from 100 to 500 μg/ml. The absorbance was measured at 517 nm using a Multiskan GO microplate Spectrophotometer (Thermo Scientific, Finland) after 20 minutes of incubation at room temperature and in the dark. Antioxidant activity was measured in duplicate using the DPPH assay.
% Radical scavenging activity = [(A0-AS)/A0] x100.
A0 – absorbance value of the control; AS – absorbance value of the test sample.
FRAP assay
The FRAP reagent was typically prepared by mixing 0.3 M acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM ferrous chloride in a 10:1:1 (v/v/v) proportion. Experiments were conducted by combining 2.5 ml of FRAP reagent with 0.5 ml of test samples at increasing concentrations (100-500 μg/ml). The absorbance readings were read at 593 nm using a multiskan GO microplate spectrophotometer (Thermo Scientific,Finland) after 20 minutes of incubation in the dark at 37°C . The FRAP test was performed in duplicate to measure antioxidant activity.
% Radical scavenging activity = [(A0-AS)/A0] x100.
A0 – absorbance value of the control; AS – absorbance value of the test sample.
Antimicrobial Activity
Determination of inhibition zone
Agar diffusion techniques with minor changes were used to test antibacterial activity according to [30]. A sterile Petri plate was filled with around 25 mL of molten MH agar (Himedia, Mumbai, India). After allowing the plates to set, a sterile cotton swab was used to transfer 100 µl of 18 hours grown (OD adjusted to 0.6) bacteria onto the plate and create a culture lawn (Himedia, Mumbai, India). The wells were created using a sterile cork-borer (6 mm) after the bacteria had set for five minutes, and the test sample was poured into the wells at varying concentrations of 100 – 400 µg/mL. The negative and positive controls were filled wells with sterile water and 30 µg/mL Azithromycin, respectively. The plates were incubated for 24 hours at 37°C in a bacteriological incubator. The diameter of the zone of inhibition surrounding the well was measured using the antibiotic zone scale to estimate antibacterial activity (Himedia, Mumbai, India).
Micro broth dilution assay – minimum inhibition concentration
The 96-well microtiter plate with cover was used to test MH broth for antibacterial screening as per [31] with required modifications. Plant samples (Stalk and Leaf) were developed at a concentration that was double that of the final concentration. In the first wells in row A–B, 200 μl of the produced extract (Stalk) in broth was introduced (in column 1). Rows A–B in column Using a micropipette, twofold serial dilutions were performed systematically down 2–11 contained 100 μl of broth alone, whereas rows A–B in column 12 had 200μl. columns 1–10 (from rows A–B). 100 μl was withdrawn from the starting concentrations (columns 1–10 in rows A–B) and transferred to the next column with the 100 μl broth, adequately mixed, and the operation was repeated until the last column (10) was reached, while the last 100 μl was discarded. This makes the total amount of the extract in all the test wells to 100μl, with the exception of the 12th column, which included 200 μl of broth as a sterility control. To achieve the necessary final inoculum load of 5 × 105 CFU/mL, an equivalent amount (100 μL) of 1 × 106 CFU/mL bacterial [S. aureus] inoculum was put into all wells except the 12th column. Column 11 was used as the growth control (extract-free). The leaf extract was applied in rows F–G in the same way, using the same technique of preparation. The extract concentrations were 2000 μg/mL to 4 μg/mL, and the microtiter plate was incubated for 24 hours at 37°C. In the broth dilutions, the Minimum Inhibitory Concentrations were calculated visually as the lowest concentrations of the extract at which no bacterial growth could be seen with the naked eye.
Determination of minimum bactericidal concentrations (MBC)
The MBC was calculated using the approach described in [32]. 10 μl of culture was collected from each of the first three broth cultures that exhibited no growth in the MIC tubes and spread out on fresh nutrient agar plates. After 24 hours of incubation, the lowest concentration of extract that did not cause any bacterial growth on the solid medium was regarded as MBC values for the extracts.
Preparation of value-added product
Stalk extract of concentration 500 μg/ml was added to about 5 grams of organic aloe vera gel and mixed thoroughly. The product was stored in 4ºC.
Statistical analysis
All the experimental data that was obtained from the respective independent experiments represented as mean ± standard deviation. Microsoft Excel 2013 was used for graphical evaluations. Differences with p-values equal to or less than 0.05 were considered as statically significant.
RESULTS
Effect of solvents on the yield of different parts of broccoli
Table 1 represents the results of yield percentage which was calculated after the maceration process. It was observed that aqueous leaf extract and aqueous stalk extract had the maximum yield percentages with 2.341% and 1.973%, respectively. Whereas the lowest yield percentage was recorded in chloroform extract in the case of leaf and in hexane extract in the case of stalk.
Maceration is one of the best methods to obtain the yield percentage. Finding yield percentage is important in any product developmental experiment as yield percentage shows the amount of phytochemicals that can effectively be extracted from the sample material (leaf and stalk) using suitable solvents. Higher the yield percentage implies higher the amount of phytochemicals resulting in higher productivity.
Table 1. Yield of different parts of broccoli.
Effect of broccoli on phytochemicals
Figure 1A showcases the comparison of total carotenoids, chlorophyll b and chlorophyll a content in the leaf sample using three different solvents namely aqueous, chloroform and hexane. This graph was plotted with the values that were obtained after the quantitative analysis. With the help of this graph, it is evident that all the three phytochemicals are present in higher quantities in the chloroform extract of the leaf. Contrastingly, the lowest amount of chlorophyll and total carotenoids was observed in aqueous extract and the hexane extract had the least amount of chlorophyll b. Whereas, Figure 1B showcases the comparison of total carotenoids, chlorophyll b and chlorophyll a content in the stalk sample using the same three different solvents. By observing the plotted graph, unanimously hexane extract of the stalk sample contains the higher amounts of required phytochemicals. Whereas the lowest content of these phytochemicals is seen in the chloroform extract.
Gas chromatography-mass spectrometry is a quantitative analysis which is performed to check for the presence of beneficial compounds in a sample. As represented in Table 2, from the given leaf sample it was recorded that the sample contains compounds like Nonacosane, Tetratriacontane, n-Tetracosanol-1 and many more compounds which can exhibit antioxidant, antibacterial, antimicrobial activities, and cytotoxic properties.
As represented in Table 3, the stalk extract contained compounds such as Nonacosane, Pentane, 3-bromo-3-methyl, 15-Nonacosanone which acts as preservatives and raw material in the cosmetic industry and also as recreational drugs and steroids.
The significant peak in both the leaf and the stalk sample’s graph is shown by a compound called Nonacosane, with a retention time of 29.343 (Table 2 and Figure 2) and 29.313 (Table 3 and Figure 3), respectively. This significant peak signifies that the compound is present higher in quantity and eluted highly by the given sample.
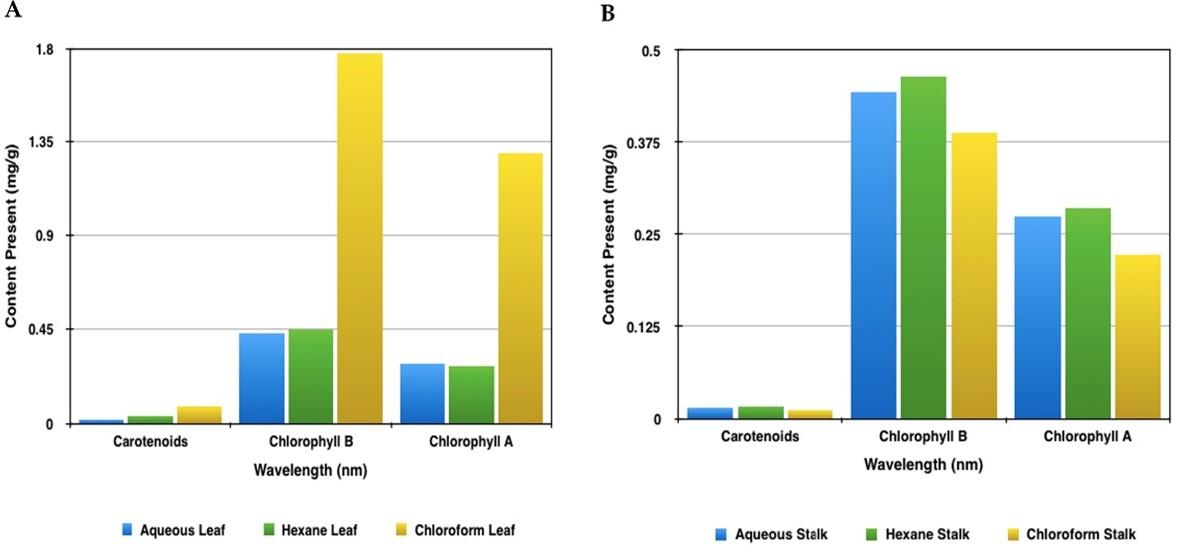
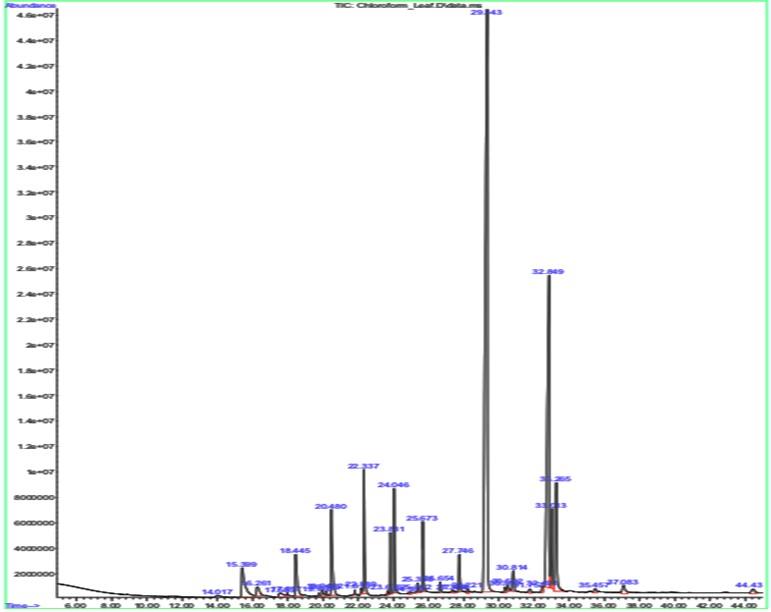
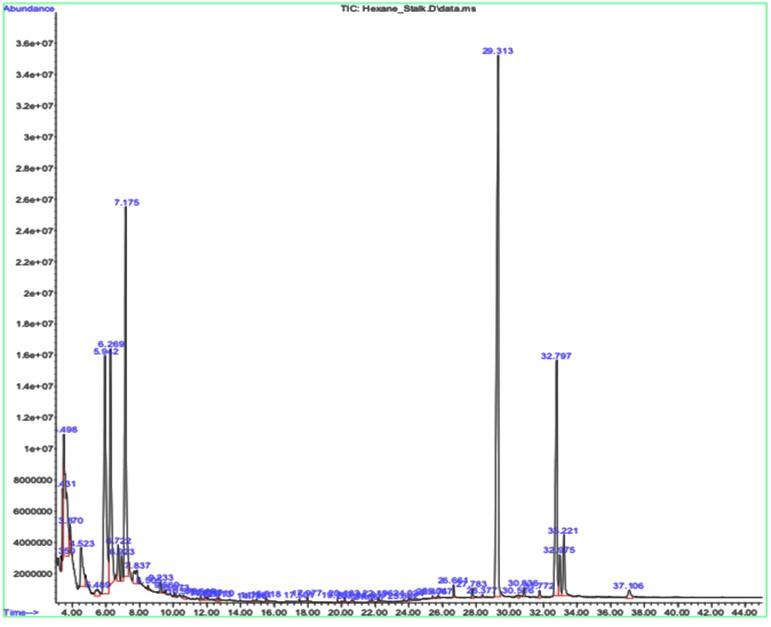
Table 2. GCMS results for leaf sample.
Table 3. GCMS results for stalk sample.
Effect of broccoli waste on inflammatory activities
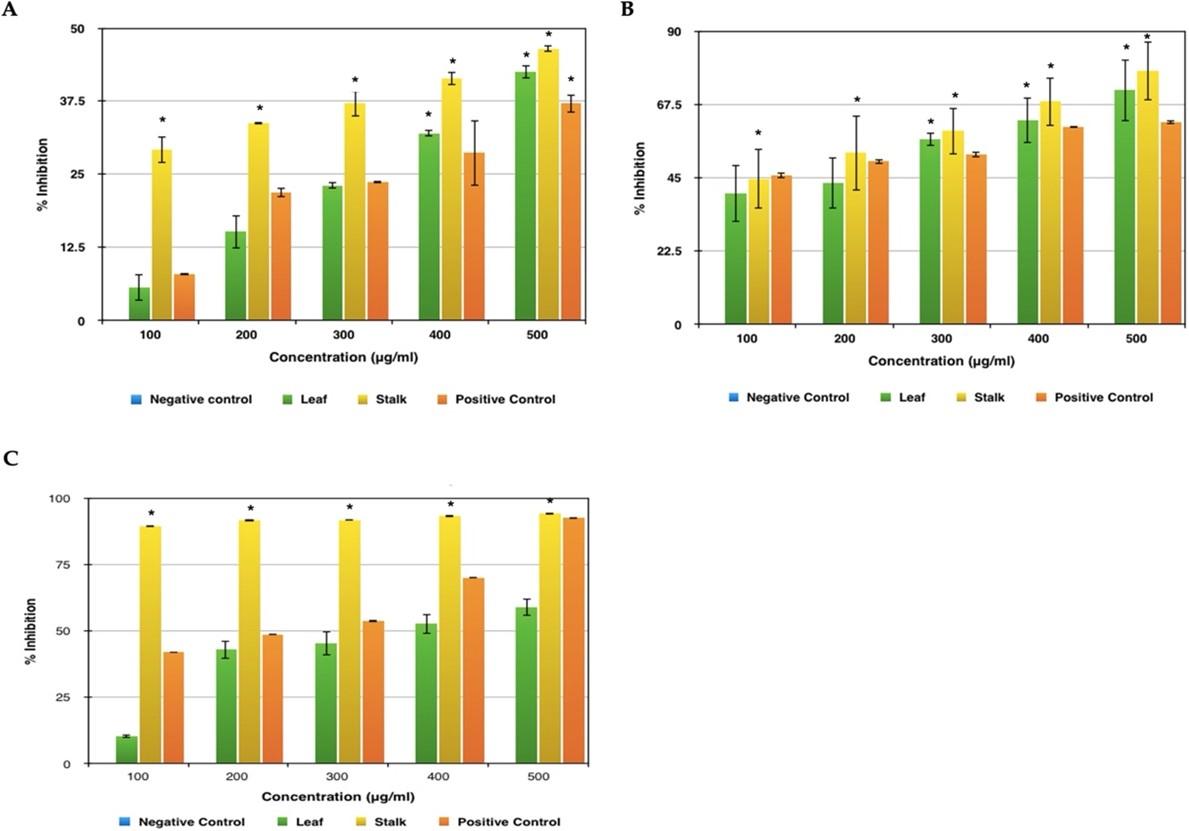
Figure 4A represents the results obtained by conducting the anti-inflammatory test such as albumin denaturation activity and it was found that supreme inhibition of 46% was seen in stalk extract and 42 % was recorded for leaf extract. Both highest peaks were observed at a concentration of 500 μg/ml. As far as the standard used is concerned, aspirin showed the maximum impediment of 37% at the same concentration. The inhibition shown by the plant extract is more when compared to the standard used which means that these plant extracts protect the structure of the protein from denaturing, hence proving that it has a better anti-inflammatory activity.
From Figure 4B, it is perceived that in both the leaf and stalk extract there is an observable exhibition of antiproteinase activity at 100 μg/ml concentration itself. But still, the maximum inhibition was recorded at the concentration of 500 μg/ml with leaf sample inhibiting around 71% and the stalk extract inhibiting around 78%. As far as the standard is concerned, aspirin was seen with a maximum inhibition of 62% in the same concentration. In this test too, the given plant sample shows higher activity than the standard used proving that the sample has a better anti-inflammatory activity.
The extract was effective in inhibiting the heat induced haemolysis at different concentrations. The results showed that stalk extract at concentration 100, 200, 300, 400 and 500 µg/ml and leaf extract at concentration 500µg/ml significantly protected (p<0.05) the erythrocyte membrane against lysis induced by heat (Figure 4C). Aspirin 500µg/ml offered a significant protection against the damaging effect of heat solution. The maximum inhibition was recorded at the concentration of 500 μg/ml with leaf sample inhibiting around 58% and the stalk extract inhibiting around 95%. As far as the standard is concerned, aspirin was seen with a maximum inhibition of 93% in the same concentration. In this test, the given broccoli stalk sample shows higher activity than the standard used proving that the sample has a better anti-inflammatory activity. Aspirin was taken as a standard drug for the comparison.
Effect of broccoli waste on oxidation activities
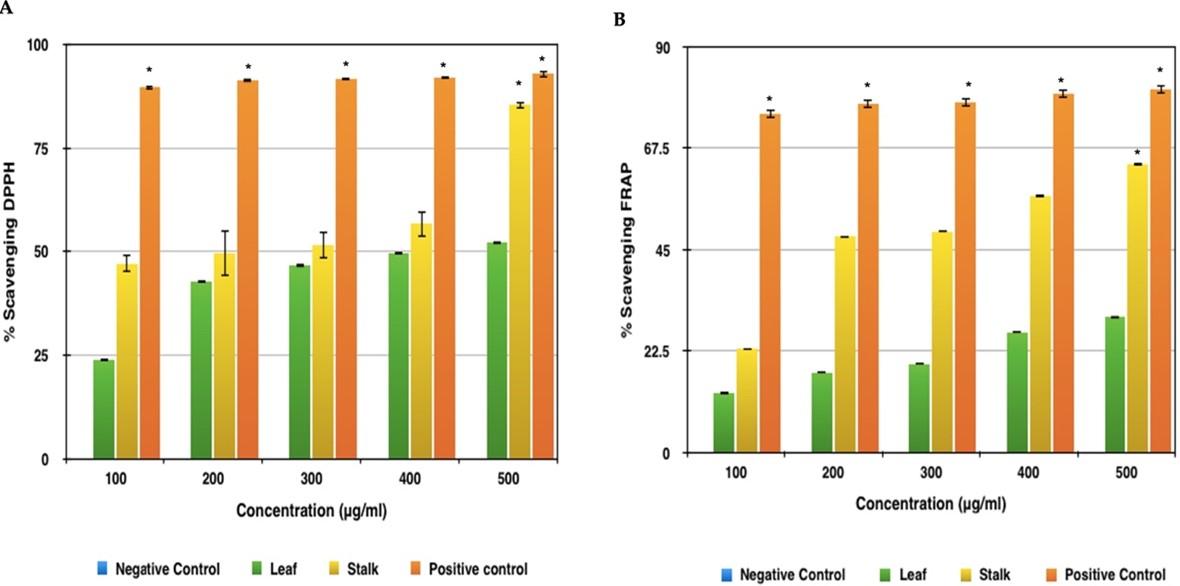
Both extracts had an increasing rate of DPPH scavenging activity, which was most likely owing to the high amount of phenolic compounds. The leaf sample had the lowest antioxidant activity as measured by DPPH in each of the trials. The leaf sample showed 52% scavenging activity at 500μg concentration (Figure 5A). The extract with the higher value was the stalk sample with 86% scavenging activity at 500μg concentration. This method established a significant difference of scavenging activity for the Broccoli samples with respect to the standard-Ascorbic Acid, which showed rising scavenging activity of up to 93% in concentrations 100μg, 200μg, 300μg, 400μg and 500μg respectively.
Broccoli leaf extract had the lowest antioxidant activity, with a 52 percent scavenging activity at 500 g concentration, according to the FRAP results (Figure 5B). While, the highest antioxidant activity corresponded to the stalk extract, both with 63.8% scavenging activity at 500 μg concentration. The difference between the Broccoli extract and the standard was obtained using this method and Ascorbic Acid showed highest scavenging activity of approximately 80.54% in concentrations 100 μg, 200 μg, 300 μg, 400 μg and 500 μg, respectively
Effect of broccoli waste on microbial activities
An agar well diffusion assay was used to determine the antibacterial activity of the material. A very good antibacterial activity was observed in both the extracts against S. aureus. Among the extracts tested, the stalk extract showed better activity than the leaf extract (Figure 6; Table 4). At a concentration of 0.1 mg/mL, a zone of 8mm was found in the stalk extract, whereas a zone of 7mm was observed in the leaf extract at a dosage of 0.2 mg/mL. At a maximum dose of 0.4 mg/mL, stalk extract inhibited the greatest zone growth of gram-positive bacteria like Staphylococcus aureus (19 mm).
The minimum inhibitory concentration (MIC) for leaf and stalk extracts were determined to be 0.5 mg/mL and 0.25 mg/mL, respectively (Figure 7A and Table 5). At 0.25 mg/mL and 0.5 mg/mL, respectively, the minimum inhibitory zones were detected in both the stalk and leaf extracts. However, when compared to the leaf extract, the stalk extract demonstrated minimal inhibitory effect against Staphylococcus aureus at a lower dose (0.5 mg/mL).
For leaf and stem extracts, the minimum bactericidal concentrations (MBC) were determined to be 0.5 mg/mL and 2 mg/mL, respectively (Figures 7B and Table 5). At a dosage of 2 mg/mL, the antibacterial activity of broccoli plant extracts revealed minimal bactericidal activity against Staphylococcus aureus in the leaf extract. However, when compared to the leaf extract, the stalk extract demonstrated minimal bactericidal efficacy against Staphylococcus aureus at a lower dose (0.5 mg/mL).
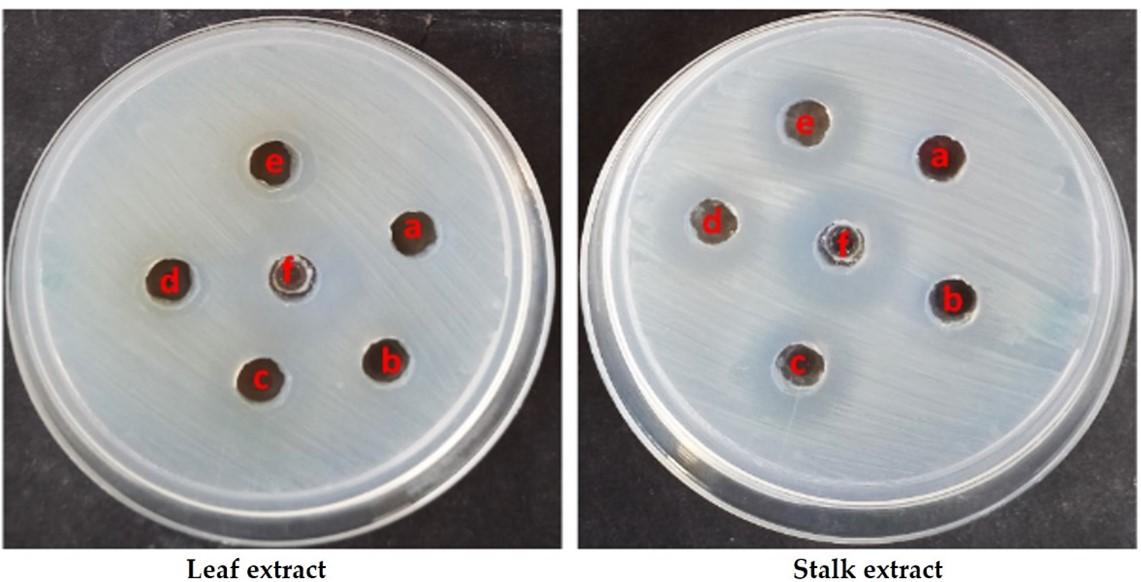
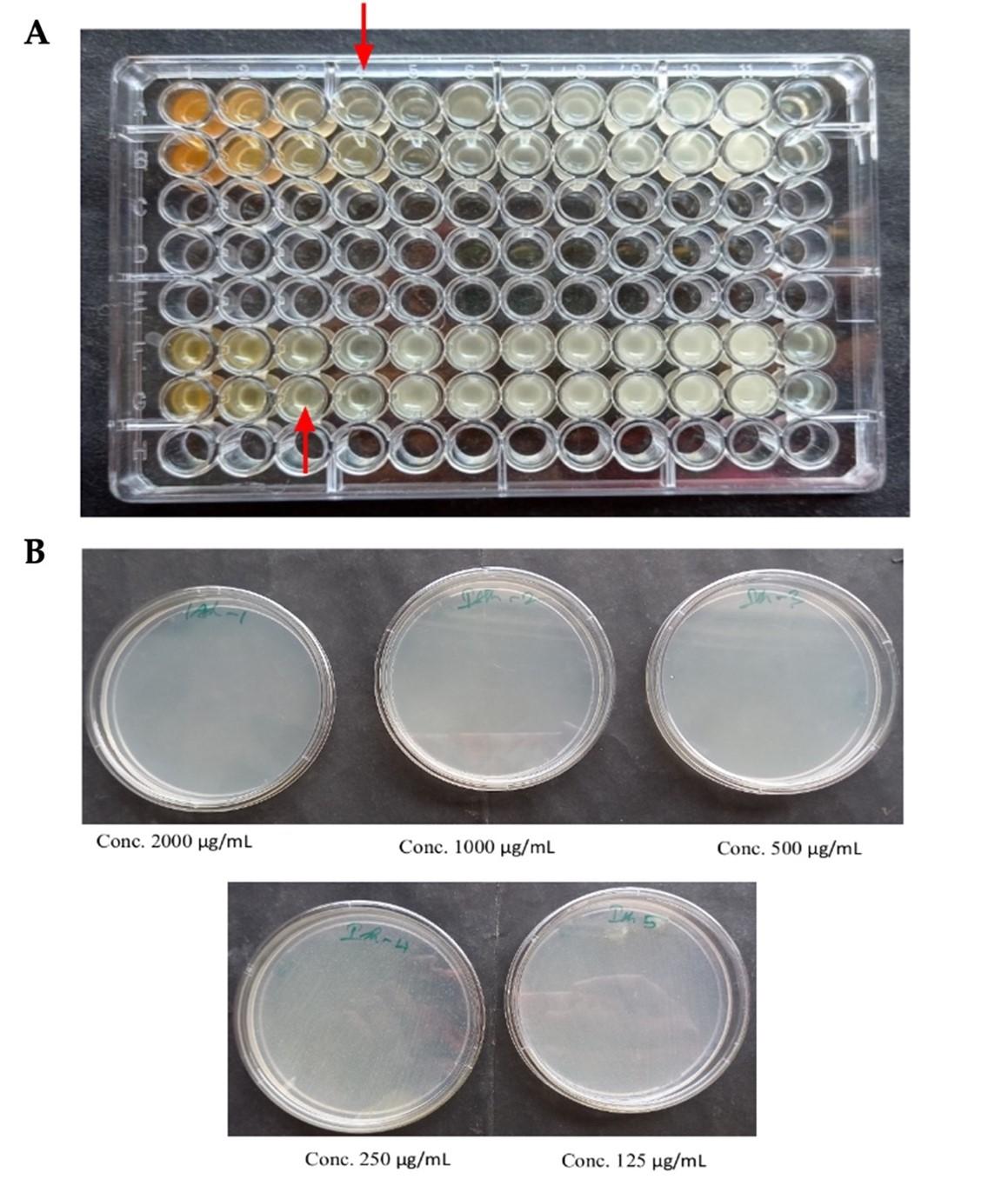
Table 4. Antibacterial activity of test samples against S. aureus.
Table 5. Stalk extract test sample: minimum bactericidal concentrations activity against S. aureus.
DISCUSSION
The impressive findings of compounds like Nonacosane, Tetratriacontane, n-Tetracosanol-1, showed the ability to exhibit antioxidant, antibacterial, antimicrobial activities, and cytotoxic properties after performing gas chromatography-mass spectrometry of the samples.
Denaturation of protein is a process where proteins tend to get structurally damaged. Usually, the secondary and the Tertiary structures are the ones which get disrupted by any strong compound or external stress. So, to investigate the process of anti-inflammatory activity, the potentialities of the chosen plant extract to impede the process of denaturation was studied [24]. The plant extract showed remarkable results in inhibiting albumin denaturation, and it was found that the Broccoli wastes showed successful inhibition in heat induced albumin denaturation.
Serine proteinases are predominantly sourced from neutrophils, and these are restricted at lysosomes. It has also been formerly disclosed that these leukocyte proteinases take part in a very significant role in the progression of tissue rupture via any inflammatory reaction. To prevent further damage to the cells, a remarkable level of protection must be provided by these proteinase inhibitors [26]. This test was conducted to check the antiproteinase activity of the plant sample which showed excellent anti-inflammatory activity.
Lysosomes disintegrate and release constituent enzymes during inflammation, resulting in a range of illnesses. By blocking the release of lysosomal enzymes or stabilizing the lysosomal membrane, nonsteroidal anti-inflammatory medications (NSAIDs) have positive effects [23]. Hemolysis and membrane lysis with haemoglobin oxidation occur when red blood cells (RBC) are exposed to harmful agents such as hypotonic media, heat, methyl salicylate, and phenylhydrazine. Because the human erythrocyte (HRBC) membrane resembles a lysosomal membrane component, inhibition of hypotension and heat-induced lysis of the erythrocyte membrane has been adopted as a measure of the mechanism of anti-inflammatory activity of broccoli extract. The hemolytic effect of hypotonic fluid is associated with the excessive accumulation of fluid inside the cell, leading to the rupture of its membrane. Damage to the erythrocyte membrane makes cells vulnerable to secondary damage from free radical-induced lipid peroxidation. Membrane stabilization results in the prevention of leakage of serum proteins and fluids into tissues during periods of increased permeability caused by inflammatory mediators. Broccoli stalk extract stabilizes the erythrocyte membrane by preventing the release of lytic enzymes and active inflammatory mediators [33]. The human erythrocyte membrane was protected from hypotonic and heat-induced lysis by broccoli stalk extract.
DPPH assay is frequently used in the laboratory to determine the free radical capture capacity of purified phenolic compounds and natural plant extracts because they are fast, easy, and inexpensive. The DPPH assay measures the ability of a compound to function as a free radical scavenger or hydrogen donor and showed rising scavenging activity of up to 93% in sample leaf and stalk extract [34].
The FRAP assay assesses the antioxidant’s ability to reduce the ferric tripyridyltriazine (Fe3 + TPTZ) complex to yield coloured ferric tripyridyltriazine (Fe2 + TPTZ). The release of hydrogen atoms causes free radical chain breakage [35]. The stalk extract showed the highest antioxidant activity with excellent scavenging activity.
Staphylococcus aureus is a facultative anaerobic, immobile, spore-free, gram-positive cocci that causes skin infections. It is the cause of various human infections, including superficial skin lesions. In this study, we evaluated the potential of broccoli plant leaf and stem extracts to treat acne. Polyphenols, which play a crucial part in the defensive system against phytopathogens, may be the source of the extract’s antibacterial activity. Gallic acid has released toxicity to numerous bacteria and exerted a significant antioxidant capacity due to its structural features, such as the hydroxyl group (OH) of gallic acid. Our study also revealed that the Broccoli stalk extract showed stronger antimicrobial effect than the leaf extract at a comparatively lower concentration of the sample [36].
CONCLUSION
On comparing the yield percentages of the Broccoli extracts using the three different solvents – water, hexane, and chloroform, it was observed that aqueous extract had the highest yield. It is a safe solvent with less to no toxicity and is also economical. Lack of toxicity in the aqueous extract proves its reliability to function as a cosmetic product or as a functional food. The anti-inflammatory and antioxidant activities of broccoli phytochemicals indicated anti-aging properties of the Broccoli stalk sample. Our study also revealed that the Broccoli stalk extract showed stronger antimicrobial effect than the leaf extract at a comparatively lower concentration of sample against S. aureus. This infers that Broccoli stalk has excellent anti-acne properties and can be used in cosmetic products. Incorporating broccoli into cosmetics at optimal concentrations not only improves physicochemical quality, but also improves nutritional value and bioactivity properties. Adding broccoli stalk extract into aloe vera gel did not have a concerning effect on the overall quality, texture or the color of the gel. Through our results we can conclude that after performing certain toxicity tests we can incorporate our research findings into creams, lotions, serums, and other cosmetic products to tackle acne, inflammation, and other concerning skin conditions.
AUTHOR CONTRIBUTIONS
JL was involved in conception and design of experiments. AR, AN and SD contributed to perform the experiments and also to analyse the data. SS contributed to the drafting of the manuscript. Finally, JL approved the current script of the manuscript.
ACKNOWLEDGEMENT
The authors are thankful to Prof. C. Muthamizchelvan, Pro-Vice-Chancellor, Engineering and Technology, Dr M. Vairamani, Dean, School of Bioengineering, Prof. R.A. Nazeer, HOD, Department of Biotechnology, SRM Institute of Science and Technology for providing facilities to carry out the research work.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Martinez Lopez BC. Martinez Ballesta MC, Moreno DA, Carvajal, M, Garcia VC. Growing hardier crops for better health: Salinity tolerance and the nutritional value of broccoli. J Agric Food Chem. 2009; 57: 572–578.
- [2]Ballesta MC, Dominguez PR, Moreno DA, Muries B, Alcaraz – Lopez C, Bastias E, Garcia-Viguera C, Carvajal M. Minerals in plant food: Effect of agricultural practices and role in human health. A review. Agron Sustain Dev. 2010; 30: 295–309.
- [3]Arnaiz E, Bernal J, Martin MT, Nozal MJ, Bernal JL, Toribio L. Supercritical fluid extraction of free amino acids from broccoli leaves. J Chromatogr A. 2012; 1250: 49–53.
- [4]Borja-Martinez M, Lozano-Sanchez J, Borras-Linares I, Pedreno MA, Sabater-Jara AB. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants. 2020; 9(12): 1195.
- [5]Dominguez-Perles R, Moreno DA, Carvajal M, Garcia-Viguera C. Composition and antioxidant capacity of a novel beverage produced with green tea and minimally-processed byproducts of broccoli. Innov. Food Sci Emerg Technol. 2011; 12: 361–368.
- [6]Drabinska N, Ciska E, Szmatowicz B, Krupa-Kozak U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2018; 267: 170-177.
- [7]Coman V, Teleky BE, Mitrea L, Martau GA, Szabo K, Calinoiu LF, Vodnar DC. Bioactive potential of fruit and vegetable wastes. Adv Food Nutr Res. 2020; 157–225.
- [8]Hooper L, Cassidy A. A review of the health care potential of bioactive compounds. J Sci Food Agric. 2006; 86: 1805–1813.
- [9]Ares AM, Nozal MJ, Bernal J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J Chromatogr A. 2013; 1313: 78–95.
- [10]Barbulova A, Colucci G, Apone F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics. 2015; 2: 82–92.
- [11]Selvamuthukumaran M, Shi J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual Saf. 2017; 1: 61–81.
- [12]Ares AM, Nozal MJ, Bernal JL, Bernal J. Optimized extraction, separation and quantification of twelve intact glucosinolates in broccoli leaves. Food Chem. 2014; 152: 66-74.
- [13]Butkute B, Taujenis L, Norkeviciene E. Small-seeded legumes as a novel food source”. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules. 2019; 24: 133.
- [14]Gupta C, Prakash D, Gupta S. Relationships between bioactive food components and their health benefits” Introduction to Functional Food Science Textbook. 1st ed. Create Space Independent Publishing Platform; Scotts Valley, CA, USA; pp. 66–85. 2013.
- [15]Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010; 2: 611–625.
- [16]Le TN, Sakulsataporn N, Chiu CH. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals. 2020; 13: 82.
- [17]Galanakis CM, Tsatalas P, Galanakis IM. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind Crops Prod. 2018; 111: 30–37.
- [18]Nagarajan J, Krishnamurthy NP, Ramanan RN, Raghunandan ME, Galanakis CM, Ooi CW. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chem. 2019; 296: 47–55.
- [19]Galanakis CM. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci Technol. 2018; 79: 98–105.
- [20]Naviglio D, Scarano P, Ciaravolo M, Gallo M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods. 2019; 8(7): 245.
- [21]Shivaji S, Dronamaraju VL. Scenedesnus rotundus isolated from the petroleum effluent employs alternate mechanisms of tolerance to elevated levels of Cadmium and Zinc. Sci Rep. 2019; 9: 8485.
- [22]Tripathi AK, Gautam M. Biochemical parameters of plants as indicators of air pollution. J Environ Biol. 2007; 28(1): 127–132.
- [23]Moon JK, Kim JR, Ahn YJ, Shibamoto T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J Agric Food Chem. 2010; 58(11): 6672-6677.
- [24]Mizushima Y, Kobayashi M. Interaction of anti‐inflammatory drugs with serum preoteins, especially with some biologically active proteins. J of Pharma Pharmacol. 1968; 20: 169‐ 173.
- [25]Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010; 2(1): 146-155.
- [26]Oyedepo OO, Femurewa AJ. Anti‐protease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscorpioidea and Tetrapleura tetraptera. Int J of Pharmacong. 1995; 33: 65‐69.
- [27]Sadique J, Al-Rqobahs WA, Bughaith, EIGindi AR. The bioactivity of certain medicinal plants on the stabilization of RBS membrane system. Fitoterapia. 1989; 60: 525-532.
- [28]Shinde UA, Kulkarni KR, Phadke AS, Nair AM, Dikshit VJ, Mungantiwar MN Saraf. Mast cell stabilizing and lipoxygenase inhibitory activity of Cedrus deodara (Roxb.) Loud Wood Oil. Ind J Exp Biol. 1999; 37(3): 258-261.
- [29]Altisent R, Plaza L, Alegre I, Vinnas I, Abadias M. Comparative study of improved vs traditional apple cultivars and their aptitude to be minimally processed as ‘ready to eat’ apple wedges. LWT ‐ Food Science and Technology. 2014; 58: 541–549.
- [30]Holder IA, Boyce ST. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns. 1994; 20: 426-429.
- [31]Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 2008; 3(2): 163–175.
- [32]Olajuyigbe OO, Afolayan AJ. Antimicrobial potency of the ethanolic crude bark extract of Ziziphus mucronata Willd. subsp. mucronata Willd. Afr J Pharmacy Pharmacol. 2012; 6(10): 724-730.
- [33]Anosike CA, Obidoa O, Ezeanyika, LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru: Journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2012; 20(1): 76.
- [34]Rahman MM, Islam MB, Biswas M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015; 8: 621.
- [35]Rajurkar, Nilima S, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm sci. 2011; 73(2): 146-51.
- [36]Taylor TA, Unakal CG. “Staphylococcus Aureus” Treasure Island (FL): Stat Pearls Publishing, https://www.ncbi.nlm.nih.gov/books/NBK441868/#_NBK441868_pubdet. 2022.