Evaluation of diagnostic accuracy of NS1 antigen and oxidative stress for Dengue virus infection in Bangladeshi population
Abstract
The dramatic rise of global incidence of dengue infection has generated an urgent yet an unmet need for an accurate and validated diagnostic test for DENV infection. A total of 81 serum samples were enlisted to dengue NS1 ICT test and dengue NS1 ELISA test and oxidative stress markers including NO, MDA and SOD activity were measured. Out of 81 samples, 41 were found positive by ELISA (DENV group; n=41) whereas 28 were found positive by ICT test. Out of 81 samples; 40 were found negative (control group; n=40) by ELISA whereas 53 were found negative by ICT test. There were 28 samples which were found positive by both ICT and ELISA test. There were 40 samples which were found negative by both ICT and ELISA. The sensitivity and specificity of ICT test were 68.29% and 100% when compared with ELISA. The positive cases of dengue were confirmed by platelet count having significantly higher level of platelet counts (P<0.0001) in dengue patients compared to that of control. Dengue patients had significantly higher levels of NO (P=0.0007), MDA (P<0.0001) and SOD activity (P<0.0001) compared to those of control. Among dengue patients, a positive correlation was found between MDA level and hematocrit percentage (R2 = 0.8107; P<0.001) and between MDA level and NO level (R2 = 0.4954; P<0.001). The study has unveiled that though the rapid ICT test is cost-effective and with satisfactory specificity, the sensitivity is not up to the mark and possibility of misdiagnosis by this rapid test kit is significant.
INTRODUCTION
Dengue is recognized as one of the deadliest viral diseases with the potential to cause life threatening infections in human. It is an arthropod-borne disease responsible for causing an array of clinical manifestations in humans. The Aedes aegypti mosquito is recognized as the most common vector of dengue. The A. aegypti mosquito is normally found in urban areas and unlike other mosquitoes, A. aegypti bites mainly during daytime and before twilight. Dengue is prevalent in hot, steamy, sweltering tropical climates worldwide especially in semi-urban and urban regions.
During 2013 to 2017, Dhaka city experienced highest number of deaths due to dengue compared to other districts in Bangladesh. In this period, 14121 dengue cases were reported throughout the country [1]. Dengue virus is mainly of 4 serotypes named DENV-1, DENV-2, DENV-3, and DENV-4 which are antigenically diverse. All four serotypes have similarities since they share approximately 65% of their genomes [2]. Recently, another serotype has emerged which is named DENV-5 [3]. Although being antigenically different, all the serotypes exert same disease with same clinical symptoms. Among the four dengue serotypes, DENV-1 and DENV-2 are the most predominant ones found in the circulation in three metropolitan cities of Bangladesh [4]. Clinical manifestation due to dengue infection is very much irrespective of dengue virus serotypes but it can vary depending on various aspects including the immune status, genetic makeup, and age of the human host [5]. In the initial stage of dengue virus infection, mild and undifferentiated “flu-like” fever symptoms can be observed just like the other viruses of Flaviviridae family. About 20% of all infections are symptomatic and the symptoms covers a broad scale of mild to severe clinical indices [6].
There are different ways to diagnose dengue and several serological techniques are available. Although many methods are available, they differ in accuracy, sensitivity and specificity. It’s a major problem in identifying and measuring the severity of the disease. Sometimes patients are asymptomatic and so they remain undiagnosed. Regions where dengue is not endemic or predominant, dengue symptoms are clinically mistaken as other disease . This is one of the main reason behind the low number of reported case in Africa [7]. Dengue patients are misdiagnosed as a febrile disease when they don’t have any typical symptoms or they catch dengue outside the dengue season. Combination of extensive amount of diagnosis and improvement of accuracy are therefore needed for improving surveillance on dengue.
Molecular methods have provided efficiency in terms of identifying DENV. Techniques like RT-PCR and nucleic acid hybridization are widely used with great success in diagnosing DENV infection. The nonstructural protein 1 (NS1), generated by all the flaviviruses, is a conserved glycoprotein secreted as hexamer from the cells infected by DENV [8]. Since IgM, IgG takes a few days to be generated whereas NS1 is present in acute viraemic phase of infection, NS1 detection becomes highly useful for early diagnosis of dengue [9]. Serum sample taken within 1-3days after the infection might show IgM negative result which is misleading but will show NS1 positive result. Characteristics like early occurrence, specificity to dengue and exuberance in sera make it a perfect candidate for rapid diagnostic test [10].
The Dengue NS1 Rapid Test is a qualitative, membrane strip-built immunoassay for the identification of NS1 antigen in human serum [11]. The rapid test membrane is pre-antibody coated (NS1 specific antibody) on the test line region and employs a separate control to confirm assay flow and conduction. The appearance of a red line at the test line confirms the presence of NS1 antigen. The Dengue NS1 antigen ELISA is a vastly sensitive, rapid, and reliable assay. It uses sandwich-type immunoassay to detect low levels of NS1 in serum. The presence of NS1 antigen is confirmed by the colorimetric response obtained using an enzyme-conjugate and a substrate. The values obtained for the negative and positive sera serve as guidelines as to determining if a sample contains NS1 antigen.
Oxidative Stress is a phenomenon while protective cellular responses emerge against the invasion of foreign objects into the cell [12] and it occurs due to alterations in the magnitudes of reactive oxygen species (ROS) or oxidants including hydroxyl radicals (HO.), superoxide anions (O2) and hydrogen peroxide (H2O2) [13]. Balancing ROS production within a specifc homeostatic level is central for the consistent upkeeping of cells, their production, and immune responses [14].
The capacity of DENV to trigger oxidative stress in humans has been explored [15]. Like HIV and HCV, DENV creates oxidative stress through the end product NADPH oxidase (NOX). This stimulates the inflammatory cytokine response, resulting in the pathogenesis of DENV infection [16]. Antioxidants are generated to restore homeostasis [17]. Stimulation of IRF3/7/STAT1, an antiviral and inflammatory network mediated by NF-κB, and Nrf-2-dependent transcription of antioxidant genes are the chief actions that occur after DENV infection [18]. DENV infection activates the unfolded protein response to combat ER stress and this results conditions that are appropriate for maintaining viral infection [19]. In agreement with this, recent experimental findings have suggested that controlling ER stress could be a probable method to prophylactic treatment against flavivirus infections [20].
Since dengue NS1 rapid ICT test kit is used more, we wanted to evaluate the efficacy of dengue NS1 rapid ICT test kit and compare dengue NS1 rapid ICT test kit with dengue NS1 ELISA kit. It would justify whether the usage of the rapid test kit for mass testing is reliable or not. We also wanted to analyse the oxidative stress observed in DENV patients and find a correlation between oxidative stress markers with various hematological parameters. It would help us to find a prognostic marker for dengue and help us to diagnose dengue patient effectively. We also tried to find a correlation between Dengue symptoms and preventive measures taken with the occurrence of Dengue fever. Our another target was to investigate whether there is any correlation between different hematological parameters and oxidative stress markers which may help design drug for dengue patients.
METHODS AND MATERIALS
Study subjects
The study comprised of a cohort of 41 patients diagnosed with DENV at Dhaka University Medical Center. A cohort of 40 individuals who had satisfactory platelet count, no fever and negative result in dengue NS1 ELISA were enrolled as controls. Dengue patients suffering from infectious disease, renal dysfunction, diabetes, impaired liver or autoimmune disease, hypo or hypertension were excluded from the study. The control subjects did not have a prior history of dengue infection and cardiovascular diseases. Full consent was obtained from both the control and the patient groups prior to blood collection and were kept enlighten about the goals of the study. The guidelines of the Ethical Review Committee of the Faculty of Biological Sciences, University of Dhaka, were stringently adopted throughout the sample collection (20190602/ERC_biosciencedu) procedure.
All the study subjects completed a questionnaire before blood collection which included generalized information on age, gender, weight, fever duration, various disease symptoms and several demographic factors including knowledge, attitude and practice related to DENV infection.
Separation and storage of samples
The study was conducted from June 2019 to July 2020. About 10 mL of peripheral blood was collected from the study participants with the aid of an expert technician and the collected blood samples were allowed to stand at RT for few minutes in vacutainer tubes. The samples were then centrifuged for 10 minutes at 3,000 rpm and the resulting serum were then collected using micro pipettes so that no red blood cells could remain in it. Appropriate aliquots of serum were then stored in microcentrifuge tubes and were stored at -200C until analyzed.
Dengue NS1 rapid ICT test
The Dengue NS1 Rapid ICT Test, which is a qualitative, membrane strip-built immunoassay for the identification of NS1 antigen in human serum was used in the study. The rapid test involved the pre-coat of the membrane with a NS1 specific antibody on the test line region and utilized a separate control to assure assay flow and performance. During testing, sample was added directly to the sample well and running buffer to the buffer well. If NS1 antigen is present, a red line would appear at the test line. The red line at the control region should always appear if the assay is performed correctly. The presence of this red line verified that proper flow had occurred, and catastrophic failure of the conjugate had not occurred.
This technique was carried out according to the protocol provided with the kit (SD Bioline, Korea). At the beginning, the forehand of a study subject was cleaned with alcohol swab and blood was drawn from a vein of that part and the blood was collected in an EDTA tube (K3 EDTA tube, India). Two drops of blood was then placed in the sample well and another two drops of buffer solution was added in the buffer well of the test strip without further delay. The solution migrated upward on the membrane via capillary action to react with the anti-NS1 antibody on the membrane. The results were read subsequently after 15 minutes and were interpreted according to the position of the test line described above. The presence of the red line in the control region indicated that the test was a valid one. The presence of red line in both the control and test region indicated that the sample was dengue NS1 Ag positive. The presence of red line in the test region only marked the test result as an invalid one. No red line in any of the region also defined the test as invalid.
Dengue NS1 ELISA (Enzyme-linked immunosorbent assay) test
The Dengue NS1 antigen ELISA which is a highly sensitive, rapid, and reliable sandwich-type immunoassay (SD Biosensor, India) was utilized in the study. In this assay, controls and unknown serum samples were diluted in sample dilution buffer, containing secondary antibody, and incubated in micro wells.
Microtitration plates were coated overnight at 40C with monoclonal anti-dengue NS1 antibodies. The samples were added in the coated wells and incubated for 1 hour at RT. After washing to remove unreacted and unbound material, monoclonal antibody-HRP conjugate was added. The wells were washed further to eradicate unbound components and the bound enzyme was detected by adding substrate. The reaction was stopped after specified time with acid and the absorbance was determined for each well at 450 nm with an ELISA reader (Benchmark Scientific, USA). The cutoff value was calculated by the given formula and absorbance of all the wells were compared with the cutoff value. Any sample having absorbance more than the cutoff value was considered reactive.
50 μL sample dilution buffer was added to each well followed by the addition of 100 μL sample or control in different wells. The plate was incubated for 60 minutes at 37 oC after which each well was washed by filling approximately 350 µL diluted wash buffer. Next 100 µL diluted conjugate was added in each well and incubated for 30 minutes at 37 °C. Later, wells were washed with wash buffer and 100μL substrate was added in each well and incubated at room temperature for 15 minutes in dark. Then reaction was stopped by adding 100μL stop solution and the absorbance was measured at 450nm within 30 min of stopping the reaction.
Calculation of sensitivity, specificity and accuracy of the dengue NS1 rapid ICT test
The sensitivity and specificity of the NS1 rapid ICT test kit was calculated based on the NS1 ELISA test result.
Mathematically this can be stated as:
Sensitivity= (TP*100) / (TP+FN)
Where, TP= True positive = the number of cases correctly identified as patient
FN= False negative = the number of cases incorrectly identified as healthy
Specificity= (TN* 100) / (FN+TN)
Where, TN= True negative = the number of cases correctly identified as healthy
FN= False negative = the number of cases incorrectly identified as healthy
Accuracy of a test is its ability to differentiate the patient and healthy cases correctly. Mathematically, this can be stated as:
Accuracy= (TP+TN) / (TP+TN+FP+FN)
Detection of thiobarbituric acid reactive substances
Thiobarbiturate reactive substances (TBARS) are the low-molecular-weight end products, whose main component is malondialdehyde (MDA), that are formed during the decomposition of lipid peroxidation products. These MDA react with thiobarbituric acid to form a fluorescent red adduct which was measured spectrophotometrically. The serum concentration of TBARS was used as an index of lipid peroxidation and oxidative stress. In this study, TBARS level was determined according to the method of Yagi et al [21, 14].
An aliquot of 2 mL of working TBAR reagent (Sigma-Aldrich) was added to 1mL sample (100 μL plasma + 900 μL PBS saline), followed by an addition of 30 μL of 50 mM butylated hydroxy toluene (BHT) (The Chemical Co.), which was then incubated for 15 minutes in a boiling water bath. It was then refrigerated in a stream for 15 minutes and centrifuged for 10 minutes at 3000 rpm at RT and the supernatant was collected to measure the absorbance at 535 nm. Then the MDA concentration of the sample was measured using a standard curve.
Estimation of nitric oxide
Endothelium-derived NO level was determined using the Griess Reagent System. The Griess reaction is a two-step diazotization reaction in which the NO-derived dinitrogen trioxide (N2O3) generated from the acid-catalyzed formation of nitrous acid from nitrite (or autoxidation of NO) reacted with sulfanilamide to produce a diazonium ion which was then coupled to N-(1-napthyl) ethylenediamine to form a chromophoric azoproduct that absorbed light strongly at 548 nm.
33.3 μL of Griess Reagent (BioVision), 100 μL of the nitrite-containing sample and 865 μL of deionized water were mixed, which was then incubated for 30 minutes at RT. The final absorbance was measured at 548nm.A photometric reference sample was prepared by mixing 100 μL of Griess Reagent and 2.9 mL of deionized water which was used to construct a standard curve. Absorbance of the nitrite-containing sample was measured at 548 nm relative to the reference sample.
Detection of superoxide dismutase activity
Superoxide dismutase (SOD) is an enzymatic antioxidant, which was measured spectrophotometrically by the method established by Markland et al (1974). At an alkaline pH, pyrogallol underwent autoxidation and produced a superoxide anion as an intermediate. SOD inhibited the autoxidation of pyrogallol to o-quinone, a yellow-colored product. The rate of autoxidation inhibition was directly proportional to the level of SOD.
A 1.5 ml reaction mixture was prepared containing 1250μL 50mM Tris-HCl buffer (pH 8.2), 50 μL 1mM EDTA, 50 μL enzyme, 100 μL 0.4mM pyrogallol (Otto Chemie Pvt. Ltd.) and 50 μL distilled water. All reagents except pyrogallol was added into the blank and sample cuvettes (quartz) and mixed properly. The spectrophotometer (Shimadzu UV-VIS) was set to the time scan mode at 420nm. Pyrogallol solution (100 μL) was added in the sample cuvette and the absorbance was recorded at every 10 seconds interval for two minutes. Temperature of the room was maintained at 30±2 o C throughout the entire study. SOD activity was calculated by the equation (Ma et al., 2009): SOD activity (U/ml) = [(Vp-Vs)/(Vpx0.5)] x [Vt/Vs] x n
Statistical analysis
Data analyses were performed using GraphPad Prism for windows, version 8.0.1 software. Results were shown as the mean ± standard deviation. The Mann-Whitney U-test was used for comparisons of different types of blood cells between control and patient group (nonparametric data). Relationships between nitric oxide, SOD activity, lipid peroxidation and blood cells were evaluated using Pearson correlation and the results are expressed as coefficient of determination R2. P-value of less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study subjects
Forty-one dengue patients (mean age of 23.78 ± 7.482 years) were included in this work. The baseline characteristics of the population are profiled in Table 1. The control group comprised of 70% male and 30% female whereas the DENV patient group comprised of 61% male and 39% female. The mean ± SD age range of the control group was 26.1 ± 11.7 years ranging from 12 years to 67 years whereas for DENV patient group it was 23.78 ± 7.482 years ranging from 16 years to 55 years. The mean weight of the control group and the DENV patient group was measured 58.4 ± 11.77 and 58.85 ± 19.48 respectively. None of the control group had any fever, whereas all the patients had fever for 2.8 days on average ranging between 1-5 days. The mean values of red blood cell were 5.172 ± 0.458 and 5.528 ± 0.984 in control and DENV patients group respectively with a P value 0.041. The control group had a hematocrit value of 41.37 ± 5.52 % with a range from 27.9 to 51.2 % whereas the DENV patient group had a hematocrit level of 49.35 ± 6.22 % with a range from 26.3 to 58.6 %. The hematocrit value was significantly higher (P <0.0001) in DENV patients compared to that of control. The mean value of white blood cell of the control group was 7664 ± 1650 cells/µL with a range from 4460 to 13210 cells/ µL while the DENV patient group showed a mean value of 5994 ± 2538 cells/µL with a range from 2880 to 13330 cells/ µL. The mean WBC value of DENV patient group was significantly lower (P=0.0005) than that of the control group. The control group had a mean neutrophil percentage of 77.61 ± 11.09 ranging from 43.6 to 92.3 %. A mean neutrophil percentage of 60.36 ± 8.186 were reported in the DENV patient group ranging from 47.2 to 80.9%. The mean neutrophil value of DENV patient group was significantly lower (P<0.0001) than that of the control group. The mean lymphocyte percentage of the control group was found 31.57 ± 7.89 with a range from 11.7 to 44.9 % whereas the DENV patient group had a mean lymphocyte percentage of 27.1 ± 12.65 with a range 5.1 to 53.1%. The mean percentage of monocyte, another member of leukocyte was found 4.929 ± 1.615 in control group with a range of 3.1 to 11.4 %. The DENV patient group had a mean monocyte value of 5.371 ± 2.002% ranging from 2.6 to 12.2 %.
Table 1. Comparison of the baseline characteristics between control subjects and dengue patients.
General information about the dengue symptoms and preventive measures
The proportion of study subjects that had different types of DENV related symptoms and had taken different preventive measures are shown in Table 2 which suggests that in the control group, 62.5% participants used mosquito net to protect them from encountering DENV whereas only 41.3% participants of the patient group participants used mosquito net which is significantly lower (P=0.0027) than that of the control group. Among the control group participants, 30% used insecticide spray as a preventive measure compared to 17% among DENV patient group, which is significantly lower (P=0.03) than that of the control group. Among the DENV patient group, 40.7% had sweating problems compared to 20.2% among control group participants which is significantly higher (P=0.002) than that of control group. Among the DENV patient group, 13.8% encountered rash problem and 34.5% had joint pain complication compared to 8.25% and 4% among control group participants respectively. The joint pain was significantly higher (P<0.0001) in DENV patients compared to the control group. The DENV patient group had significantly higher percentage of waist pain (P=0.0043), muscle pain (P=0.0002), back pain (P=0.0072) and eye pain (P=0.0009) compared to those of the control group.
Table 2. General information on dengue symptoms and preventive measures for study subjects.
Platelet level in control and patients
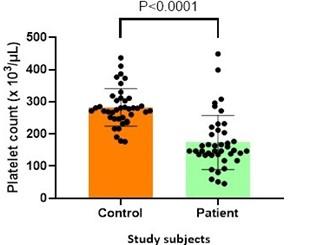
The number of Platelets was counted for both control and DENV patient group and were subjected to statistical analysis. The mean platelet count for the control group was 282800 ± 58260 cells/µL ranging from 176000 cells/µL to 437000 cells/ µL. The mean platelet count for the DENV patient group was 173500 ± 84040 cells/µL with a range between 45000 cells/µL to 449000 cells/µL. The mean platelet count for the DENV patient group was significantly lower (P <0.0001) than that of the control group (Figure 1).
Comparison between dengue NS1 rapid ICT test kit and dengue NS1 ELISA
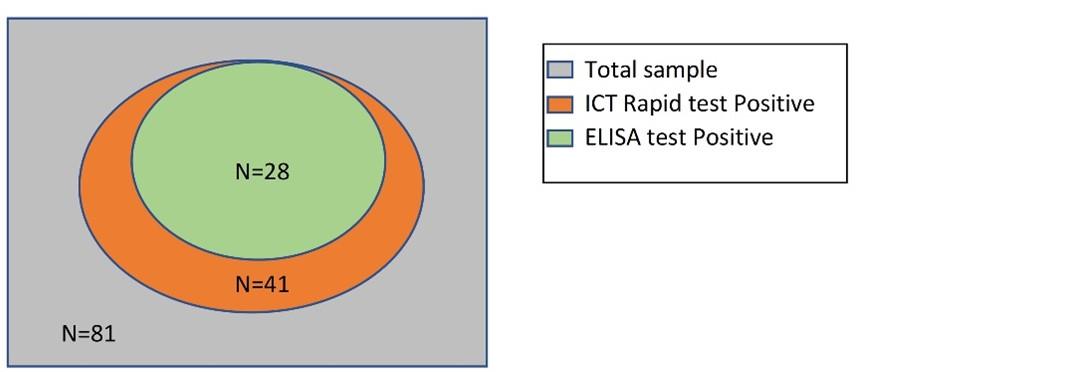
Among 81 study subjects, 28 individuals showed positive result in DENV NS1 rapid ICT test assay whereas 53 individuals showed negative result. Among 81 study subjects, 41 individuals showed positive result in DENV NS1 ELISA assay whereas 40 individuals showed negative result. 28 individuals showed positive result in both DENV NS1 rapid ICT test assay and DENV NS1 ELISA assay and 40 individuals showed negative result in both DENV NS1 rapid ICT test assay and DENV NS1 ELISA assay. 13 individuals showed positive result in DENV NS1 ELISA assay but not in NS1 rapid ICT test assay. There was no individual who showed positive result in NS1 rapid ICT test assay but not in DENV NS1 ELISA assay. Figure 2 summarizes a Venn diagram representing the positive, negative, and total cases in DENV NS1 rapid ICT test assay and DENV NS1 ELISA assay and the details are segregated in Table 3.
Table 3. Summary of DENV NS1 rapid ICT test assay and DENV NS1 ELISA.
Sensitivity, specificity and accuracy of the dengue NS1 rapid ICT test
Sensitivity is the proportion of true positives tests out of all DENV patients. Specificity is the percentage of true negatives out of all subjects who do not have DENV infection. Accuracy is the ability to segregate the DENV patients and healthy subjects correctly. The sensitivity and specificity of the NS1 rapid ICT test kit was calculated based on the NS1 ELISA test result.
The sensitivity of NS1Rapid ICT test kit was calculated = (28*100) / (28 + 13)
= 68.29%
The Specificity of NS1Rapid ICT test kit was calculated = (40*100) / (0+40)
=100%
The Accuracy of NS1Rapid ICT test kit was calculated = (28+40) * 100 / (28+40+0+13)
= 83.95 %
Hence, the sensitivity, specificity, and accuracy of the Dengue NS1 rapid ICT test was found 68.29%, 100% and 83.95 %, respectively.
Correlation between oxidative stress markers and hematological parameters in study subjects
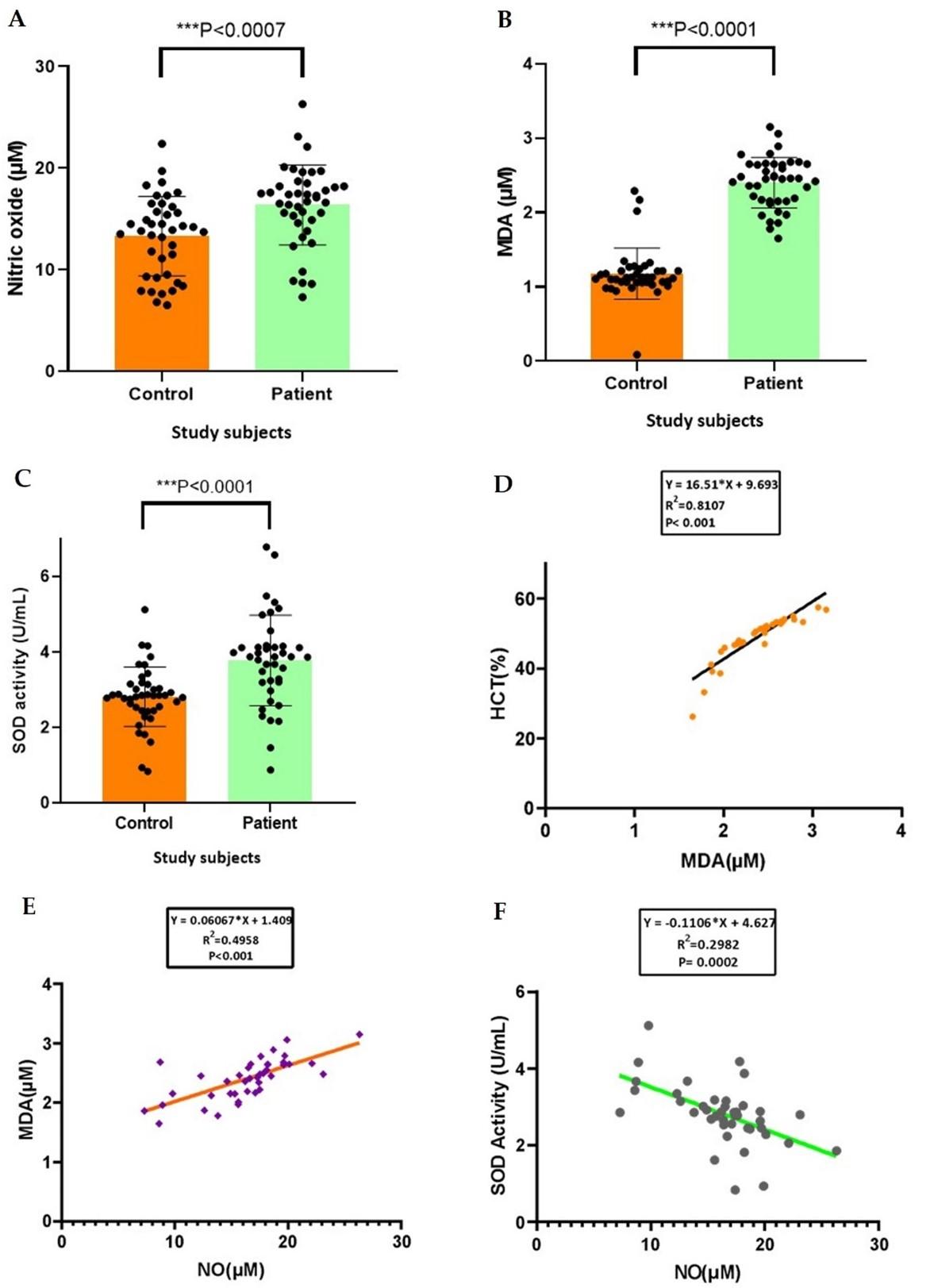
In this study, DENV patients showed significantly higher levels of nitric oxide, lipid peroxidation and SOD activity compared to the control subjects. It was found that the mean nitric oxide level of the patient group was 16.37 ± 3.94 μM whereas the control group had 13.3 ± 3.9 μM (Figure 3A). The thiobarbituric acid reactive substance of the control group showed a mean value of 1.18 ± 0.34 μM which is lower than the mean value of the patient group (Figure 3B). The mean SOD activity in the patient group was 2.8 ± 1.19 U/mL and in the control group was 1.84 ± 0.77 U/mL (Figure 3C). A positive correlation (R2 = 0.8107) was found between MDA level and hematocrit level in DENV patients (Figure 3D). Another positive correlation (R2 = 0.4954) was found between nitric oxide and MDA level in DENV patients (Figure 3E). They also showed a significant negative correlation (R2 = 0.2982) between the activity of superoxide dismutase (SOD) and nitric oxide level (Figure 3F).
DISCUSSION
Bangladesh has experienced unprecedented rise in reported dengue cases in recent years especially in 2019 and it is predicted that it might become more fatal in the next two or three years by going through genetic mutation. Most cases of dengue are identified depending upon signs and symptoms alone, producing considerable ambiguity due to the broad-spectrum and with no particular symptoms. There are several methods used to identify this mosquito borne virus. It can be detected clinically and established by a range of methods, including anti-DENV antibodies, non-structural protein 1 (NS1) antigen or DENV-specific nucleic acid detection. Validation of dengue identification is useful in managing compassionate clinical care, predominantly for uncharacterized cases and plummeting the need for costly investigations and treatments for other diagnoses. However, diagnostic methods differ in their sensitivity and specificity, indicating that not all reported cases of dengue are likewise accurate. At present, various dengue NS1 Antigen rapid test kit is used frequently to identify this virus since it is very easy to use and provides results quickly. But there is always a doubt about its accuracy, sensitivity and specificity compared to other methods.
The intent of the present study was to determine how accurately the clichéd dengue NS1 rapid test kit works. One of the targets of this study was to determine the sensitivity and specificity of the dengue NS1 rapid ICT test kit compared to traditional dengue NS1 ELISA. In this study 41 people were tested positive for dengue NS1 in ELISA test and only 28 of them showed positive results in dengue NS1 rapid ICT test kit. So, the rapid kit showed a sensitivity of 68.29% and a specificity of 100% while compared with NS1 antigen capture ELISA. The accuracy of the kit was 83.95% relative to DENV NS1 ELISA. Though the specificity is very satisfactory, the sensitivity is not up to the mark. Since there is still no well-known drug which works against dengue virus, it is very important to accurately identify the ones which are affected. Rapid test kits cannot perform well enough as the ELISA technique due to some limitations. As DENV rapid ICT test kit uses membrane bound antibody and depends on the lateral movement of blood sample and buffer over the membrane, sometimes this movement gets hampered resulting in failure of NS1 antigen-antibody complex formation and shows false negative result. Another drawback of this rapid ICT test kit is it cross reacts with zika virus and some other flaviviruses and so can show false positive results [22]. On the other hand, NS1 ELISA technique is more robust, accurate and target specific and it provides a lot more reliable and credential results.
Reports of complete blood count and differential blood count were collected, and comparative analysis was done. The mean age of the control group and the DENV patient group were close to each other 26.1 years and 23.78 years respectively. The mean weight of both the groups were even closer to each other scoring 58.4 and 58.85 kg respectively showing the even distribution of control and sample group.
The DENV patient group had a mean platelet count of 173500/µL which is significantly lower (P< 0.001) than the control group’s mean platelet count of 282800/µL. This finding resembles with the observation of Chaloemwong et al. [23] where thrombocytopenia was observed in dengue patients. Platelet consumption during ongoing coagulopathy process and activation of the complement system might be the reasons behind this thrombocytopenia [24]. It has also been confirmed that DENV patients produce anti-platelet antibodies of the IgM isotype [25]. Another study later confirmed that platelets from DENV-infected patients displayed characteristic signs of the intrinsic pathway of apoptosis, which comprise augmented surface phosphatidylserine exposure, mitochondrial depolarization, and caspase-9 and caspase-3 activation [26]. Leucopenia was also observed in the patient group compared to the control group [27]. Control group had a mean white blood cell count of 7664/µL whereas the DENV patient group had a mean white blood cell count of 5944/µL which was significantly lower than the control group (P= 0.0005). Similar observation has been reported in several other studies [28, 23]. A hypothesis regarding the incidence of the leukopenia in the cases of dengue infection is that it is caused by the destruction or impediment of myeloid progenitor cells as the bone marrow inspection exhibited mild hypocellularity. Suppression of bone marrow region is the underlying reason behind this complexity [28]. Hematocrit level of the DENV patient group was found comparatively higher than the control group which is in accordance with the finding of Chaloemwong et al., 2018 [23]. Neutrophil percentage was predominant in the control group compared to the DENV patient group. This result agreed with a previous study [23] which showed that dengue patients encountered neutropenia. The mean value of monocyte percentage of the DENV patient group was found higher than the mean monocyte percentage of the control group. A hypothesis as to why there was an upsurge in monocytes is that monocytes and macrophages are portions of the primary immune system which perform phagocytosis of microorganisms and show the resulting carried antigen to the T helper cells [23]. The DENV patient group also had a lower mean percentage of lymphocyte compared to the control group. The increased binding of neutrophils and platelets to infected endothelial cells may explain neutropenia and thrombocytopenia in dengue patients [30].
The general information of preventive measures and the symptoms of the DENV patients and the control subjects were collected in a preformed questionnaire. It showed that lesser proportion of DENV patients (41.3%) used mosquito net during day and nighttime compared to the percentage of the control group (62.5%). DENV patients were also more reluctant to use protective material like aerosol spray. Apart from hematological alteration, higher proportion of patients reported to suffer from symptoms like sweating, rash and pain in joint, muscle, waist, back and eye.
Nitric oxide level of the patient group was found significantly higher than the control group. Similar result was found by Hapugaswatta et al. in their study [31]. NO induces vasorelaxation with reduced systemic vascular resistance and therefore blood pressure [32] and excessive NO production could induce shock like syndrome [33]. The complex events that address the vascular leakage and hemorrhagic manifestations in DHF could be modified by NO. In this regard, several reports indicate that NO can inhibit viral replication [34, 35] and it has been demonstrated to play a role in the transmission of dengue virus–specific suppressor signal. In addition, NO has been involved in the innate immunity against dengue-infected cells [36]. So, finding an elevated level of nitric oxide in the DENV patient group was very relevant.
Lipid peroxidation is a multifaceted process comprising three phases including initiation, propagation, end-decomposition. Development of peroxides, particularly lipid ones, is a result of the stimulation of O2, the development of reactive species and the damage of natural system’s protection. In living environments, the most promising substrate for peroxidation is characterized by polyunsaturated fatty acids (PUFA), machineries of cell and subcellular membranes. Since aldehydes are one of the most stable products of lipid peroxidation, malondialdehyde (MDA) was measured as a marker of lipid peroxidation. The mean malondialdehyde level of the DENV patient group was found significantly higher (P< 0.0001) than that of the control group indicating that there was oxidative stress and imbalance of reactive oxygen species. This finding was very similar to the findings of Cherupanakkal et al. and Soundravally et al. [37, 16].
Superoxide dismutase (SOD) is an enzymatic antioxidant that catalyzes the superoxide anion into molecular oxygen by single electron transfer, which decreases the cellular damage from excessive concentration of ROS produced in oxidative stress of dengue. This study found the SOD activity in DENV patients to be significantly higher than in the control subjects (p<0.0001). This finding supported the observation of Anez el al [38] where increased serum nitric oxide level was found in dengue patients. Since there was increased level of oxidative stress and reactive oxygen species, superoxide dismutase enzymes increased their activity to compensate with the augmented oxidative agents and balance the oxidative and anti-oxidative status.
In this study, a positive correlation was found between lipid peroxidation and hematocrit percentage in DENV patient group. Due to lipid peroxidation, vascular leakage of plasma can occur resulting in high percentage of hematocrit. However, this correlation was only found significant in the DENV patient group. A similar correlation was found by Soundravally et al. [39] reporting an increase in lipid peroxidation positively correlated with the hematocrit percentage in DENV patient serum.
Statistical correlation analysis shed lighter on the association between lipid peroxidation and nitric oxide level. This study showed a positive correlation between them (P< 0.001) which was significant. An amplified superoxide concentration and its downstream consequences such as hydrogen peroxide, peroxynitrite, and hydroxyl radicals appear mostly important in facilitating the augmented generation of NO and can react with superoxide to form peroxynitrite and with thiols and metal centers in proteins to form nitrosyl adducts [40]. It has also been presented to impede with the disulfide-bond development and result in the buildup of misfolded proteins in the endoplasmic reticulum, resulting stress and ROS production [41]. It is acknowledged that disproportionate ROS production leads to macromolecule oxidation, bringing about in a free radical attack on membrane phospholipids with resulting membrane damage via induction of lipid peroxidation. So, it supports our finding of positive correlation between lipid peroxidation and nitric oxide.
The present study found a significant negative correlation between SOD activity and Nitric oxide. In the literature, no study was found analyzing these two biomarkers in dengue patients for oxidative stress analysis. In vivo development of the reaction products of NO• is retained small by its speedy elimination through reactions with oxy-hemoglobin in red blood cells and by scavenging of O2 – by superoxide dismutase SOD [42]. Thus, low SOD activity enhances superoxide anion levels and consequently peroxynitrite. Henrotin et al. [43] calculated the effect of pro-inflammatory cytokines on cellular antioxidant defense mechanisms. They observed that inflammation described by TNF-α overproduction is linked to amplified oxidative stress consistent with a diminished SOD activity.
The study sheds light on the efficiency and accuracy of the dengue NS1 rapid ICT test kit which are in vogue in Bangladesh. This study has found that though the specificity of the rapid test kit is satisfactory, the sensitivity is not up to the mark and possibility of misdiagnosis by this rapid test kit is significant. Although rapid test kit is much more cost-effective and takes less amount of time to produce results, misdiagnosis can lead a person’s life to a very complex situation from where it would become very tough to recover. So, attention should be given on improving its accuracy and efficiency. Several oxidative stress markers’ levels were also found higher, and more emphasis should be given on studying the correlations between hematological parameters and oxidant-antioxidant imbalance to understand the pathophysiology of the virus and invent possible medicine.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONS
TR designed and supervised the overall research work; TR and SKK performed the research work; TR and SKK wrote the manuscript and analyzed the data; FS and MM revised the manuscript and generated ideas in the conduct of the lab works; TR, FS and MM critically overviewed the manuscript. All authors revised and approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Kundu SK, Seraj MF, Mohasin M and Rahman T. Dengue Virus: A Review on Epidemiology, Clinical Manifestation, Diagnosis and Pathogenesis, Journal of Infectious Diseases & Travel Medicine. 2020; 4 (2): 000142.
- [2]Azhar EI, Hashem AM, El-Kafrawy SA, Abol-Ela S, Abd-Alla AMM, Sohrab SS, et al. Complete genome sequencing and phylogenetic analysis of dengue type 1 virus isolated from Jeddah, Saudi Arabia. Virology journal. 2015;12(1):1.
- [3]Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Medical journal, Armed Forces India. 2015;71(1):67–70.
- [4]Muraduzzaman AKM, Alam AN, Sultana S, Siddiqua M, Khan MH, Akram A, et al. Circulating dengue virus serotypes in Bangladesh from 2013 to 2016. Virusdisease. 2018;29(3):303–7.
- [5]Zhang X-Y, Huang H-J, Zhuang D-L, Nasser MI, Yang M-H, Zhu P, et al. Biological, clinical and epidemiological features of COVID-19, SARS and MERS and AutoDock simulation of ACE2. Infectious Diseases of Poverty. 2020; 9(1):99.
- [6]Yacoub S, Lam PK, Vu LHM, Le TL, Ha NT, Toan TT, et al. Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: An observational study. The Journal of Infectious Diseases. 2016;214(5):697–706.
- [7]Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue.Nature. 2013;496(7446):504–7.
- [8]Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients Journal of Clinical Microbiology. 2000; 38(3):1053–7.
- [9]Janeczko D, Czyzyk A, Kopczynski J, Krzyzanowski M. Risk factors of cardiovascular death in diabetic patients. Diabetic Medicine. 1991; 8 Spec No:S100-3.
- [10]Raafat N, Blacksell SD, Maude RJ. A review of dengue diagnostics and implications for surveillance and control. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2019; 113(11):653–60.
- [11]Pal S, Dauner AL, Mitra I, Forshey BM, Garcia P, Morrison AC, et al. Evaluation of dengue NS1 antigen rapid tests and ELISA kits using clinical samples. PloS one. 2014; 9(11):e113411.
- [12]Sahnoun Z, Jamoussi K, Zeghal KM. Free radicals and antioxidants: human physiology, pathology and therapeutic aspects. Therapie. 1997; 52(4):251–70.
- [13]He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 2017;44(2):532–53.
- [14]Kamruzzaman M, Choudhury TZ, Rahman T, Islam LN. A cross-sectional study on assessment of oxidative stress in coronary heart disease patients in Bangladesh.World Journal of Cardiovascular Diseases. 2019; 09(05):331–42.
- [15]Rojas M, Zhang W, Xu Z, Lemtalsi T, Chandler P, Toque HA, et al. Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. Plos One. 2013;8(12):e84357.
- [16]Soundravally R, Hoti SL, Patil SA, Cleetus CC, Zachariah B, Kadhiravan T, et al. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence.The International Journal of Infectious Diseases. 2014;18:68–72.
- [17]Rahman T, Khanam A-R, Shekhar T. Recurrent Indoor Environmental Pollution and Its Impact on Health and Oxidative Stress of the Textile Workers in Bangladesh. Environmental Health Insights. 2020; 14:1178630220938393.
- [18]Nasirudeen AMA, Wong HH, Thien P, Xu S, Lam K-P, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLOS Neglected Tropical Diseases. 2011;5(1):e926.
- [19]Yu C-Y, Hsu Y-W, Liao C-L, Lin Y-L. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. Journal of Virology. 2006; 80(23): 11868–80.
- [20]Chan KR, Gan ES, Chan CYY, Liang C, Low JZH, Zhang SL-X, et al. Metabolic perturbations and cellular stress underpin susceptibility to symptomatic live-attenuated yellow fever infection. Nature Medicine. 2019;25(8): 1218–24.
- [21]Yagi K. Lipid peroxides and related radicals in clinical medicine. Advances in Experimental Medicine and Biology. 1994; 366:1–15.
- [22]Wang SM, Sekaran SD. Early diagnosis of Dengue infection using a commercial Dengue Duo rapid test kit for the detection of NS1, IGM, and IGG. The American Journal of Tropical Medicine and Hygiene. 2010; 83(3):690–5.
- [23]Chaloemwong J, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, et al. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study.BMC Hematology. 2018;18(1):20.
- [24]Simmons CP, Farrar J, Chau NVV, Wills B. Current concepts dengue. The New England Journal of Medicine. 2014; 366.
- [25]Honda S, Saito M, Dimaano EM, Morales PA, Alonzo MTG, Suarez L-AC, et al. Increased phagocytosis of platelets from patients with secondary dengue virus infection by human macrophages. The American journal of tropical medicine and hygiene. 2009;80(5):841–5.
- [26]Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, et al. Generation of IgM anti-platelet autoantibody in dengue patients. Journal of Medical Virology. 2001;63(2):143–9.
- [27]Hottz ED, Oliveira MF, Nunes PCG, Nogueira RMR, Valls-de-Souza R, Da Poian AT, et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. Journal of Thrombosis and Haemostasis. 2013;11(5):951–62.
- [28]Lin, SF, Liu HW, Chang CS, Yen JH, Chen TP. 1989. Hematological aspects of dengue fever. Gaoxiong Yi Xue Ke Xue Za Zhi= = The Kaohsiung journal of medical sciences. 1989. 5(1), 12-16.
- [29]Phan DT, Ha NT, Thuc LT, Diet NH, Phu LV, Ninh LY, et al. Some changes in immunity and blood in relation to clinical states of dengue hemorrhagic fever patients in Vietnam. Haematologia (Budap). 1991;24(1):13–21.
- [30]Butthep P, Bunyaratvej A, Bhamarapravati N. Dengue virus and endothelial cell: a related phenomenon to thrombocytopenia and granulocytopenia in dengue hemorrhagic fever. The Southeast Asian Journal of Tropical Medicine and Public Health. 1993;24 Suppl 1:246–9..
- [31]Hapugaswatta H, Wimalasekara RL, Perera SS, Premaratna R, Seneviratne KN, Jayathilaka N. Expression of nitric oxide synthase and nitric oxide levels in peripheral blood cells and oxidized low-density lipoprotein levels in saliva as early markers of severe dengue. BioMed Research International. 2021;2021:6650596.
- [32]Umans JG, Levi R. Nitric oxide in the regulation of blood flow and arterial pressure. Annual Review of Physiology. 1995;57(1):771–90.
- [33]Valero N, Espina LM, Añez G, Torres E, Mosquera JA. Short report: increased level of serum nitric oxide in patients with dengue. The American journal of tropical medicine and hygiene. 2002; 66(6):762–4.
- [34]Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. Journal of Virology. 1995;69(4):2208–13.
- [35]Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo MA. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. Journal of virology. 1995;69(11):7001–5.
- [36]Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352(9132):971–7.
- [37]Cherupanakkal C, Samadanam DM, Muthuraman KR, Ramesh S, Venkatesan A, Balakrishna Pillai AK, et al. Lipid peroxidation, DNA damage, and apoptosis in dengue fever: DNA DAMAGE, MDA, AND APOPTOSIS IN DENGUE. IUBMB Life. 2018;70(11):1133–43..
- [38]0Añez G, Valero N, Mosquera J. Role of nitric oxide in the pathogenesis of dengue. Dengue Bulletin. 2007;31:118–23.
- [39]Soundravally R, Sankar P, Bobby Z, Hoti SL. Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidation. Platelets. 2008;19(6):447–54.
- [40]Liu G, Chai C, Cui L. Fluoride causing abnormally elevated serum nitric oxide levels in chicks. Environmental Toxicology and Pharmacology. 2003;13(3):199–204.
- [41]Barbier O, Arreola-Mendoza L, Del Razo LM. Molecular mechanisms of fluoride toxicity. Chemico-Biological Interactions. 2010;188(2):319–33.
- [42]Naseem KM. The role of nitric oxide in cardiovascular diseases. Molecular Aspects of Medicine. 2005;26(1–2):33–65.
- [43]Henrotin YE, Bruckner P, Pujol J-PL. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747–55.