Increased CD73 expression is associated with poorly differentiated Gleason score and tumor size in prostate cancer
Abstract
There are few prostate cancer prognostic biomarkers. However, clinical difficulties in distinguishing between aggressive and non-aggressive tumors have been observed. CD73 is a 70-kDa glycosylphosphatidylinositol-linked ecto-enzyme that reduces antitumor immunity in mouse models of tumor, particularly prostate cancer. It’s believed to be a promising biomarker for predicting the clinical development and prognosis of certain tumor types. Its function in prostate cancer, however, is unknown. This study aims to investigate the hypothesis that CD73 may be used as a biomarker in prostate cancer diagnosis and/or prognosis. Nuclear and cytoplasmic CD73 staining has been evaluated by immunohistochemistry using benign and malignant prostate tissues. The immunohistochemical study showed nuclear and cytoplasmic CD73 staining in cancerous and non-cancerous prostate tissues. Increased CD73 staining was shown in prostate cancer tissues compared to benign prostate tissues. A negative association between CD73 expression and Gleason scores has been observed. However, increased cytoplasmic CD73 staining was significantly associated with increasing tumor size. This finding suggests that CD73 may have a role in cancer development or aggressiveness, indicating that more research is needed to better understand its function and determine whether it might be used as a diagnostic biomarker for prostate cancer.
INTRODUCTION
Prostate cancer (PCa) represents one of the most serious health problems in the world, with a high fatality rate [1, 2]. This disease can affect millions of men and represents the second greatest cause of cancer-related death, with an incidence of 300,000 cases/ year in the USA after skin cancer, 41,000 deaths/year after lung cancer [3]. Approximately 95% of PCa cases are diagnosed with acinar adenocarcinoma which is derived from the prostate gland glandular regions [4, 5]. However, there are only 5 % of PCa cases diagnosed histopathologically as a ductal adenocarcinoma which begins in the cells lining prostate gland ducts [6].
The Gleason grade system, which is developed in the 1960s and 1970s by Dr Donald Gleason, represents the most widely used histopathological grading scheme for measuring PCa development [7, 8]. This system can be divided into five different Gleason grades (1-5) based on a review of the prostate’s histopathological architecture which specifies how much of the prostate tissue seems normal or abnormal [8]. This system is based on how closely the cancer tissue resembles normal tissue when seen under a light microscope. For example, less aggressive cancer are more likely to seem like healthy tissue, but more aggressive cancer are more likely to spread to other parts of the body and don’t look like healthy tissue [8]. Because PCa is a heterogeneous disease with several histopathological patterns in the same PCa sample, a Gleason score is calculated by adding the two most common Gleason grades: primary and secondary, which are assigned separately for biopsy and prostatectomy [8]. Gleason score of 10 represents the highest score in this system [8]. In this system, the first number assigned is the most prevalent grade found in cancer. For instance, if it is expressed as 3+4=7, it signifies that the majority of the tumor is grade 3 and just a little portion is grade 4, and the two are added to provide a Gleason score of 7. In addition, Gleason score of 7 (4+3) means that the majority of the tumor is grade 4 and a few sections are grade 3. If all cancer sections are the same grade (for example, grade 3), the Gleason score is 3+3=6 [8]. However, this system isn’t always able to distinguish between aggressive and non-aggressive tumors [9]. The tumor-node-metastasis (TNM) system, which is developed by the American Joint Committee on Cancer/International Union Against Cancer (AJCC/ UICC), is another system used to diagnose and progress PCa. This system is based on the PCa size and the extent of its dissemination [2, 10]. This system has the benefit of being able to evaluate the prognosis of PCa patients as well as determine the expected progression of their disease [11], as well as act as a guide for patient treatment planning. However, this system is unable to predict which patients would relapse following the first therapy and which will remain in remission.
The evidence of a loss of basal cells is a crucial step in accurately diagnosing PCa [12]. However, the H&E staining may be unable to accurately identify basal cells in prostate glands [13] and because of that, it is necessary to find a biomarker that can confirm the presence of basal cells in prostate glands. Biomarkers that are expressed in PCa, rather than being lost, are also used. Rare biomarkers have been recognized for PCa diagnosis/prognosis and there are clinical difficulties in distinguishing between prostate gland disorders such as cancerous vs. non-cancerous and localized vs. metastasized PCa. Therefore, identifying new PCa biomarkers has become a priority.
Anti-tumor immune biomarkers may have a role in tumor diagnosis and prognosis, according to much research [14,15]. One of the most well-known immunosuppressive pathways implicated in the development of tumor is the CD73–adenosinergic pathway[16, 17]. CD73, also known as ecto-5′-nucleotidase (ecto-5′-NT, EC 3.1.3.5), is a 70-kDa glycosyl-phosphatidylinositol (GPI)-linked ecto-enzyme that reduces antitumor immunity in mouse models of tumor, particularly PCa [18]. CD73, a protein that catalyzes the conversion of AMP to adenosine, is overexpressed in a variety of cancers [19]. Its expression is regulated by a variety of variables and processes such as proliferation, migration, and invasion [20]. It has a role in regulating cancer cell proliferation, migration, and invasion in vitro, tumor angiogenesis, and tumor immune evasion in vivo, according to much evidence [21]. CD73 has been a popular therapeutic target due to its critical function in cancer. Targeted inhibition of CD73 in mouse models has recently been shown to be a promising cancer therapeutic strategy in the future. A study found that The CD73–adenosinergic pathway can be activated by tissue hypoxia and soluble factors present in the TME, such as type I IFNs, TNFα, IL1b, TGFβ, and Wnt activators [22]. Another study showed that CD73 deficiency may be linked to reducing in PCa growth and an increase in CD8 T cell infiltration [23] , suggesting CD73 could be linked to PCa progression and reduced antitumor immunity. Endothelial and epithelial cells, as well as a minority of lymphocytes, particularly regulatory T cells, express CD73. CD73, formerly known as a lymphocyte differentiation antigen, has been discovered to operate as a lymphocyte signaling and adhesion molecule [24]. According to the previous finding, CD73 may have a key role in the developing tumor and may be linked to a poor prognosis in a variety of cancers; however, its prognostic significance in PCa remains unknown. Therefore, the current study aims to assess CD73 immunostaining in cancerous and non-cancerous prostate tissues as well as to establish if its expression correlates with prostate clinical parameters such as grade and stage.
MATERIALS AND METHODS
Patients and ethics statement.
The study was accepted by the ethics board of Al Hussein Teaching hospital, Thi-Qar governorate, Iraq (Thi-Qar 2021159 in 7/12/2022). The total number of prostate tissue samples in the study was 96. Seventy-five formalin-fixed, paraffin-embedded tissue samples from radical prostatectomy or transurethral resection of the prostate (TURP) specimens which reviewed to establish Gleason score and stage of samples by histopathologist were used in this study, whereas twenty- one benign prostate tissue samples were also used as a control. These tissue samples were obtained from Al-Hussein teaching hospital’s histopathology department, Thi-Qar city, Iraq. Tonsil tissue samples was used as a positive control for Anti CD73 antibodies. Negative control, no primary antibody add, was also used in this study. A diagnostic H&E section was prepared to identify the tissues architecture by histopathologists. Table 1 summarizes the clinical data of benign and malignant prostate samples.
Immunohistochemistry
Immunohistochemistry was used to stain the benign and malignant prostate tissue sections using two independent anti-CD73 antibodies (Mouse monoclonal, dilution 1:25, Abcam, cat. number Ab3380) and anti-CD73 Rabbit polyclonal, 1:200; Abcam, cat. number Ab3380). Pretreatment steps were used before IHC. paraffin-embedded prostate tissue sections (5 μm) were cute, deparaffinized by use of Histoclear, and rehydrated through graded alcohols (100%, 95%, 70%, respectively. The tissue sections were then permeabilized 0.5% triton X-100 in PBS (phosphate buffer saline), subjected to heat-induced epitope retrieval in a citrate buffer, pH 6.0 with 0.05% Tween 20 for 30 minutes at 90°C, followed by a 20 mins cool down.
Drops of 3% H2O2 (Dako peroxidase) were added on the tissue section in a humid chamber to block endogenous peroxidase activity. Additionally, 10% normal goat serum with 0.05 bovine serum albumin solution was prepared in PBS and then drops of the solution were added to tissue sections. The primary CD73 antibody diluted in Dako antibody diluent (Dako, Ely, UK) was added on the tissue sections and incubated overnight at 4°C and then washed three times for 10 mins each. The next day, the secondary antibody was then added on the tissue section and incubated for 30 minutes at room temperature. As a chromogen, diaminobenzidine tetrahydrochloride was used to view the reaction products using the EnVision+Kit (K400611-2 and K401011-2, Dako, Ely, UK) following the manufacturer’s instructions. Finally, hematoxylin (H-3401, Vector Laboratories, Peterborough, UK) was used to counterstain the sections. These sections were mounted in DPX (Sigma-Aldrich, Gillingham, UK). Nikon Eclipse E800 brightfield illumination was used to see stained tissues, and a Nikon Digital Sight DS-U1 CCD Digital camera was used to take pictures.
Immunohistochemical analysis
To assess the CD37 Immunostaining in prostate tissue samples, 5 random images were taken and then scored using a semi-quantitative scoring system for cytoplasmic CD73 staining. The percentage scoring of cytoplasmic CD37 immunoreactive was as follows: 0 (0%), 1 (1-25%), 2 (26-50) and 3 (>50%). The cytoplasmic CD37 intensity was scored as negative (0), weak (+1), moderate (+2), or strong (+3). The final score for each case represents the sum of the proportion and intensity scores, which ranged from 0 to 6 [25].
Statistical analysis
The mean, standard error, and standard deviation data were calculated using GraphPad Prism version 8.00 for Windows, GraphPad Software, La Jolla, California, USA, www.graphpad.com. The unpaired t-test and one-way ANOVA with Tukey’s multiple comparisons tests were used for statistical analysis. P<0.05 was considered significant.
RESULTS
CD73 expression in benign and malignant prostate tissues
CD37 immunostaining was examined on benign and malignant prostate tissues. The immunohistochemistry results revealed cytoplasmic CD73 staining in both groups with varying degrees of signal strength, ranging from strong and widespread (Figure 1B, arrow) to moderate (Figure 1A & C, arrows) to weak (Figure 1D, arrow) or Negative (Figure 1E, arrow). Because CD73 is located in the cytoplasm of tonsil cells, this study used normal tonsil tissues as a positive control for anti-CD73 [26], and IHC revealed cytoplasmic CD73 staining in tonsil cells, as predicted (Figure 1F, arrow). There was no significant background staining in prostate tissue in the negative control (NC) group, which did not utilize a primary antibody (Figure 1G, arrow).
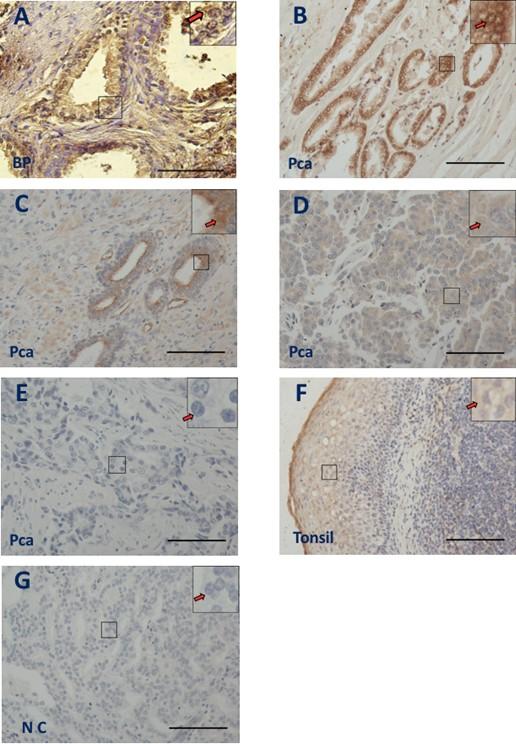
Increased CD73 expression is associated with poorly differentiated Gleason score and tumor size in PCa
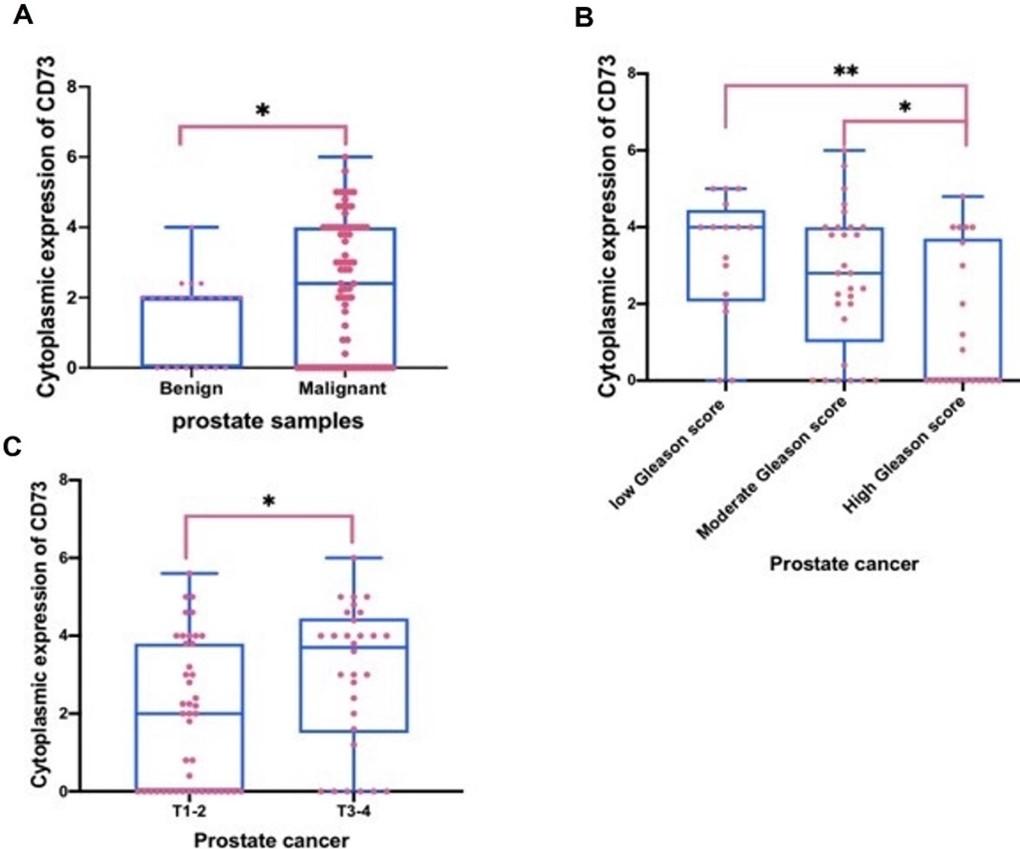
Quantification of the IHC staining revealed that CD73 staining was increased significantly in PCa tissues compared to benign prostate tissues (p=0.0344) (Figure 2A and Table 1). CD73 expression was negatively associated with increasing Gleason score, using an ANOVA test (p=0.0072) (Figure 2B and Table 1). When comparing PCa patients with a high Gleason score to those with a low (p=0.0081) or intermediate (p=0.0469) Gleason score, further analysis utilizing multi-comparison (Tukey) testing revealed that cytoplasmic CD73 staining was considerably reduced (Figure 2B and Table 2). In contrast, there was a positive association between cytoplasmic CD73 immunostaining and clinical stage T (T3-4 vs. T1-2) (P= 0.0144) (Figure 2C and Table 2), but not associated with other clinical stage parameters, including Metastasis (M1 vs M0) and Lymph node metastasis (N1vs N0) (p=0.8191& 0.9650, respectively) (Table 2).
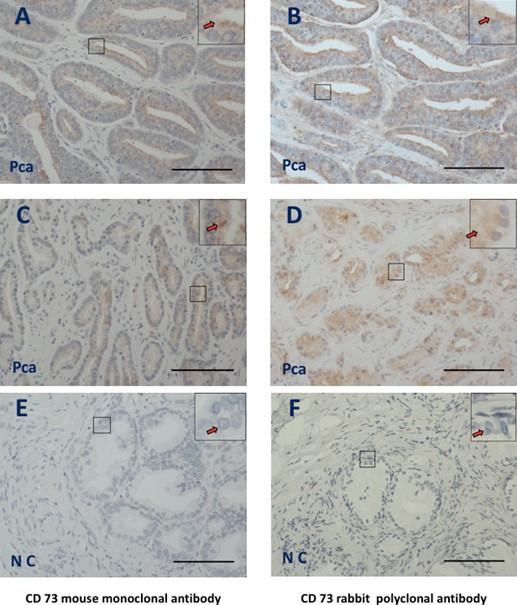
Table 1. Clinical data of benign and malignant prostate samples.
Table 2. Cytoplasmic CD73 staining in prostate tissue samples as compared to clinical data.
Validation of CD73 expression on prostate tissues samples
IHC was then performed on tissue sections from identical locations of prostate samples to establish that the two independent CD73 antibodies (mouse monoclonal and rabbit polyclonal) produced a similar staining pattern. Using a mouse monoclonal CD37 antibody (Figure 3A and C, arrows) and a rabbit polyclonal antibody, IHC results revealed a cytoplasmic staining pattern in PCa tissues (Figure 3B and D, arrows). PCa revealed no background staining with negative control (Figure 2E and F, arrows).
DISCUSSION
The adenosine pathway has been an interesting topic in cancer research in recent years because of increasing evidence suggesting its role in the development of cancer and metastasis [27]. This study examined the CD73 expression in benign and malignant prostate tissue samples using IHC as a potential biomarker for PCa diagnosis, prognosis and therapy. The current data revealed increased cytoplasmic CD73 immunostaining in PCa tissues compared to benign prostate tissues. This is consistent with other CD73 data from different types of tumors, including breast [21], colorectal [28], ovarian [29] and salivary gland tumors [30] suggesting CD73 may have an important role in cancer formation and development because of its role as a novel immunoinhibitory protein which plays an important function in tumor growth and metastasis. In addition, a previous study revealed that the major role of CD73 in normal tissues is to convert extracellular ATP to immunosuppressive adenosine in conjunction with CD39 to inhibit excessive immune response. Tumors, on the other hand, use the CD73-mediated adenosinergic pathway to defend themselves against immunological attacks [31]. Other studies have found the extracellular adenosine produced by CD73 on malignant cells is enough to mediate immune evasion, allowing cancer growth and metastasis to occur [23, 32]. In addition, it has been found that CD73 can regulate the cell cycle, apoptosis, and signaling pathways such EGFR, b-catenin/cyclin D1, VEGF, and AKT/ERK to enhance tumor cell proliferation [33]. In addition, Leclerc and his colleagues found that increased expression of CD73 in the epithelial cells of the prostate can reduce CD8 T cell immunosurveillance and turn them into tumor-promoting cells [22]. Another study has also found that reduction of CD73 by reprogramming Th17 cells may enhance the antitumor effects through increasing their effector function [25]. Taken together, Increased CD73 expression increased may promote prostate cancer growth.
Furthermore, the purpose of this study was to see if there was a link between CD73 immunostaining and Gleason score. The result of this study showed that CD73 immunostaining was negatively associated with increasing Gleason score. This data has in agreement with the previous studies. For example, it has been found that CD73 is reduced in endometrial carcinoma cells of poorly differentiated and advanced-stages in compared to low-grade malignancies, suggesting the protective role of CD73-derived adenosine on epithelial integrity in normal endometrium [34]. Another study on urothelial bladder cancer has found that increased CD73 immunostaining is negatively associated with poorly differentiated grades [35]. In contrast, another study demonstrated that there was no significant association between CD73 immunostaining and differentiation of kinds of cancers, including prostate [18]. This difference may be because of using different methods and/ or different scoring systems. Taken together, CD73 appears to be linked to tumor differentiation and the loss of CD73 on epithelial cells of prostate may encourage the progression of PCa and increasing CD73 expression in tumors may represent a good prognosis indicator for patients with PCa.
This study looked at the association between CD73 immunostaining and PCa clinical stage. The current data showed a positive correlation between CD73 immunostaining and tumor size (T1-2 vs T3-4). This data was agreed with the previous studies on colorectal carcinoma [36] and papillary thyroid carcinoma [20], suggesting Increased CD73 may promote the growth of kinds of cancer, including PCa. These studies suggest that CD73 stimulates the development of human cancer cells via EGFR and the ß-catenin/cyclin D1 signaling pathway, according to all of the findings [36]. In contrast, this data was not agreed with another study which showed increased CD73 was significantly associated with lymph node metastasis [18]. This difference may be because of using different antibodies, antigen retrievals, scoring systems or different populations. In addition, another reason which may explain these differences is that the sample size of lymph node metastatic PCa (M1 and N1) in this study was lower than non-lymph node metastatic PCa (M0 and N0) (Table 1). Taken together, this data may suggest that increased CD73 expression seems to be linked to PCa progression and prognosis and might be a useful biomarker for PCa.
In conclusion, increased CD73 staining in PCa is negatively associated with Gleason score and positively associated with tumor size. This early evidence suggests that CD73 may have a role in the development and progression of PCa. CD73 might be a new potential biomarker for PCa. Further study is also needed to validate these data using a second independent antibody with a large cohort or using an RNAscope to detect the mRNA level of CD73 in cancerous and non-cancerous prostate tissues. In addition, it will be very fascinating to investigate the functional role of CD73 in prostate cell lines using tissue culture.
ACKNOWLEDGEMENT
The authors would like to thank Cancer research unit in the college of Medicine at the University of Thi-Qar for providing use of imaging. The authors thank all staffs at Al-Hussein Teaching hospital for collecting samples.
AUTHOR CONTRIBUTIONS
Dhafer wrote the first manuscript, did the experimental techniques, designed the entire study, and performed the statistical analysis. Collecting data was done by Dhafer and Rash. Writing, review and editing: Dhafer, Rasha, and Sada. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. The Journal of pathology. 2009; 217(2): 299-306.
- [2]Kirby RS. Prostate cancer. 7th ed. ed. Abingdon: Abingdon : Health Press. 2012.
- [3]Swami U, McFarland TR, Nussenzveig R, Agarwal N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer. 2020; 6(8): 702-715. doi: 10.1016/j.trecan.2020.04.010. Epub 2020 Jun 10. PMID: 32534790.
- [4]Dunn MW and Kazer MW. Prostate Cancer Overview. Seminars in Oncology Nursing. 2011, 27(4): 241-250.
- [5]Bagnall P. Diagnosis and treatment of prostate cancer. Nursing times. 2014; 110(9): 12-15.
- [6]Stajno P, Kalinowski T, Ligaj M. and Demkow T. An incidentally diagnosed prostatic ductal adenocarcinoma. Cent European J Urol. 2013; 66(2):164-167. doi:10.5173/ceju.2013.
- [7]Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. 1966; Rep. 50
- [8]Matoso, A. and Epstein, J.I. Grading of prostate cancer: past, present, and future. Current urology reports. 2016; 17(3): 1-6.
- [9]Penney KL, Stampfer MJ, Jahn JL, Sinnott JA, Flavin R, Rider JR, et.al. Gleason grade progression is uncommon. Cancer research. 2013; 73(16): 5163-8.
- [10]Edge SB and Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. 2010; 17(6): 1471-1474.
- [11]Goldstein AS, Huang J, Guo C, Garraway IP & Witte ON. Identification of a cell of origin for human prostate cancer. Science (New York, N.Y.). 2010; 329(5991): 568.
- [12]Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA. Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol. 2002; 26(9):1161-8. doi: 10.1097/00000478-200209000-00006. PMID: 12218572.
- [13]Varma M, Lee MW, Tamboli P, Zarbo RJ, Jimenez RE, Salles PG. et al. Morphologic criteria for the diagnosis of prostatic adenocarcinoma in needle biopsy specimens. A study of 250 consecutive cases in a routine surgical pathology practice. Archives of pathology & laboratory medicine. 2002; 126(5): 554-61.
- [14]Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014; 32: 3436–48.
- [15]Saad F, Miller K. Current and emerging immunotherapies for castration- resistant prostate cancer. Urology. 2015; 85: 976–86.
- [16]Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets. 2014; 18:863–8.
- [17]Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015; 7: 277.
- [18]Jiang T, Xu X, Qiao M, Li X, Zhao C, Zhou F, et al. Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer. 2018; 18:267. 10.1186/s12885-018-4073-7.
- [19]Zhang B. CD73 promotes tumor growth and metastasis. Oncoimmunology. 2012; 1(1):67-70. doi:10.4161/onci.1.1.18068.
- [20]Jeong YM, Cho H, Kim TM, Kim Y, Jeon S, Bychkov A and Jung C.K. CD73 Overexpression Promotes Progression and Recurrence of Papillary Thyroid Carcinoma. Cancers (Basel). 2020; 12(10):3042.. doi:10.3390/cancers12103042.
- [21]Chen S, Wainwright D A, Wu J D, Wan Y, Matei D E, Zhang Y et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy. 2019; 11(11), 983–997. https://doi.org/10.2217/imt-2018-0200.
- [22]Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin Cancer Res. 2016; 22(1):158-66. doi: 10.1158/1078-0432.CCR-15-1181. Epub 2015 Aug 7. PMID: 26253870.
- [23]Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-cd73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Aca Sci U S A . 2010; 107:1547-1552.
- [24]Zhang B.) CD73: a novel target for cancer immunotherapy. Cancer Res. 2010; 70(16):6407–6411.
- [25]Chatterjee S, Thyagarajan K, Kesarwani P, Song JH, Soloshchenko M, Fu J, Bailey SR, et al. Reducing cd73 expression by il1beta-programmed th17 cells improves immunotherapeutic control of tumors. Cancer Res. 2014; 74:6048-6059.
- [26]Sharpe B, Alghezi DA, Cattermole C, Beresford M, Bowen R, Mitchard J, Chalmers AD. A subset of high Gleason grade prostate carcinomas contain a large burden of prostate cancer syndecan-1 positive stromal cells. Prostate. 2017; 77(13):1312-1324. doi: 10.1002/pros.23391.
- [27]Turiello R, Pinto A, Morello S. CD73: A Promising Biomarker in Cancer Patients. Front Pharmacol. 2020; 16; 11:609931. doi: 10.3389/fphar.2020.609931. PMID: 33364969; PMCID: PMC7751688.
- [28]Hajizadeh F, Masjedi A, Heydarzedeh Asl S, Karoon Kiani F, Peydaveisi M, et al. Adenosine and adenosine receptors in colorectal cancer. Int Immunopharmacol. 2020; 87:106853. doi: 10.1016/j.intimp.
- [29]de Leve S, Wirsdörfer F and Jendrossek V. Targeting the Immunomodulatory CD73/Adenosine System to Improve the Therapeutic Gain of Radiotherapy. Front Immunol. 2019; 5;10:698. doi: 10.3389/fimmu.2019.00698.
- [30]Ranjbar MA, Ranjbar Z, Zahed M and Nikookar N. CD73 a novel marker for the diagnosis of benign and malignant salivary gland tumors. J Clin Exp Dent. 2019; 1;11(3):e213-e218. doi: 10.4317/jced.54918. PMID: 31001389; PMCID: PMC6461735.
- [31]Roh M, Wainwright DA, Wu JD, Wan Y, and Zhang B. Targeting CD73 to augment cancer immunotherapy. Curr Opin Pharmacol. 2020; 53:66-76. doi: 10.1016/j.coph.2020.07.001. Epub 2020 7. PMID: 32777746; PMCID: PMC7669683.
- [32]Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. Cd73 on tumor cells impairs antitumor t-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010; 70:2245-2255.
- [33]Gao ZW, Dong K. and Zhang HZ. The roles of CD73 in cancer. Biomed Res Int. 2014 :460654. doi: 10.1155/2014/460654.
- [34]Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K Jr and Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest. 2016; 126(1):220-238. doi:10.1172/JCI79380.
- [35]Wettstein MS, Buser L, Hermanns T, Roudnicky F, Eberli D, Baumeister P, et al. CD73 Predicts Favorable Prognosis in Patients with Nonmuscle-Invasive Urothelial Bladder Cancer. Dis Markers. 2015; 785461. doi: 10.1155/2015/785461.
- [36]Wu R, Chen Y, Li F, Li W, Zhou H, Yang Y et al. Effects of CD73 on human colorectal cancer cell growth in vivo and in vitro. Oncol Rep. 2016; 35(3):1750-6. doi: 10.3892/or.2015.4512. Epub 2015 Dec 23. PMID: 26708311.