Cognitive enhancing properties of aqueous leaf extract of Vigna unguiculata in ketamine-induced memory damage in mice
Abstract
Conventional remedies for managing Alzheimer’s disease (AD) and related cognitive deficits are not curative but relieve the symptoms and cause adverse side effects. Alternatively, use of herbal therapies to manage cognitive illnesses has increased substantially. Vigna unguiculata is commonly utilized for nutritional benefits and management of neurological disorders in herbal medicine. The present study evaluated cognitive enhancing potential of V. unguiculata leaf aqueous extract in mice with ketamine-induced AD-like cognitive deficits. Cognitive performance indicated by step-through latency was assessed using passive avoidance test. Anti-acetylcholinesterase (anti-AChE) and antioxidant potential of the extract were determined using brains of the test animals. Further, phytochemical constituents of the extracts were determined using LC-MS. Aqueous extract of V. unguiculata leaf demonstrated significant prowess in combating cognitive deficits in the test animals. This was evidenced by significantly higher (p<0.001) step-through latencies in extract-treated mice than the untreated cognitively damaged mice. Moreover, cognitively impaired mice given the studied extract exhibited significantly less (p<0.001) malondialdehyde levels and AChE activity than the negative control mice. This result confirmed antioxidant and anti-AChE properties of V. unguiculata, indicating its potential to attenuate oxidative stress in the brain and augment cholinergic transmission. Notably, some conventional therapies for cognitive disorders, especially AD, are AChE inhibitors. The studied extract contained phytocompounds such as flavonoids and phenolics with confirmed antioxidant and anti-AChE activities, thus, its cognitive enhancing efficacy could be attributed to these phytoconstituents. Collectively, this study upholds V. ungiculata usefulness in management of cognitive illnesses.
Keywords
INTRODUCTION
Cognitive damage presents a major risk for development of neurological disorders such as Alzheimer’s disease (AD), the most common type of dementia [1]. Alzheimer’s disease is a multifactorial condition characterized by gradual memory decline, compromised cognitive functioning and personality changes resulting from neuronal damage in the frontal cortex and hippocampus [2]. The major neurochemical aberrations observed in AD condition include deterioration of cholinergic function in the central nervous system and imbalanced redox homeostasis [2]. Also, AD’s pathogenesis is characterized by deposits of amyloid-β plaques and neurofibrillary tangles in the brain [3]. Some lines of research hypothesize that amyloid deposition in the brain is the fundamental etiology of AD [1] whereas others propose that tau hyperphosphorylation (pTau) is the principal pathogenic mechanism that initiates AD [4]. What is universally accepted is that all these factors are collectively involved in neuropathogenesis observed in AD [3].
Many approaches have been pursued to develop agents for managing and treating AD [5]. To some degree, these attempts have successfully produced several agents with efficacy in alleviating AD symptoms [5]. Some of the commonly used agents for management of AD include memantine (N-Methyl-D-aspartate antagonist) [6], donepezil, galantamine, rivastigmine, and tacrine (acetylcholinesterase inhibitors) [7]. Unfortunately, clinical use of these drugs is associated with numerous side effects such as cardiovascular complications, muscle cramps, and urinary incontinence [7], among others. Further, the current pharmacotherapy for AD targets alleviating symptoms but cannot eradicate the disease and the cure for AD remains elusive [5]. In this regard, search for novel curative but safe anti-Alzheimer’s agents remain an attractive area of vigorous research.
Several potential remedies are being investigated for alternative sources of AD therapy [5]. Markedly, there is considerable interest in using nutritionally bioactive foods as an imperial approach to prevent and manage cognitive disorders [8]. Application of nutritional agents to address neurodegenerative diseases has been unequivocally practiced [8,9]. Indeed, Hippocrates’ philosophy notes that medical interventions which include patient’s nutritional needs in treatment may boost recovery [8].
Epidemiological studies have indicated that regular consumption of legumes is likely to reduce incidences of dementia [10]. More so, numerous studies have demonstrated invaluable significance of legume-based diets for management of cognitive disorders [9,11]. Among nutritionally appreciated leguminous foods, V. unguiculata is probably the most widely consumed [9]. Its leaf and seed are commonly used as food in the tropics (Africa) [12]. The plant is endowed with numerous proteins, minerals, vitamins and dietary fiber, enhancing its nutritive and health value [11,12]. Further, it contains various classes of secondary metabolites including flavonoids, unsaturated fatty acids, steroids, saponins, terpenoids, phenolics and alkaloids [9,12]. The pharmacological relevance of these compounds in neurological ailments is widely documented [11]. Some of the classes such as flavonoids have been reported to inhibit oxidative stress and reverse cholinergic decline, all of which are major drug targets for AD therapy [11].
Consequently, its medicinal utility in traditional therapy is exemplary. It is used to manage memory impairment, stomatitis, corneal ulcers and coeliac disease [12], among others. It’s also used to relieve headaches, fever, antidote for snake bites and urinary schistosomiasis [13]. Besides, it is utilized to prevent menstrual pains, intestinal cramps, leucorrhea, diabetes and cardiac illness [14]. Locally, V. unguiculata is claimed to possess memory enhancing potency and is consumed traditionally for this purpose besides nutritional value [15]. Cowpea is given to school going children to improve their cognitive functions such as memory, attention and alertness [16]. Also, it is consumed for management of epilepsy, a recognized neurological disorder [17]. Further, V. unguiculata has been shown to express antioxidant, neuroprotective and cardioprotective properties [9,18], among others
Ethnopharmacological information [12], epidemiological evidence [10] and phytochemistry constituents [9,12] on V. unguiculata offer appealing background for its likely cognitive enhancing potential. More so, neuroprotective efficacy of V. unguiculata seed extracts has been demonstrated in Parkinson’s disease [9]. Nevertheless, no evidence has been established on the efficacy of V. unguiculata against other cognitive disorders such as AD. Furthermore, previous studies on health benefits of V. unguiculata have majorly focused on seed preparations, yet the leaf is equally consumed as food and remedy for memory deficiencies in ethnomedicine [12]. Additionally, the promising cognitive improving effects of V. unguiculate leaf, noted in folkloric medicine, have not been analytically confirmed. As well, its probable cognitive enhancing mechanisms are yet to be established.
Given the elucidated gaps, this study aimed to evaluate cognitive enhancing properties of aqueous leaf extract of V. unguiculata. Further, in the view that oxidative dyshomeostasis and aberrant acetylcholine metabolism are major hallmarks of cognitive decline in AD [3], the study evaluated the potential of the studied extract to act as an antioxidant and anti-cholinesterase agent, preserving memory integrity in AD conditions.
MATERIALS AND METHODS
Sample collection and preparation of aqueous extract
Fresh leaves were sampled from V. unguiculata plants naturally growing in Namakuyu area, Muranga County, Kenya. A taxonomist authenticated the plant at Kenyatta University Herbarium, where a voucher specimen was labeled DJK (004). The collected samples were spread out under shade for drying at 25±20C. Once completely dry, they were pulverized to a coarse powder and stored in dark, clean, clearly labeled dry plastic containers.
During extraction, 50 grams of the powdered sample was mixed with 500ml of distilled water heated to 1000C in a water bath for 90 minutes. The treatment was conducted to mimic the folkloric preparation of V. ungiuculata leaf. The mixture was intermittently shaken to allow homogeneous dispersion. After that, it was cooled to 250C, decanted, and filtered. The filtrate was freeze-dried, and the obtained extract was preserved at 40C for further study.
Experimental animals
Male Swiss Albino mice (20 ± 2g) aged between 4-5 weeks were used in this study. They were obtained from Kenyatta University animal breeding facility and moved to the experimental room, where they were provided with standard rodent pellets and water ad libitum. The animals were housed at room temperature and natural night/day cycle. They were allowed to accustom this environment for a week prior to commencing the bioassays. All the animals were handled in accordance to the American Psychological Association’s ethical guidelines on experimenting on laboratory animals [19]. Prior to conducting this study, ethical approval was granted by National Commission for Science, Technology, and Innovation (NACOSTI) (NACOSTI/P/22/17588).
Randomly selected mice were assigned to 6 groups (n=5). Treatments were administered as follows (Table 1). Prior to treatment, the animals were starved overnight, after which extract dosages were orally administered to the respective experimental groups (D, E, F) and the reference drug (Donepezil) was given to the positive control mice as outlined above. Forty-five min later, 0.2ml of ketamine (15mg/kg bw) was intraperitoneally administered to all the groups except the normal control to initiate cognitive damage. The concentrations of the studied extract were selected through a pilot test and ketamine dosage was based on previous literature [20]. Saline was used to dissolve all treatments. The treatments were given daily for 3 days.
Table 1. Treatment protocol.
Passive avoidance test
Passive avoidance test (PAT) was carried out for 6 consecutive days. The test used passive avoidance box with dimensions of 25cm x 20cm x 20cm [21]. The box comprised a brightly lit and dark compartment separated by a carton wall. The wall was fitted with a sliding door interconnecting the two chambers. An electric circuit (0.5 mA) was fixed on the surface of the dark chamber of the box.
The test was segmented into three phases: habituation, training and testing. During habituation, mice were introduced to the lit chamber of the passive avoidance box (PAB) and explored it for 20 seconds. Thereafter, the sliding door was opened, and the animals were allowed to roam in the lit and dark compartments for 300 seconds. The electric circuit was off during habituation.
After habituation, the animals were conditioned for memory development through a training session. Mice were individually positioned in the lit chamber of PAB which they explored for twenty seconds. Then, the sliding door was raised, and the mice were allowed to walk around the PAB for 300 seconds. Once the animal completely entered the dark partition with both hind legs, an electroshock of 0.5mA was administered for 10 seconds. The animals were restricted in the dark chamber for thirty seconds so that to associate the section with irritating stimuli.
Following training, memory retention testing was carried out for three consecutive days. Each mouse was administered with the respective treatment and transferred to the lit chamber of PAB for 20 seconds, after which the sliding door was opened. The animal was observed for 5 min while in PAB. The time taken (step-through latency) by the animal to enter the dark chamber with all the legs was recorded.
Between each mouse habituation, testing and training session, the box was carefully wiped with 70% ethanol to neutralize any lingering olfactory stimuli [22]. The experiments were conducted at 10.00 am throughout the study period (i.e., 24 h apart).
Ex vivo biochemical assays
On the 6th day of PAB test, the animals were euthanized, and their brains recovered. To prepare a homogenate, each mouse brain was mixed with 0.6ml sodium phosphate solution (pH 7.4, 0.1M) and centrifuged (12000 rpm) for 15 min at 4°C. The resultant homogenates were used to estimate levels of malondialdehyde (MDA) as described [23] and acetylcholinesterase (AChE) activity following the protocol outlined previously [24].
Characterization of secondary metabolites
Phytochemistry diversity of aqueous extract of V. unguiculata was explored using liquid chromatography coupled with tandem mass spectrometer (UPLC-MS/MS, Palo Alto, CA) following methods as described [25-26].
Statistical analysis
All the results were expressed as Mean ± SEM and presented in tables as well as graphs. Inferential analysis was done for passive avoidance tasks and ex vivo biochemical data. Normality was confirmed by Kolmogorov-Smirnov test, after which parametric one-way ANOVA was done for extract treated groups followed by fisher’s post hoc test for multiple pairwise comparisons. Un-paired t test was done for comparison between two treatment groups. Means with p˂0.001 (at 99% confidence interval) were statistically significant. All data were analyzed in Minitab v19.
RESULTS
Effects of aqueous leaf extract of V. unguiculata on passive avoidance
Effects of three doses (200, 300 and 400mg kg-1 bw-1) of aqueous leaf extract of V. unguiculata were investigated in memory impaired mice. Testing was carried out for three consecutive days after habituation and training. Throughout the test period, animals in the negative control group revealed significantly reduced step-through latency (P < 0.001; Table 2) compared to all other treatment groups. On the other hand, the extract demonstrated cognitive remedial effects as it increased step-through latency in cognitively damaged mice. Universally, mice treated with 200 mg/kilogram bodyweight had the optimal cognitive improvement among the extract-treated animals as they exhibited the highest step-through latency across the test duration. On the 1st and 2nd days, 200mg/kg bw of the extract restored cognition to normalcy as animals treated with this dosage and normal control mice exhibited comparable (p > 0.001; Table 2) step-through latency. Furthermore, cognitive enhancing effects of the extract doses of 200 and 400mg/kg bw were similar on the 1st day of the test as revealed by comparable step-through latency (p > 0.001; Table 2). However, on the 2nd and 3rd day, the doses of the studied extract induced different cognitive enhancing effects as evidenced by statistically varying step-through latencies (p < 0.001; Table 2). On both days, 200 mg/kg bw, significantly induced the highest step-through latency while 300 mg/kg bw exerted the lowest step-through latency.
Table 2. Effects of Vigna unguiculata aqueous leaf extract on step-through latency in mice after ketamine-induced cognitive impairment.
Effects of aqueous leaf extract of V. unguiculata on ex vivo acetylcholinesterase activity
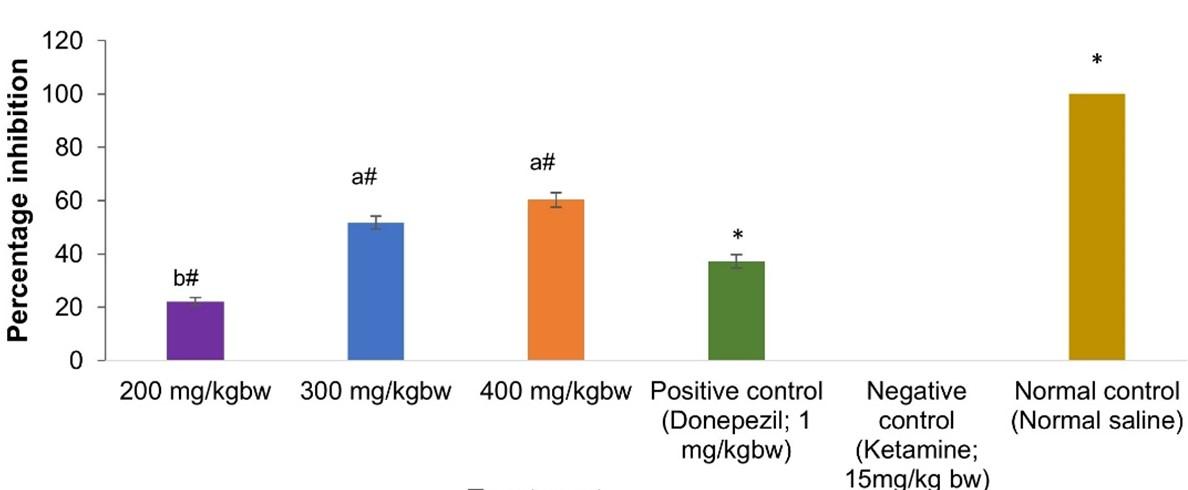
The present study investigated effects of aqueous leaf extract of V. unguiculata on AChE activity in brain homogenates of ketamine-treated mice. All the extract concentrations and donepezil demonstrated prominent inhibition of AChE activity (p< 0.001; Figure 1). Extract dose of 400mg/kg bw of V. unguiculata demonstrated the highest anti-AChE efficacy though not significantly different from 300mg kg-1 bw-1 (p > 0.001; Figure 1). Further, 300mg kg-1 bw-1 of the extract and donepezil (reference drug) exerted statistically similar anti-AChE activity (p > 0.001; Figure 1). Extract dose of 200mg/kg bw exhibited the lowest anti-AChE activity (Figure 1).
Effects of aqueous leaf extract of V. unguiculata on ex vivo MDA profile
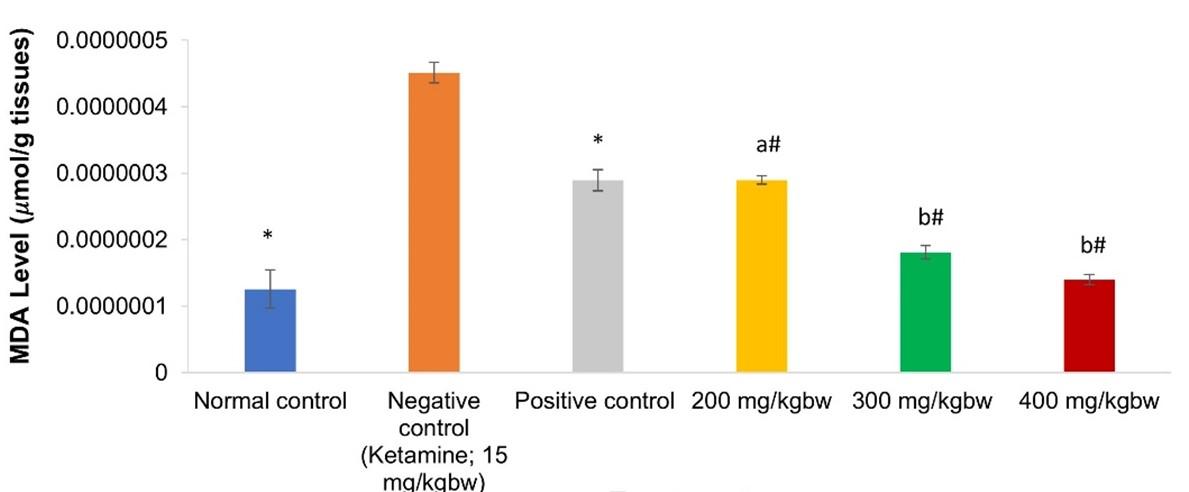
It was observed that brains of negative control mice had the highest MDA level, which was significantly different (p<0.001; Figure 2) from all extract and donepezil-treated groups. Markedly, among all the tested doses of aqueous extracts of V. unguiculata leaf, brains of the mice given 300 and 400mg/kg bw had the lowest and comparable (p>0.001; Figure 2) MDA levels. Notably, the same doses restored MDA level to normal state as it was statistically similar to the levels observed in the normal control animals (p>0.001; Figure 2). Further, levels of MDA in the brains of mice given 200mg kg-1 bw-1 of the extract were comparable to the concentration of MDA in the brains of animals treated with the reference drug (p>0.001; Figure 2).
Diversity of phytochemicals in V. unguiculata aqueous leaf extract
The secondary metabolites identified in the studied extract are shown in Table 3. The compounds identified in the extract included oleic acid, octadecanoic acid, arachidic acid, catechin, pyrogallol, baicalein, beta-sitosterol, apigenin, luteolin, campesterol, stigmasterol, gallocatechin and quercetin. Mainly, the most concentrated compounds were oleic acid and octadecanoic acid whereas the least concentrated were quercetin and arachidic acid.
Table 3. Phytocompound diversity of aqueous leaf extract of V. unguiculata.
DISCUSSION
To recollect, this study evaluated cognitive improving effects of aqueous V. unguiculata in ketamine-treated mice. Ketamine induces memory dysfunction via mechanisms observed in Alzheimer’s disease namely cholinergic dysfunction and oxidative dyshomeostasis [27]. It disrupts metabolism of major neurochemicals such as acetylcholine, leading to cholinergic decline [28]. Additionally, ketamine causes severe oxidative damage to the brain [29]. Through these events, ketamine is known to mediate hippocampal-associated memory deficits [28,29] typically observed in AD disease. Also, ketamine transiently increases tau hyperphosphorylation following clinical administration [30]. Thus, ketamine provided an adequate model for effectively initiating AD-like cognitive dysfunction as revealed by negative control animals which showed decreased step-through latency, high MDA levels and acetylcholinesterase activity.
Passive avoidance test, a fear-driven avoidance test [31], was used to evaluate memory recovery after extract intervention. Passive avoidance technique is extensively used to assess ability of animals to learn and recall aversive stimulus (electrical foot-shock) in the dark chamber of PAB. The test is conceptualized on the basis that rodents innately prefer dark to lit environment [31]. Therefore, they would naturally occupy the dark chamber of passive avoidance box. However, the dark chamber provided hostile stimuli through electroshock, hence cognitively functioning mice would recall aversive stimuli based on memory acquired during training and hesitate to re-enter the dark chamber. This technique has been widely used to assess cognitive functionality in rodents [8].
Treatment of cognitively impaired mice with aqueous extract of V. unguiculata profoundly reversed memory decline induced by ketamine as revealed by increased step-through latency. Memory formation involve processes such as acquisition, retention and retrieval of memory in hippocampus [32]. Increased step-thorough latency indicated that the extract-treated mice recalled to avoid aversive stimuli in the dark chamber where they experienced electric shocks [21,31]. Hence, cognitive improving effects of the extract reflect its efficacy to enable recall of the information developed during training.
Biochemical evaluation illuminated on the potential mechanisms through which V. unguiculata enhanced cognition. AChE is a recognized of biomarker for cognitive functionality [33,34]. The extract demonstrated anti-acetylcholinesterase efficacies in brains of cognitively damaged mice. Assay of acetylcholinestarase activity is widely utilized to screen for agents with cognitive enhancing potential. Elevated acetylcholinesterase activity is associated with cognitive decline [35–37]. AChE breaks down acetylcholine (ACh), a key neurotransmitter that acts on cholinergic receptors to mediate signal transmission in the synapse [38]. Reduced levels of ACh results in decline of cholinergic neurotransmission which produces memory deficits observed in AD patients [38]. Indeed, modulation of cholinergic neurotransmission has received considerable attention because of its direct correlation with AD [38]. Inhibition of AChE, prevents degradation of ACh resulting in rescue of cholinergic transmission ultimately correcting cognitive dysfunction [39]. Currently, novel AChE inhibitors are being sort for development of the most effective agents to relieve cognitive consequences observed in AD [39]. The outcome of the present study demonstrated that aqueous leaf extract of V. unguiculata could salvage cholinergic decline through inhibition of AChE activity. Thus, the extract is a promising candidate for management of neurological disorders such as AD.
In the present study, V. ungiuculata reversed ketamine mediated oxidative harm as revealed by low levels of malondialdehyde in brain homogenates. Malondialdehyde is a biomarker for oxidative imbalance in cellular systems [40]. Level of MDA in brain homogenates are assayed to estimates levels of oxidative stress in the brain. High MDA levels reveals elevated levels of oxidative stress in the brain [41–43]. Oxidative stress is a prominent mechanism that has been implicated in progression of neurological disorders including AD [44]. The brain is highly susceptible to oxidative assault by free radicals due to higher oxygen consumption, increased metabolism, rich content of oxidizable polyunsaturated fatty acids and relatively low antioxidant mechanism [8]. Oxidative stress damages brain cell molecular components (lipids, DNA, RNA), resulting in their apoptosis, eventually compromising learning and memory development processes [8,44]. Thus, overexpression of oxidative radicals may result in abnormal neuronal signaling in the hippocampus [44].
Antioxidant substances have been shown to limit pathogenesis and progression of neurological disorders. Indeed, antioxidant intake has been linked with low incidence of dementia [45]. As such, antioxidant substances have been considered as a promising avenue for diminishing the rate of initiation and progression of cognitive disorders [45]. Thus, the current study confirmed the potential of V. unguiculata in antioxidant therapy for neurological disorders like AD.
Cognitive remedying prowess of V. unguiculata may be ascribed to its phytochemistry constituents, as many findings have reported their therapeutic neuroprotective effectiveness. Anti-AChE and antioxidant potency of the majority of the compounds identified in the extract have been documented including pyrogallol [46], quercetin [47], catechins and [48], apigenin [49], campesterol, β-sitosterol, stigmasterol, octadecanoic acid [50], and oleic acid [51]. The antioxidant mechanisms have been shown to involve increased activity of endogenous antioxidant enzymes such as catalase, glutathione S-transferases, superoxide dismutase and glutathione peroxidase [52]. Also, phenolics and flavonoids could have inhibited oxidative stress via their hydroxyl groups, which can directly scavenge oxidative radicals [52].
Alongside the two neuroprotective mechanisms confirmed in this study, the compounds reported in the studied extract have been shown to improve cognition via additional mechanisms. For instance, flavonoids like apigenin and baicalein are known to increase levels of brain-derived neurotrophic factor, a neurotrophin that aids in neuronal proliferation and survival [53]. More so, apigenin is known to down-regulate production of βA precursor protein-cleaving enzymes, presenilin 1 and 2 proteins, which are genetic risk factor of AD disease [49]. Baicalein, luteolin, oleic acid and catechin has been revealed to inhibit Aβ-stimulated damage of neurons [48]. Additionally, these compounds as well as campesterol and phytosterols (sitosterol, campesterol and stigmasterol), have been demonstrated to inhibit neuroinflammation, a major mechanism that accelerates AD neurodegeneration [54]. Furthermore, oleic acid has been shown to promote neuronal clustering, production of the axonal growth-associated protein-43 and axonal growth, thus acting as a neurotropic factor [55]. Therefore, the cognitive improving properties of V. ungiuculata reflect the mechanism of action of its neuroprotection associated phytochemicals.
It is worth noting that cognitive ailments like AD are multifactorial disorders with mixed molecular etiologies [5]. As such, this complicates choice of the most effective targets to hit while developing therapies for neurological disorders [1]. New approaches seek to design therapeutic cocktails or single molecule directed to multiple drug targets in cognitive diseases [56]. Vigorous research is ongoing in search of multimodal agents for management and treatment of cognitive disorders [56]. In the present study, V. unguiculata demonstrated dual-modal efficacy; anti-AChE and antioxidant, through which it remedied cognitive dysfunction. Also, through review of previous studies, the extra contained secondary metabolites with diverse neuroprotective mechanisms. Taken together, it’s conceivable that V. unguiculata could enhance cognition via multiple targets. This study provides evidence that supports the promising prospects of V. unguiculata for development of multi-functional therapy for cognitive ailments.
CONCLUSION
The aqueous extract of V. unguiculata reversed ketamine-induced cognitive impairment in mice. The mechanism of cognitive enhancement by the extract was partly attributed to activation of cholinergic system by inhibiting AChE activity. Also, the extract alleviated cognitive decline via antioxidant mechanisms reducing ketamine-initiated oxidative damage in the brain. The cognitive enhancing outcomes of the extract may have been exerted by the phytochemicals present in the extract. The phytochemicals potency to block oxidative stress and enhance cholinergic neurotransmission has been documented. Thus, the present study confirms cognitive enhancing properties of aqueous extract of V. unguiculata observed in traditional use of this plant. This study presents V. unguiculata as a promising agent for management of AD as well as other related cognitive disorders. The present proposes bioassay-guided studies on cognitive enhancing potential of phytochemical constituents of V. unguiculata. Also, further studies are required to confirm cognitive enhancing efficacy of V. unguiculata in other models.
ACKNOWLEDGEMENTS
The authors appreciate Kenyatta University for allowing the study to be conducted in their laboratories. Further, the authors are grateful to Mr. Daniel Gitonga, Technical Staff, Kenyatta University, for providing technical support during experimentation in this study.
AUTHOR CONTRIBUTIONS
DJK, AMI and MPN were involved in the conception, design and performed the experiments. They also contributed to writing of the manuscript.
CONFLICTS OF INTEREST
References
- [1]Singh SK, Srivastav S, Yadav AK, Srikrishna S, Perry G. Overview of Alzheimer ’ s Disease and S
- [2]Lombardo S, Maskos U. Neuropharmacology Role of the nicotinic acetylcholine receptor in Alzheimer ’ s disease pathology and treatment. Neuropharmacology. 2014; 96: 255-262.
- [3]Spires-jones TL, Hyman BT. Review The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer ’ s Disease. Neuron. 2014; 82(4): 756-771.
- [4]Kametani F, Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer ’ s Disease. Front Neurosci. 2018; 12(25): 1–11.
- [5]Kumar A, Murleedharan C. Current and novel therapeutic molecules and targets in Alzheimer ’ s disease. J Formos Med Assoc. 2016; 115(1): 3–10.
- [6]Parsons CG, Danysz W, Dekundy A, Pulte I. Memantine and Cholinesterase Inhibitors : Complementary Mechanisms in the Treatment of Alzheimer ’ s Disease. Neurotox Res. 2013; 24(3): 358–69.
- [7]Haake A, Nguyen K, Friedman L, Chakkamparambil B. Ac ce pt ed us t. Expert Opin Drug Saf. 2020; 19(2): 147-157.
- [8]Imran I, Javaid S, Waheed A, Rasool MF, Majeed A, Samad N, et al. Grewia asiatica Berry Juice Diminishes Anxiety , Depression , and Scopolamine-Induced Learning and Memory Impairment in Behavioral Experimental Animal Models. Front Nutr. 2021; 327(7): 1–19.
- [9]Tripodi F, Lombardi L, Guzzetti L, Panzeri D, Milanesi R, Leri M, et al. Protective effect of Vigna unguiculata extract against aging and neurodegeneration. Aging (Albany NY). 2020; 12(19): 19785–19808.
- [10]Mazza E, Fava A, Ferro Y, Moraca M, Rotundo S, Colica C, et al. Impact of legumes and plant proteins consumption on cognitive performances in the elderly. J Transl Med. 2017; 15(1): 1–8.
- [11]Avanza MV, Gerardo Á, Cifuentes A, Mendiola JA, Ib E. Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea ( Vigna unguiculata ( L .) Walp . ) Obtained by Green Extraction Technologies. Agronomy. 2021; 11(1): 162-180.
- [12]Zaheer M, Ahmed S, Hassan MM. Vigna unguiculata ( L .) Walp . ( Papilionaceae ): A review of medicinal uses , Phytochemistry and pharmacology Vigna unguiculata ( L .) Walp . ( Papilionaceae ): A review of medicinal uses , Phytochemistry and pharmacology. J Pharmacogn Phytochem. 2020; 9(1): 1349-1352.
- [13]Chauke SH, Kritzinger Q. Vigna unguiculata. 2017; (44): 287–293.
- [14]Abebe BK, Alemayehu MT. A review of the nutritional use of cowpea ( Vigna unguiculata L . Walp ) for human and animal diets. J Agric Food Res. 2022; 10(2022): 1–14.
- [15]Njonjo MW. optimization of nutritional and quality properties of african cowpea ( vigna unguiculata ) leaves and other selected traditional african vegetables from farm to fork ( food science and technology ) Jomo Kenyatta University of Agriculture and Technology. 2013;
- [16]Muriuki MW. Effect of Provision of Nutritious School Meals on Educational Achievements in Secondary Schools in Kibra Sub-County in Nairobi, Kenya. African Res J Educ Soc Sci. 2021; 8(2): 10–20.
- [17]Kritzinger Q, Lall N, Aveling TAS, Wyk B Van. Antimicrobial activity of cowpea ( Vigna unguiculata ) leaf extracts. South African J Bot. 2005; 71(1): 45–8.
- [18]Janeesh PA, Abraham A. Food & Function cardiac markers , genotoxicity and gene expressions. Food Funct. 2013; 4(4): 568-574.
- [19]Perry JL, Dess NK. Laboratory animal research ethics: A practical, educational approach. Am Psychol Assoc. 2012; 2: 423–440.
- [20]Idris NF, Neill JC, Large CH. Comparison of the effi cacy of two anticonvulsants , phenytoin and valproate to improve PCP and D -amphetamine induced defi cits in a reversal learning task in the rat. J Psychopharmacol. 2009; 20(5): 1–11.
- [21]Kim J, Seo YH, Kim J, Goo N, Jeong Y, Jung H. Casticin has cognitive ameliorating effects. J Ethnopharmacol. 2020; 259: 1–39.
- [22]Eagle AL, Wang H, Robison AJ. Sensitive assessment of hippocampal learning using temporally dissociated passive avoidance task. Bio-protocol. 2016; 6(11): 1–8.
- [23]Ohkawa H, Ohishi N, Yagi K. Assay for Lipid Peroxides in Animal Tissues Thiobarbituric Acid Reaction. Anal Biochem. 1979; 95(2): 351-358.
- [24]Courtney KD, Francisco S. A new and rapid colorimetric of acetylcholinesterase determination. Biochem Pharmacol. 1961; 7(2): 88–95.
- [25]Saleem H, Htar TT, Naidu R, Nawawi NS, Ashraf M, Ahemad N. Biological, chemical and toxicological perspectives on aerial and roots of Filago germanica (L.) huds: Functional approaches for novel phyto-pharmaceuticals. Food Chem Toxicol. 2018; 123: 363–373.
- [26]Kimuni SN, Gitahi SM, Njagi EM, Ngugi MP. Antinociceptive potential of methanol leaf extracts of Cissampelos parreira Antinociceptive potential of methanol leaf extracts of Cissampelos parreira ( Linn ), Lantana camara ( Linn ) and Ocimum gratissimum ( African basil ). J Adv Biotechnol Exp Ther. 2021; 4(3): 349–364.
- [27]Nan Gİ, Atirlar ZİNÖŞ. Alzheimer disease and anesthesia. Turkish J Med Sci. 2015; 45(5): 1026–1033.
- [28]Monte AS, Souza GC De, Mcintyre RS, Soczynska JK, Vieira J, Cordeiro C, et al. Journal of Psychopharmacology. J Psychopharmacol. 2013; 27(11): 1032–43.
- [29]Ben-azu B, Aderibigbe AO, Ajayi AM. Neuroprotective effects of the ethanol stem bark extracts of Terminalia ivorensis in ketamine- induced schizophrenia-like behaviors and oxidative damage in mice. Pharm Biol. 2016; 54(12): 2871–2879.
- [30]Brouillette J, Hector A, Mcanulty C, Buée-scherrer V. Cognitive dysfunction induced by ketamine and xylazine anesthesia is associated with tau hyperphosphorylation following CaMKII activation: Molecular and cell biology/tau. Alzheimer’s Dement. 2020; 16: 1–2.
- [31]Lee HY, Weon JB, Jung YS, Kim NY, Kim MK, Ma CJ. Cognitive-Enhancing Effect of Aronia melanocarpa Extract against Memory Impairment Induced by Scopolamine in Mice. Evidence-Based Complement Altern Med. 2016; 2016: 1-8.
- [32]Abel T, Lattal KM. Molecular mechanisms of memory acquisition , consolidation and retrieval. Curr Opin Neurobiol. 2001; 11(2): 180–187.
- [33]Javier S, Small DH. Acetylcholinesterase and butyrylcholinesterase glycoforms are biomarkers of Alzheimer ’ s disease. J Alzheimer’s Dis. 2001; 3(3): 323–328.
- [34]Han S, Park J, Byun MS, Yi D, Lee JH, Young D, et al. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol Aging. 2018; 73: 21–29.
- [35]Targum SD. Biomarkers for the identification and treatment of dementia. . Psychiatry. 2008; 5(2): 51–56.
- [36]Bakhtiari, S., Moghadam, N. B., Ehsani, M., Mortazavi, H., Sabour, S., & Bakhshi M. Can Salivary Acetylcholinesterase be a Diagnostic Biomarker for Alzheimer ? J Clin diagnostic Res JCDR. 2017; 11(1): 58–60.
- [37]Marucci, G., Buccioni, M., Dal Ben, D., Lambertucci, C., Volpini, R., & Amenta F. Neuropharmacology Efficacy of acetylcholinesterase inhibitors in Alzheimer ’ s disease. Neuropharmacology. 2021; 190: 108352.
- [38]Schuster D, Spetea M, Music M, Rief S, Fink M, Kirchmair J, et al. Morphinans and isoquinolines: acetylcholinesterase inhibition, pharmacophore modeling, and interaction with opioid receptors. Bioorg Med Chem. 2010; 18(14): 5071–5080.
- [39]Dani M, Brooks DJ, Edison P. Suspected non-Alzheimer’s pathology–Is it non-Alzheimer’s or non-amyloid? Ageing Res Rev. 2017; 36: 20–31.
- [40]Ghani A, Barril C, Jr DRB, Prenzler PD. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017; 230:195–207.
- [41]Reed TT. Free Radical Biology & Medicine Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011; 51(7): 1302–1319.
- [42]Moniczewski LPA. Oxidative Stress Biomarkers in Some Rat Brain Structures and Peripheral Organs Underwent Cocaine. 2013; 92–102.
- [43]Rao YL, Aradhana BG, Poornima M, Teresa AM, Sheetal J, Murlimanju MMPB V. Comparison of malondialdehyde levels and superoxide dismutase activity in resveratrol and resveratrol / donepezil combination treatment groups in Alzheimer ’ s disease induced rat model. 3 Biotech. 2021; 11(7): 1–10.
- [44]Chen Z, Zhong C. Oxidative stress in Alzheimer ’ s disease. Neurosci Bull. 2014; 30(2): 271–281.
- [45]Bohouth E, Tahrir E. Acetylcholinesterase inhibition and antioxidant activity of some medicinal plants for treating neuro degenarative disease. African J Tradit Complement Altern Med. 2015; 12(3): 97–103.
- [46]Sarikaya SBO. Acethylcholinesterase inhibitory potential and antioxidant properties of pyrogallol. J Enzyme Inhib Med Chem. 2014; 30(5): 761–776.
- [47]Wen S, Qing H, Zhang H, Lin Y, Jun J, Luo L. Quercetin attenuates spontaneous behavior and spatial memory impairment in d- galactose – treated mice by increasing brain antioxidant capacity. Nutr Res. 2007; 27(3): 169–175.
- [48]Yu T, Zhang P, Guan Y, Wang M, Zhen M. Protective effects of luteolin against cognitive impairment induced by infusion of Aβ peptide in rats. Int J Clin Exp Pathol. 2015; 8(6): 6740–6747.
- [49]Kim Y, Kim J, He M, Lee A. Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice. Molecules. 2021; 26 (17): 1–16.
- [50]Ayaz M, Junaid M, Ullah F, Subhan F, Sadiq A, Ali G, et al. Anti-Alzheimer ’ s Studies on β -Sitosterol Isolated from Polygonum hydropiper L . Front Pharmacol. 2017; 8: 697–715.
- [51]Maghsoud-Nia L, Asle-Rousta M, Rahnema M AR. Sesame Oil and Its Component Oleic Acid Ameliorate Behavioral and Biochemical Alterations in Socially Isolated Rats. Iran J Sci Technol Trans A Sci. 2021; 45(4): 1155-1163.
- [52]Kumar S, Pandey AK. Chemistry and Biological Activities of Flavonoids : An Overview. Sci World J. 2013; 2013: 1–17.
- [53]Castrén E RT. The Role of BDNF and Its Receptors in Depression and Antidepressant Drug Action : Reactivation of Developmental Plasticity. Dev Neurobiol. 2009; 70(5): 289–297.
- [54]Utami W, Aziz HA, Fitriani IN, Zikri AT, Mayasri A ND. In silico anti-inflammatory activity evaluation of some bioactive compound from ficus religiosa through molecular docking approach. J Phys. 2020; 1563(1): 1–9.
- [55]Tabernero A, Lavado EM, Granda B VA. Neuronal differentiation is triggered by oleic acid synthesized and released by astrocytes. J Neurochem. 2001; 79(3): 606–616.
- [56]Simone K, Dias T, Viegas C. Multi-Target Directed Drugs : A Modern Approach for Design of New Drugs for the treatment of Alzheimer ’ s Disease. Curr Neuropharmacol. 2014; 12(3): 239–255.