Effect of moderate-intensity treadmill exercise under different ambient temperatures on peripheral circulatory responses in young healthy adults
Abstract
Exercise training has the potential for inducing enhancements in peripheral circulation, which can play important preventive and therapeutic roles in peripheral circulatory diseases. However, the relevant published studies show conflicting and inconclusive results. Furthermore, useful or optimum ambient temperature for this purpose has not yet been established. Therefore, we investigated the acute responses in peripheral circulation from exposure of healthy subjects to treadmill exercise under different ambient temperatures; A total of 12 young adult volunteers (males 6, females 6) randomly underwent three sessions of treadmill exercise for 30 min under three different ambient temperatures (10°C, 20°C, and 30°C), at a predetermined exercise intensity. Before and after the intervention, leg skin blood flow (SBF) was measured by laser speckle flowgraphy and hand skin temperature (ST), by digital thermometry; After the cessation of treadmill exercise, compared to the corresponding baseline values, a significant increase in SBF was observed under all ambient temperature conditions (P<0.005). During intervention, ST showed a significant decrease at 10th min of intervention under all ambient temperatures (P<0.005) with a subsequent increasing trend in it. After intervention, a significant increase in ST was observed under 20°C condition only (P<0.05). Also, after intervention, the observed increase in systolic blood pressure was less significant under 20°C condition; Treadmill exercise appears to be a useful intervention modality in inducing improvements in peripheral circulation. However, exposure to treadmill exercise at or near 20°C ambient temperature might be recommended for the purpose.
INTRODUCTION
Peripheral circulatory disorder is a common health problem, especially among the elderly [1]. Such impairments in peripheral circulation are associated with delayed or impaired wound healing or healing of soft tissue injuries and can lead to chronic wounds or ulcers [2, 3]. All these can increase the risk of functional impairments, loss of mobility, morbidity, and reduced quality of life [4].
Exercise is a simple, safe, and non-invasive intervention modality which can induce a myriad of beneficial health effects including the potential positive effects on human peripheral circulation [5]. In the literature, various methods of exercise interventions including treadmill exercise have shown the efficacy to influence peripheral circulation [6, 7]. An improvement in peripheral circulation by a simple intervention like treadmill exercise may contribute to improved ambulatory function in patients limited by a range of peripheral circulatory disorders and enable them to become more functionally independent [8].
For intervention with exercise, the ambient temperature is an important factor as exposure to unfavorable cold and/or hot ambient temperatures can be stressful to the human body [9-11] and can increase the risk of cardiovascular or respiratory disorders, or impairments of other physiological systems [12]. However, for exercise intervention, useful or optimum ambient temperature has not yet been established. On the other hand, the combined effects of exercise and unfavorable ambient temperature on human physiological responses can be considerably greater [13]. Therefore, it is important to clarify the responses in peripheral circulation from combined exposure to exercise and different ambient temperatures. However, there is a severe lack of studies investigating the patterns and extents of changes in peripheral circulation induced by exposure to exercise under different ambient temperatures. The situation has been complicated by the relevant study findings as exercise-induced changes in peripheral circulation produced inconsistent results. For example, some studies in the published literature reported an exercise-induced increase in peripheral circulation [8, 14-16]. In contrast, others reported no change or a decrease in in the latter among study subjects exposed to exercise [17-19]. Therefore, there is an urgent need for better understanding of exercise-induced responses in peripheral circulation under different ambient temperature conditions.
Accordingly, the purpose of the current study was to explore the acute effects of intervention with moderate exercise protocol using a treadmill under three different ambient temperatures (10°C, 20°C, and 30°C) on peripheral circulation in young healthy subjects.
MATERIALS AND METHODS
Selection of subjects
This is a single-group repeated measures study conducted among young healthy adults. The present study is part of a larger research project the purpose of which was to examine the acute effects of intervention with moderate exercise protocol under three different ambient temperatures (10°C, 20°C, and 30°C) on various physiological parameters. The study participants were recruited from Yamaguchi University School of Medicine via poster advertisements and word of mouth. The selection criteria for the study subjects were as follows: no reported neurological, musculoskeletal, cardiovascular, or connective tissue disorders or any other known diseases that would prohibit exposure to treadmill exercise; no history of surgery within a year; not habitual cigarette smokers etc. A total of 12 young adult volunteers (males 6, females 6) were invited to participate in the study, and all of them were able to complete all the experimental sessions. The median with 25th and 75th percentiles for age and BMI of the study subjects were males, 21.0 (20.8 to 22.3) years and 19.3 (17.9 to 20.2) kg/m2, respectively; females, 21.0 (20.8 to 22.0) years and 18.9 (17.7 to 21.7) kg/m2, respectively. Written informed consents were obtained from all the study participants before their participation in the current study. The study protocol was approved by the relevant institutional review board of Yamaguchi University School of Medicine (approval no. H2021-104, dated 22-09-2021).
All the study participants refrained from eating or drinking (e.g., tea or coffee) for at least 3 h, and from strenuous physical activity, smoking or alcohol consumption for at least 12 h before the beginning of an experimental session. The subjects were asked to enter the experiment room after voiding. In the laboratory, they were instructed to be barefoot and wear light indoor clothing including two each for the upper and lower parts of the body.
Experimental design
The experimental sessions were carried out randomly on three different days which were separated by at least 24 h. For each subject, the sessions were set approximately at the same time of day. Upon arrival, all subjects were asked to take off their shoes and socks. Then they underwent acclimatization for a period of 15 min in the experiment room with a room temperature maintained at a randomized experimental condition of 10°C, 20°C, or 30°C (±1°C). During acclimatization, the subjects sat comfortably on a height-adjustable chair without physiological or psychological stress. Systolic blood pressure (SBP) was measured at 10th min after the start of adaptation.
At the end of the acclimatization period, initial measurements of heart rate variability and vibrotactile perception of foot were performed (not reported in this paper). Then the participants were asked to stand on the treadmill (MS194176, BTM, Guangdong, China) in complete upright posture and to look forward. While standing, the study subjects positioned their both arms hanging down in a relaxed manner and in close contact to the upper body. At this time, the sensors of the thermistor were attached to the dorsal surface of the middle of left hand. For this purpose, adhesive tape was used without causing any tape tension or compression of the underlying tissue, and the recording of skin temperature (ST) began. After a measurement period of at least 5 min and confirmation of a stable leg skin blood flow (SBF), the latter was measured twice from the dorsum of right foot with an interval of 1 min between the measurements. Then facing forward, the subjects underwent the intervention, which consisted of an initial warm-up treadmill run at 2 km/h for 5 min, followed by a constant speed run at a speed that was set at 50-70% of each subject’s maximum heart rate [20, 21] for 25 min. The exercise intensity was determined based on the following formula:
[(220 – age – resting heart rate) × % of maximum heart rate + resting heart rate] [20, 22].
After the cessation of the intervention, the study subjects maintained the upright position, and SBF was measured once immediately after intervention. This was followed by the measurements of ST for 5 min. Then the measurement of SBP was conducted again while they were seated on the chair.
Equipment and measurements
To measure SBF from the dorsal region of right foot, a commercially available laser speckle flowgraphy system (LSFG-ANW, Softcare Co., LTD., Fukuoka, Japan) was used, which is a non-contact measurement modality and considered to be useful in the evaluation of foot SBF [23, 24]. For the measurements with LSFG, the manufacturer’s instructions were followed. The recording unit of the Flowgraphy system was attached to the movable arm of a stand, and the latter was firmly fixed to the floor. During measurements of SBF, the recording unit was positioned above the right foot of the study subjects at a distance of 19 cm and an angle of 20°. The LSFG system incorporates a charge coupled device (CCD) camera with a resolution of 600 × 480 pixels and can capture the fluctuation in the random interference pattern (speckle pattern) of laser light reflected from the measured tissues. The values of blood flow in the LSFG system is a relative value, and are expressed in arbitrary units known as Mean Blur Rate (MBR). MBR is defined as the time-averaged moving speckle pattern of the recorded frame-shaped flows for a duration of 4 s at a rate of 30 frames/s. To minimize the effects of respiration and movement on SBF, we advised the subjects to breathe gently and to stand still during the measurements of it.
To measure ST from the middle of the dorsum of left hand and room temperature, digital thermistors (SZL-64, Technol seven, Kanagawa, Japan) were used, which had a measurement accuracy of ±0.15 °C [25]. The thermistors were connected to a scanner unit (X115, Technol seven, Kanagawa, Japan) and a high accurate data logger (K730, Technol seven, Kanagawa, Japan). The temperature data were printed on the recording paper at an interval of 1 min.
Measurements of resting arm SBP were performed with the subjects in sitting posture and their arm supported at the heart level, using an arterial pressure measurement device (HEM-705IT, OMRON, Kyoto, Japan) following the recommended guidelines for this purpose [26].
Data processing and statistical analysis
For recording of SBF, we scanned a rectangular skin region (270-300 × 170-200 pixels) on the dorsum of right foot located just proximal to the metatarsophalangeal joints and between the edges of the foot. The captured images were processed and analyzed by the LSFG analysis software version 3.0.36.0 (Softcare Co., LTD., Fukuoka, Japan).
The values of ST measured for 5 min and SBF measured twice before the intervention were averaged separately for all the 3 experimental conditions. These averaged values were considered as the baseline values for the corresponding conditions.
The continuous variables of this study were verified for normal distributions using Kolmogorov-Smirnov and Shapiro-Wilk tests and were expressed as median with 25th and 75th percentiles (for non-normal distributions) or mean and standard error (for normal distributions) in the text and figures. The differences between the exposure periods and conditions were assessed by Wilcoxon signed-rank tests for SBF and SBP, and by the Paired t-test and repeated measures analysis of variance (ANOVA) for ST, with Bonferroni corrections for multiple comparisons as necessary. We considered all statistical tests for the data used in this study as two-tailed and set the significance level at a value of P<0.05. The statistical analyses of data were performed using the software package SPSS version 22 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Effect of moderate-intensity treadmill exercise on changes in SBF
Figure 1 represents the values of SBF obtained before and after intervention under each of the three ambient temperature conditions. When the data analysis was performed including only the baseline measurements of SBF before intervention, the values did not differ significantly under the experiment conditions of 10°C, 20°C, and 30°C (P>0.05). Compared to the corresponding baseline values, there was a considerable elevation in SBF after the cessation of intervention, and the increase was similarly significant under all experimental conditions (P<0.005).
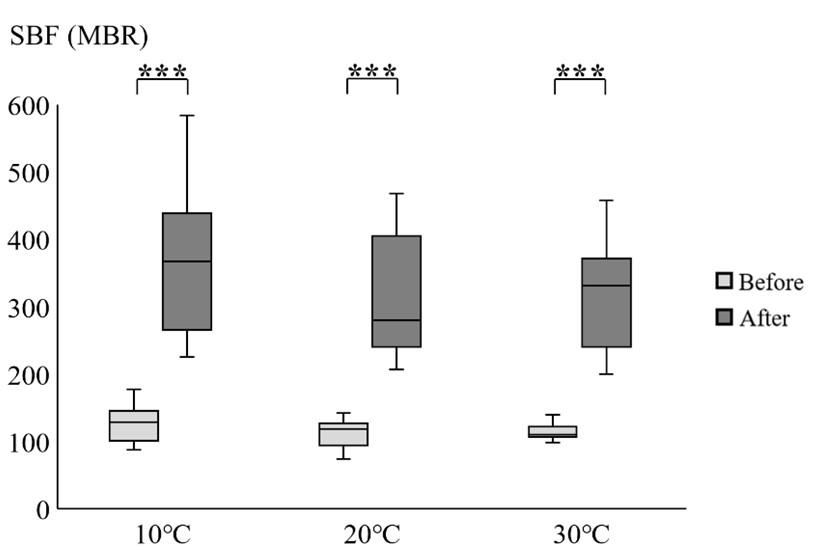
Effect of moderate-intensity treadmill exercise on changes in ST
Figure 2 shows the values of ST at left hand obtained before, during (2 time points: at 10th min and 25th min of intervention) and after intervention under each of the three ambient temperature conditions. The baseline value of ST under 10°C condition was significantly lower than that under 20°C and 30°C conditions (P<0.005; results not shown); similarly, the value under 20°C was also significantly lower than that under 30°C (P<0.005; results not shown). During intervention, compared to the corresponding baseline values, ST showed a significant decrease at 10th min of treadmill exercise intervention under all ambient temperatures (P<0.005), and then followed the trend towards a recovery under all conditions. After intervention, compared to the corresponding baseline (before-intervention) value, ST showed a significant increase under 20°C only (P<0.05).
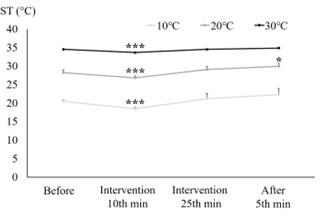
Effect of moderate-intensity treadmill exercise on changes in SBP
Figure 3 displays the values of SBP obtained before and after intervention under each of the three ambient temperature conditions. The corresponding baseline value of SBP under 20°C and 30°C conditions was lower than that under 10°C condition investigated in this study (P<0.05; results not shown), and no difference in SBP was found between the former two conditions. After the intervention, we observed a significant increase in SBP under all experimental conditions. However, compared to other two conditions, the observed increase in SBP was less significant under the 20°C condition.
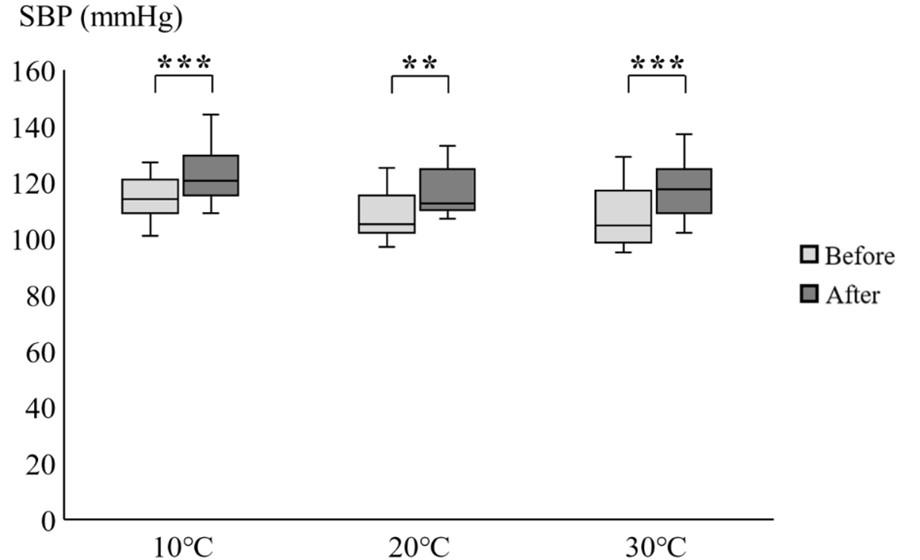
DISCUSSION
In this study, we investigated the acute effects of moderate treadmill exercise under three different ambient temperatures on peripheral circulation in both upper and lower limbs of healthy adults. The results revealed an improvement in peripheral circulation that was induced by exposure to the treadmill exercise protocol used in this study.
In the previous research works, data regarding the effects of exercise on peripheral circulation produced mixed results. A number of investigators observed that exercise did not produce any significant increase peripheral blood flow in healthy subjects or patient population [17-19]. By contrast, other studies investigating the peripheral circulatory responses exhibited vasodilatation and an increase in local blood flow from exposure to exercise [8, 14-16]. Differences in exercise intensity, protocols and methods as well as adherence to exercise intervention programs might be responsible for the observed differences in peripheral circulation in those studies.
As observed, there was a significant increase in SBF under all ambient temperatures. Our findings of an increase in peripheral SBF are consistent with several existing reports demonstrating improved peripheral circulation after exercise. Rozanski et al. reported an increase in finger pulse-wave amplitude responses to treadmill exercise in healthy subjects using a plethysmographic device [27]. In another study, a group of healthy young military recruits was exposed to a 10-week period of rigorous training including endurance exercise, which resulted in a significant enhancement in brachial artery flow-mediated dilation [6]. In a randomized controlled trial by Gardner et al., the researchers observed that exercise rehabilitation with supervised intermittent treadmill walking increased maximal calf perfusion by up to 30% in patients with peripheral arterial occlusive disease, and that improved peripheral circulation contributes to improved ambulatory function [8].
Intervention with exercise involves multiple and complex physiological processes that produce an increase in peripheral circulation. In the literature, a number of mechanisms underlying the effects of exercise on peripheral circulation have been suggested. During exercise, the locally produced vasodilatory metabolites might attenuate the effects of neural sympathetic inputs termed as functional sympatholysis [28]. Exercise-induced attenuation in α-adrenoreceptor responsiveness might also contribute to the observed increases in SBF of the lower extremity [28]. It has been hypothesized that exercise causes an increase in muscle metabolic demand and oxygen consumption of the extremity, and contraction and relaxation of the precapillary sphincters with subsequent vasodilation [24]. Also, such exposure to treadmill exercise probably results in shear stress of endothelial cells, and this can cause enhanced production of nitric oxide, which is a potent vasodilator [29, 30]. Therefore, enhancements in SBF observed in this study are probably caused by exercise-induced interactions of various neural signals, mediators, and hormones with the involvement of both local and central Vaso regulatory mechanisms [24, 27].
In this study, running exercise with the treadmill caused a change in the circulation of the upper extremity also. It has been mentioned that reflex control of the exercising muscle vasculature plays a role in vascular conductance with systemic effects and in perfusion of the tissues [28]. In line with this, in a study by Linke et al., 4 weeks of cycle leg exercise induced a positive effect on forearm radial artery responses [31]. In another study, a 3-month, home-based walking intervention in older men demonstrated vasodilation in the forearm like that observed in younger adults [29]. All these suggest a systemic effect of exercise training on peripheral vascular reactivity [31].
On the other hand, we observed a decrease in ST after 10 min of intervention under all ambient temperature conditions. Such a decrease in ST is thought to be the consequence of a reflex vasoconstriction [32] and the findings are consistent with those of others [32, 33]. However, following the intervention, ST showed a trend of recovery under all ambient temperatures with a significantly higher ST, compared with the corresponding baseline value, only under the 20°C condition. The temperature 20°C is considered to be within the range of thermal comfort zone of clothed humans (known as thermoneutral temperature) and has minimal effects on both the sympathetic and parasympathetic nervous systems [34]. It might be possible that being within the thermoneutral temperature, 20°C had a lesser effect on the sympathetic activity [34], which contributed to a larger increase in ST following the intervention.
In the literature, the significance of changes in SBP induced by moderate-intensity treadmill exercise has been reported [35]. Therefore, in this study, we compared the responses in SBP before and after the intervention under different experimental conditions. After intervention, we observed a significant increase in SBP under all experimental conditions. Our results of such changes in exercise induced SBP do not contradict the results in healthy subjects shown in the published literature [36, 37]. However, as the baseline value of SBP was lower and the increase in SBP was less significant under the 20°C condition, we postulate that treadmill exercise under 20°C condition may be less stressful to the body.
This study has some potential limitations and cautions are required while interpreting the findings. First, the sample size of this study was relatively small. Second, apparently healthy young adults were recruited for this study; therefore, the generalizability of current findings to other populations may be limited. Third, lack of relevant published data limits the comparison of current findings with those of others and their appropriate application in practice. Lastly, in this study, we investigated the acute effects of exposure to treadmill exercise on various physiological parameters. In contrast, exposure to treadmill in sports, exercise, and rehabilitation centers are usually long-term chronic interventions, and this issue needs to be considered in future investigations.
CONCLUSIONS
Treadmill exercise at or near 20°C ambient temperature could be beneficial to the human body. In future, the efficacy of treadmill exercise in improving peripheral circulation at or near 20°C ambient temperature needs to be investigated and confirmed in patients with impairments in peripheral circulation.
ACKNOWLEDGEMENT
This research received no external funding.
AUTHOR CONTRIBUTIONS
SW, MM and NY were involved in conception of this study. SW, MM and RH developed the methodology of the experiments. SW, NY, RH and YN contributed to performing the experiments. The data were analyzed by SW, MM, HT and HS. SW, MM, JS and RW were involved in the preparation of the original draft. YN, HT, and TT contributed to critical review and editing of the manuscript. All authors have read and agreed to the submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015; 116: 1509-1526.
- [2]Mayfield JA, Caps MT, Reiber GE, Maynard C, Czerniecki JM, Sangeorzan BJ. Trends in peripheral vascular procedures in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2001; 38: 347-356.
- [3]Scioli MG, Bielli A, Arcuri G, Ferlosio A, Orlandi A. Ageing and microvasculature. Vasc Cell. 2014; 6: 19.
- [4]Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, et al. Optimal exercise programs for patients with peripheral artery disease: A scientific statement from the American heart association. Circulation. 2019; 139: e10-e33.
- [5]Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012; 167: 1-12.
- [6]Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, et al. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999; 33: 1379-1385.
- [7]McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009; 301: 165-174.
- [8]Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001; 49: 755-762.
- [9]Yao Y, Lian Z, Liu W, Shen Q. Experimental study on physiological responses and thermal comfort under various ambient temperatures. Physiol Behav. 2008; 93: 310-321.
- [10]Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol. 2011; 173: 1013-1021.
- [11]Ahn N, Kim K. The influence of obesity and ambient temperature on physiological and oxidative responses to submaximal exercise. Biol Sport. 2014; 31: 139-144.
- [12]Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015; 386: 369-375.
- [13]Sureda A, Mestre-Alfaro A, Banquells M, Riera J, Drobnic F, Camps J, et al. Exercise in a hot environment influences plasma anti-inflammatory and antioxidant status in well-trained athletes. J Therm Biol. 2015; 47: 91-98.
- [14]Alpert JS, Larsen OA, Lassen NA. Exercise and intermittent claudication. Blood flow in the calf muscle during walking studied by the xenon-133 clearance method. Circulation. 1969; 39: 353-359.
- [15]Feinberg RL, Gregory RT, Wheeler JR, Snyder SO Jr, Gayle RG, Parent FN 3rd, et al. The ischemic window: a method for the objective quantitation of the training effect in exercise therapy for intermittent claudication. J Vasc Surg. 1992; 16: 244-250.
- [16]Herrero AJ, Menéndez H, Gil L, Martín J, Martín T, García-López D, et al. Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord. 2011; 49: 554-559.
- [17]Sørlie D, Myhre K. Effects of physical training in intermittent claudication. Scand J Clin Lab Invest. 1978; 38: 217-222.
- [18]Johnson PK, Feland JB, Johnson AW, Mack GW, Mitchell UH. Effect of whole-body vibration on skin blood flow and nitric oxide production. J Diabetes Sci Technol. 2014; 8: 889-894.
- [19]Zetterquist S. The effect of active training on the nutritive blood flow in exercising ischemic legs. Scand J Clin Lab Invest. 1970; 25: 101-111.
- [20]Goit RK, Pant BN, Shrewastwa MK. Moderate intensity exercise improves heart rate variability in obese adults with type 2 diabetes. Indian Heart J. 2018; 70: 486-491.
- [21]Karavirta L, Tulppo MP, Nyman K, Laaksonen DE, Pullinen T, Laukkanen RT, et al. Estimation of maximal heart rate using the relationship between heart rate variability and exercise intensity in 40-67 years old men. Eur J Appl Physiol. 2008; 103: 25-32.
- [22]Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between % heart rate reserve and % VO2 reserve in treadmill exercise. Med Sci Sports Exerc. 1998; 30: 318-321.
- [23]Kikuchi S, Miyake K, Tada Y, Uchida D, Koya A, Saito Y, et al. Laser speckle flowgraphy can also be used to show dynamic changes in the blood flow of the skin of the foot after surgical revascularization. Vascular. 2019; 27: 242-251.
- [24]Mahbub MH, Hase R, Yamaguchi N, Hiroshige K, Harada N, Bhuiyan ANH, et al. Acute effects of whole-body vibration on peripheral blood flow, vibrotactile perception and balance in older adults. Int J Environ Res Public Health. 2020; 17: 1069.
- [25]Mahbub MH, Harada N. Digital blood flow and temperature responses in palmar and dorsal skin induced by short-term vibration exposure while grasping a vibratory handle. Int Arch Occup Environ Health. 2008; 81: 889-897.
- [26]Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: A scientific statement from the American heart association. Hypertension. 2019; 73: e35-e66.
- [27]Rozanski A, Qureshi E, Bauman M, Reed G, Pillar G, Diamond GA. Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation. 2001; 103: 2084-2089.
- [28]Wray DW, Fadel PJ, Keller DM, Ogoh S, Sander M, Raven PB, et al. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol. 2004; 559: 675-684.
- [29]DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000; 102: 1351-1357.
- [30]Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998; 98: 2709-2715.
- [31]Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001; 37: 392-397.
- [32]Nakayama T, Ohnuki Y, Niwa K. Fall in skin temperature during exercise. Jpn J Physiol. 1977; 27: 423-437.
- [33]Torii M, Yamasaki M, Sasaki T, Nakayama H. Fall in skin temperature of exercising man. Br J Sports Med. 1992; 26: 29-32.
- [34]Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol. 2017; 13: 458-465.
- [35]Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001; 33: S484-S494.
- [36]Wolthuis RA, Froelicher VF Jr, Fischer J, Triebwasser JH. The response of healthy men to treadmill exercise. Circulation. 1977; 55: 153-157.
- [37]Jackson DH, Reeves TJ, Sheffield LT, Burdeshaw J. Isometric effects on treadmill exercise response in healthy young men. Am J Cardiol. 1973; 31: 344-350.