HLA-B*0702 class-I allele, anti-FSH, anti-LH, and vitamin D3: Potential links with polycystic ovary syndrome in women of Erbil city, Iraq
Abstract
Polycystic ovary syndrome (PCOS) is a widespread endocrine-reproductive-metabolic condition with severe implications for females’ health. The role of four essential parameters on PCOS, including human leukocyte antigens (HLA) represented by the HLA-B*0702 allele, anti-follicle stimulating hormone (anti-FSH) antibodies, anti-luteinizing hormone (anti-LH) antibodies, and vitamin D3 was investigated. A total of 100 samples were collected from Kurdish women attended the maternity teaching hospital and some private hospitals/laboratories in Erbil City from October 2021 to January 2022. The samples were genotyped using a PCR-based technique with specific sequence primers. The levels of anti-FSH and anti-LH antibodies were determined using an enzyme-linked immunosorbent assay (ELISA), while vitamin D3 levels were measured by an electrochemiluminescence (ECL) test on Cobas e411 immunoassay system. The odds ratio (OR) of 2.167 at a 95% confidence interval (CI) of 0.8167 to 6.330, indicated an essential link between the HLA-B*0702 allele and a risk of PCOS. Anti-FSH and anti-LH antibodies were significantly greater in PCOS patients, notably infertile women, than in healthy controls. A significant positive linear correlation was observed between antibodies against FSH and LH in patients. Most patients had hypovitaminosis D3 with a significant difference compared to controls. The results indicate that the HLA-B*0702 allele is associated with PCOS susceptibility and could be used as an immunogenetic marker. Besides, it supports the idea that anti-FSH, and anti-LH antibodies are naturally presence antibodies in PCOS patients instead of signs for autoimmunity. It also suggests that women suffer from PCOS are more prone to develop vitamin D3 deficiency.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a quite multifactorial disease in which genetic susceptibility, socio-economic status, ethnicity, metabolic and lifestyle, besides inflammatory and immunological responses, are all implicated and interact [1]. PCOS affects females of childbearing age and causes an abnormality in a female’s reproductive hormones, which can lead to ovarian issues. Despite the widespread prevalence of PCOS and its negative effect on women’s health, the exact cause is unknown [2]. One of the implicated immunogenetic factors is the major histocompatibility complex (MHC), also known as HLA system in human. The HLA is a vital component of the human immune system which is controlled by a gene on chromosome 6. They are highly polymorphic, with several distinct alleles that allow them to fine-tune the immune system [3]. Some of HLA alleles raise the likelihood of contracting certain diseases and their variance may emphasize the importance of HLA in imparting an immunogenetic proclivity to produce ovarian cysts [4, 5].
In a variety of situations, the ovaries may become the victim of an autoimmune attack, releasing autoantibodies that destroy healthy cells and molecules due to insufficient progesterone levels and an inability to manage the frequency of GnRH/LH pulses, resulting in excess estrogen production [6, 7]. Anti-FSH antibodies were found in high concentrations in infertile women [8]. Such antibodies may interfere with FSH activity and interact with it to form immune complexes, causing its removal, or it may interfere with FSH interacting with its own receptors [9]. Because FSH and LH have similar structures, functions, and secretion sites, antibodies against both are associated [10-12]. Vitamin D3, on the other hand, has sparked a lot of debate due to its association with various health problems, including PCOS; however, its role remains unknown until today [13, 14].
The current work aimed to highlight the role of HLA-B*0702 (HLA Class-I) in the possibility of imparting immunogenetic propensity to develop PCOS among Kurdish women. It also intended to assess serum levels of anti-FSH antibodies, anti-LH antibodies, and vitamin D3 in PCOS and healthy women.
MATERIALS AND METHODS
Study subjects
A total of 100 Kurdish women, aged 15-37 years, who were attended the Maternity Teaching Hospital and some private hospitals/laboratories in Erbil City, Kurdistan region of Iraq participated in this case-control study. Sixty women were clinically diagnosed with PCOS by the clinics and hospitals’ consultant medical staff using Rotterdam criteria [15] and the remaining 40 participants were healthy women whom had regular menstrual cycles and no other signs or symptoms of PCOS. Participants who satisfied the inclusion criteria for this work were requested to answer screening questions about the most frequent symptoms of PCOS. Weight and height were measured, and the body mass index (BMI) was computed. Furthermore, age, ethnicity, family history of PCOS, underlying chronic conditions, medications and supplements intake, and other general information were all recorded. In this study, PCOS-like disorders including thyroid disease, hyperprolactinemia, and non-classical congenital adrenal hyperplasia were ruled out [16].
The medical ethics committee approved and authorized the current study as per the order no. (154), dated 11/11/2021. Verbal consent to participate in the research was obtained through interviews with participants, and their anonymity and privacy have been kept confidential.
Assessment of DNA yield and HLA-genotyping
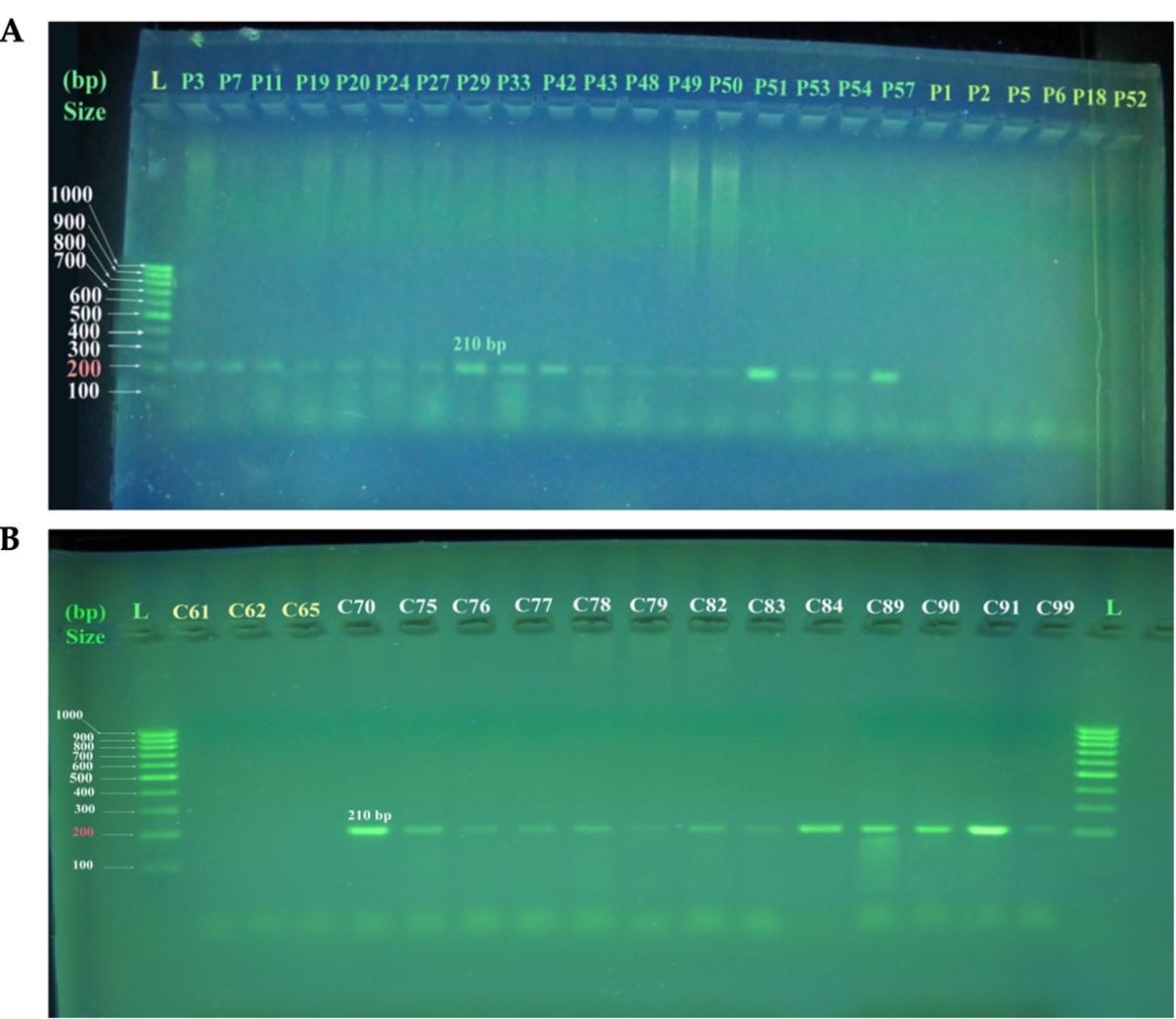
A total of 5 ml of blood samples in EDTA tubes from 100 participants (60 PCOS patients and 40 healthy controls) were used for genomic DNA extraction using Blood DNA Preparation-Solution kit (Jena Bioscience, Germany) as directed by the manufacturer. Qualification and quantification of genomic DNA were performed using agarose gel electrophoresis and Nano-Drop™ (Thermo Scientific, USA) spectrophotometer. The HLA-B*0702 allele was genotyped using a PCR-based technique with specific sequence primers (Thermo Scientific, USA). The primers (Integrated DNA Technologies (IDT), Canada) used in this study were forward: 5′-ACTCCATGAGGTATTTCTACACCT-3′ and reverse: 5′-TCTGTGCCTGGGCCTTGT-3′ [17]. A total of 25μL volume of PCR master mix (Promega, USA) reaction was achieved as directed by the manufacturer. The PCR program began with an initial DNA denaturation at 95°C for 5 minutes, followed by 35 cycles at 95°C for 30 seconds (denaturation), 63°C for 30 seconds (primer annealing), and 72°C for 1 minute (extension). To extend all PCR fragments, final extension step was set at 72°C for 5 minutes. After PCR amplification, the PCR-DNA amplicons were separated using 2.0% of agarose gel which was stained with safe nuclear staining dye before being visualized using a UV-Transilluminator (UVP, USA). The (210 bp) represent the appearance of the HLA-B*0702 allele gene polymorphism.
Detection of anti-FSH and anti-LH antibodies using ELISA
These procedures were carried out using commercially available ELISA kits (as directed by the manufacturer) for both human anti-FSH antibodies (Catalogue No. RDEEH4473) and human anti-LH antibodies (Catalogue No. RDEEH4472) purchased from AL Shkairate Establishment-Jordan, USA origin.
Detection of vitamin 25(OH)D using Cobas e411
Serum 25(OH)D levels were tested for (100) samples using ECL test on Roche Diagnostic Cobas e411 immunoassay system with a vitamin D kit from (Germany, Catalogue No. REF05894913190) as directed by the manufacturer.
Statistical analysis
GraphPad Prism 8.0.1 (244) software (San Diego, California, USA) was used to analyze the data statistically. The normal distribution was verified by the Shapiro-Wilk and the D’Agostino tests. Demographic and clinical parameters were compared by independent t-test (two-tailed) and ANOVA test. Fisher’s exact test was utilized to evaluate and compare the HLA-B*0702 allele and its frequency in cases and controls. The OR and its 95% CI were computed to demonstrate the relationship of the mentioned allele with the risk of PCOS. Youden’s index was utilized to compute the most appropriate cut-off value for ELISA tests using the Receiver Operating Characteristic (ROC) curve and the corresponding area under the curve (AUC). Associations among immunological parameters were determined by Pearson correlation (P.C). The results are presented as mean ± SD and the significance level is denoted as * P <0.05; ** P <0.01; or **** P <0.0001.
RESULTS
Distribution of demographic and clinical characteristics of the participants
Out of 100 participants, 84% had been married for ≥1 year. The age mean ± SD of the studied population was 25.02±4.135 for patient and 25.8±5.239 for controls, indicating no significant difference (P-value ≥0.05). The majority of PCOS patients (71.67%) were aged 18 to 26 and 30% had a PCOS history in their families. More than half of patients (70%) had high BMIs; however, 57.5% of healthy controls had high BMIs, indicating a significant difference (P-value <0.05). About 47% of PCOS patients experienced primary infertility, while around 33% experienced secondary infertility as indicated in Table 1.
Most of the PCOS patients (95%) experienced menstrual irregularities, with amenorrhea accounting for 13.33% and oligomenorrhea accounting for 81.67% and about 93.33% of them met the modified Ferriman-Gallway (mF-G) score criterion for mild hirsutism from the upper lip, chin, and lower abdomen [18], in addition to mild acne, which accounted for about 62%. Trans-abdominal ultrasound findings revealed that 76.67% of patients had increased size of one or both ovaries with PCO morphology as shown in Table 2.
In addition, the mean serum levels of LH and LH/FSH ratios in PCOS women were significantly different (P-value <0.0001) from the control group, however FSH were not statistically significant (P-value ≥0.05). LH/FSH levels greater than 2 were found in 86.67% of patients, which is frequently requested to assist with PCOS diagnosis as illustrated in Table 3.
Table 1. Demographic characteristics-based distribution of the study participants.
Table 2. Clinical characteristics-based distribution of the study participants.
Table 3. Serum levels of FSH, LH and LH/FSH ratios of the study participants.
HLA-B*07:02 allele genotyping
Figure 1 shows an agarose gel electrophoresis (2.0%) revealing 210bp Uniplex PCR products corresponding to amplification of HLA-B*07:02 allele typing for selected samples of PCOS patients and healthy control women. Figure 1A represents PCOS patients, with lanes P3 to P57 displaying positive PCR products (DNA amplicon sizes 210bp) and lanes P1 to P52 displaying negative PCR products (no amplification). While Figure 1B represents healthy control women with lanes C61, C62, and lane C65 show the negative PCR products (no amplification) and lanes C70 to C99 show the positive PCR products (DNA amplicon sizes 210bp).
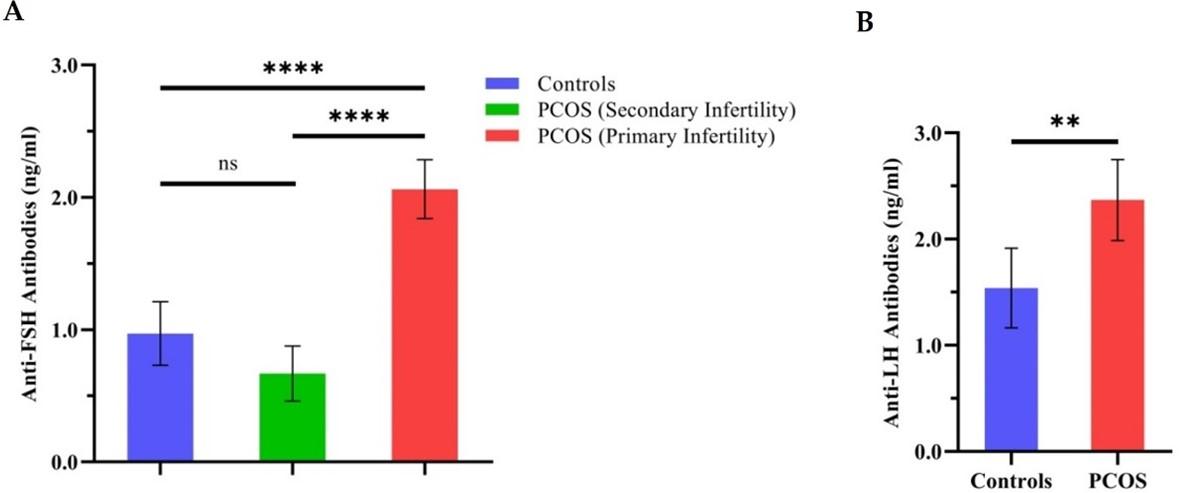
Anti-FSH and anti-LH antibodies levels in PCOS patients with primary and secondary infertility compared to healthy controls
Figure 2A demonstrates one-way ANOVA comparisons of anti-FSH antibodies mean levels with (95% CI bars) in PCOS patients with primary and secondary infertility versus healthy controls. Anti-FSH antibodies were highly significant in primary infertile patients with PCOS than in secondary infertile patients and healthy controls (P-value <0.0001). While Figure 2B shows a comparison of anti-LH antibodies in PCOS patients versus controls, in which serum anti-LH levels in patients were highly significant than controls (P-value <0.01). The ROC curve and the corresponding AUC were also computed to show the link between clinical sensitivity and specificity for possible cut-off values for both ELISA tests. The results show that anti-LH antibodies, as an immunological diagnostic value, can discriminate PCOS patients from healthy subjects (AUC=0.67, 95% CI: 0.5523 to 0.7822, P-value <0.05). Anti-FSH antibodies, on the other hand, had poor immunodiagnostic value in this case (AUC=0.6167, 95% CI: 0.4966 to 0.7367, P-value ≥0.05).
Serum levels of vitamin D3 of the study participants
Hypovitaminosis D3 was found in about 97% of patients and 75% of healthy controls with mean serum 25OHD levels of 16.33±7.722 and 22.62±11.47ng/ml, respectively, revealing a significant difference (P-value <0.01) with the majority of PCOS patients having a deficient to severe vitamin D3 deficiency (n=41; 68.33%) as shown in Table 4.
Table 4. Levels of vitamin 25(OH)D clusters in the study participants.
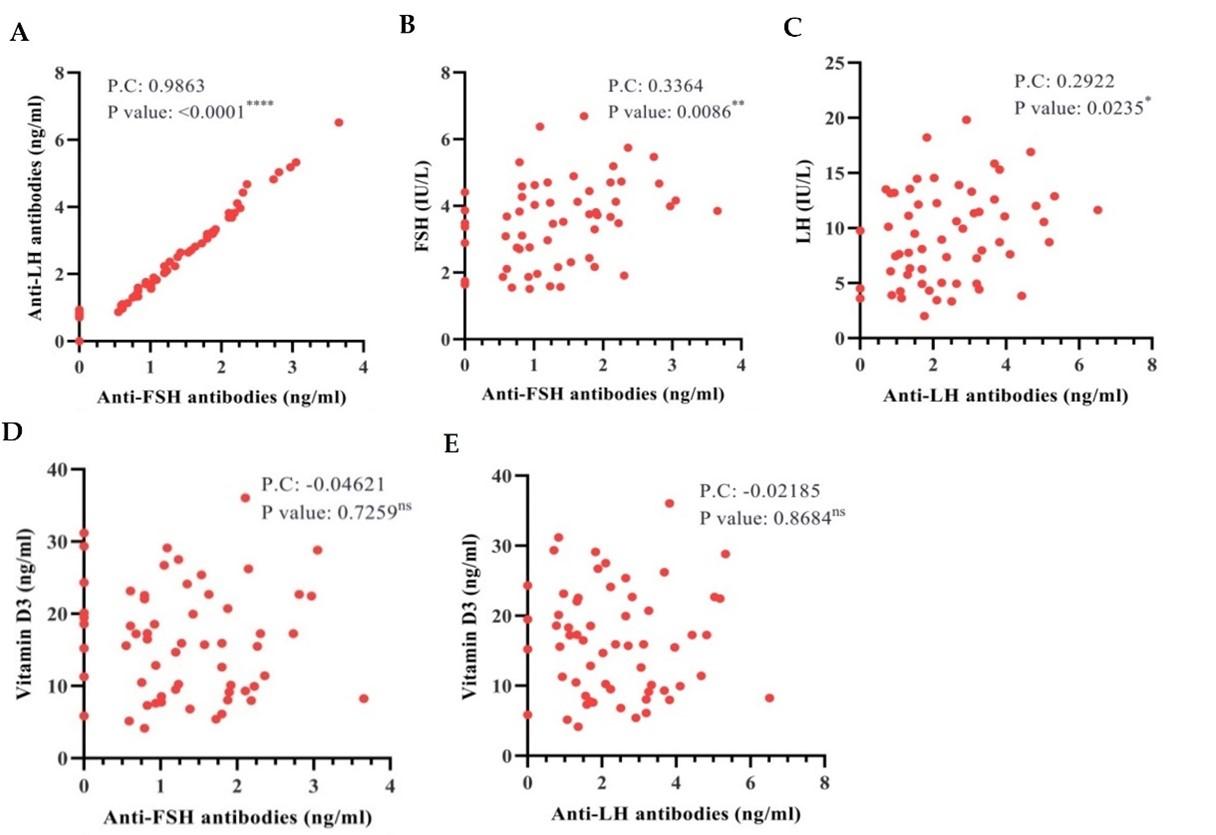
Correlations among immunological parameters in PCOS patients
Figure 3 displays scatter diagrams with varying Pearson correlations among studied parameters in PCOS patients. The findings (Figure 3A) indicate a perfect positive linear correlation between anti-FSH and anti-LH antibodies (P.C value = 0.9863, P-value <0.0001). Anti-FSH antibodies and FSH (Figure 3B) shows a significant positive correlation (P.C value = 0.3364, P-value <0.01), as were anti-LH antibodies and LH (P.C value = 0.2922, P-value <0.05) (Figure 3C). Furthermore, there were non-significant negative correlations (P-value ≥0.05) of anti-FSH as well as anti-LH antibodies with vitamin D3 (P.C value = -0.04621 and -0.02185), as illustrated (Figure 3D and E), respectively.
DISCUSSION
Several pathophysiological factors play a major role in PCOS, which is a serious health issue contributes to a variety of implications in the individual patient [19]. The results on age revealed no significant difference between patients and controls, which is in line with recent research findings [20]. The results also indicate that the disease was more prevalent in younger women who constituted the majority of PCOS patients in this study; this was also concluded by previous researchers [21-23]. The PCOS diagnostic criteria and implications are age-related, as the women’s ovaries age, their androgen production may decrease [24, 25]. The genetic foundation of PCOS differs amongst families but is connected to a common pathway [26] and the disparities might be attributed to changes in sample size, characteristics, as well as the method used to describe each variable. Accordingly, 30% of patients had a PCOS history in their families, while 70% of them had BMIs that were significantly higher than healthy women. A recent study shows that 76% of PCOS patients are overweight or obese [27]. Furthermore, recent Iraqi research confirmed the high BMI in women with PCOS [11, 28, 29]. PCOS is an obesity-related condition associated with metabolic issues [30]. However, in this study, 57.5% of healthy women had a high BMI. Perhaps this is due to lifestyle factors in our society, such as a high-fat diet, lack of exercise, stress, and sleep problems.
The findings also revealed that 95% of PCOS patients had oligomenorrhea and amenorrhea. Similarly, previous research indicated that 93% of patients had irregular periods [31]. Menstrual cycle irregularities are regarded as an important characterization of PCOS. In addition, (93.33%) of patients met the (mF-G) score criterion for mild hirsutism and about 62% of them had mild acne which is comparable to the 67.3% [32]. According to Neubronner et al, being overweight or obese had an additional impact on hirsutism in PCOS women [33]. Due to the variety of phenotypes associated with PCOS, only 77% of patients had polycystic ovary morphology on trans-abdominal ultrasound.
In the current study, FSH levels remained within normal limits, indicating no significant difference (P-value ≥0.05). LH levels, on the other hand, were significantly higher in patients than in controls, which is consistent with recent research findings [34]. De Leo and colleagues also stated that PCOS is also accompanied by high LH pulse amplitude and an abnormal LH response to GnRH, while FSH levels remain relatively normal [35]. The pituitary gland disruption causes overproduction of LH, which leads to GnRH disorder and thus an incorrect LH to FSH ratio [36]. Accordingly, a significant increase was recognized in LH to FSH ratio in patients that was mostly greater than two which considered to be one of the distinguishing features of PCOS and frequently requested to assist in the diagnosis of PCOS [37, 38]. In PCOS patients, anti-FSH antibodies and FSH were found to have significant positive correlation, as were anti-LH antibodies and LH. Moreover, antibodies against FSH were found to be significantly related to those against LH, indicating a perfect positive linear correlation since Pearson correlation value (0.9863) is close to one [39]. Luteinizing Hormone in synergy with FSH induces follicular growth and ovulation, both of which are secreted at the same site (anterior pituitary gland) and both share a common chain and are homologous, thus antibodies directed against one hormone may cross-react with the other [40].
One of the most important findings in this study was the association of the HLA-B*0702 allele polymorphism with PCOS in Kurdish women. The resulted odds ratio 2.167 at a 95% CI of 0.8167 to 6.330, indicated a positive association of the HLA-B*0702 allele with the risk of PCOS. An odds ratio greater than (1) indicates increased risk, implying that the disease is more frequent with exposure and thus the exposure may be harmful [41]. This link between the HLA-B*0702 allele and PCOS has not been seen in other regions of the world, and it may be unique to our geographical area. The extensive polymorphism and the haplotypic inheritance of HLA design leads to different distributions among different ethnicities as the HLA system is linked to autoimmune, infectious, and endocrine related conditions including PCOS [42]. Previous research in Baghdad/Iraq highlighted the relationship between HLA antigens (Class I) and PCOS, with the results confirming a link between the HLA-B7 alleles and susceptibility to PCOS. The research was carried out on a group of Iraqi Arab women using commercially available kit based on the serological HLA-typing known as micro lymphocytotoxicity test [5]. Screening of HLA antigens frequencies in various communities across the world found a variety of observations. HLA-A*11, A*31, and B*54 were significantly linked to PCOS in Korean women (OR of 2.79, 95% CI; 1.07–7.30, OR of 6.05, 95% CI; 1.23–29.85 and OR of 6.40, 95% CI; 1.70–24.09), respectively [42, 43], while in Japanese women the HLA-A*11and DRB1*0403 were the risk alleles for PCOS (OR of 2.16, 95% CI; 1.09–4.26 and OR of 2.63, 95% CI; 1.15–5.98), respectively [44]. The disparity in results may be explained by the different HLA distributions among ethnic groups, and the variation may be partially attributable to PCOS phenotypic-related features, like many other multi-factorial conditions. The mechanism behind the link between PCOS and HLA alleles is unknown and remains a subject of hot debate. One theory adopts a direct link between a specific HLA allele and disease pathogenesis, particularly the critical role of T-lymphocytes in immune response. Another idea suggests a link disequilibrium between the causative gene and susceptible HLA allele [42]. In this study, HLA-B*0702 genotyping is considered a new study in this domain since this allele has never been associated to PCOS, although the association was not reached statistically significant because of the limited sample size. This finding will provide data about the immunogenetic basis of PCOS development and clinical characteristics in our geographical area. Larger population studies can help in more clearly identifying relevant links.
Anti-FSH and anti-LH antibodies are two other important parameters examined in this study. The levels of antibodies against FSH were not significantly different between cases and controls, but after categorizing PCOS patients based on infertility status (primary or secondary), infertile patients had significantly higher levels of anti-FSH than healthy controls. Anti-LH antibodies also were higher significantly in patients than controls. An earlier research confirmed the presence of high levels of anti-FSH antibodies in infertile women [45] and antibodies of two kinds have already been recognized: anti-FSH antibodies that exist naturally and anti-FSH antibodies produced by exogenous gonadotropins. An immune system modification may have occurred and the antigen implicated in their formation is either FSH circulating in female body or in seminal fluid [40]. It could also be caused by a genetic defect in reproductive tract mucosal tolerance, which causes anti-FSH IgG and IgA antibodies in the serum to accumulate in the preovulatory follicle, impairing oocyte maturation [6]. Few studies have focused on antibodies against FSH and LH and their relationship to PCOS. A highly significant difference in antibodies levels against FSH and LH was found in PCOS women versus controls [10-12]. Researchers discovered that when hormone levels rise above their physiological levels, auto-antibodies against the hormones form [46]. Thus, the outcomes of this study support the idea that such antibodies in PCOS patients are naturally occurring [47]. Ludwig et al, on the other hand, confirm that such auto-antibodies are frequently found in healthy individuals and are not always pathogenic [48]. Naturally existing antibodies against FSH were discovered in both endometriosis and PCOS patients who had not had ovarian stimulation via IVF. Anti-FSH (IgG, IgM and IgA) antibodies were also found in healthy non-pregnant females but at a lower incidence and the physiological hormonal levels stay under a critical threshold point for the stimulation of relating autoimmune responses [6]. The area under the ROC curve is in the (0.5-1.0) range, with the minimum value representing the efficiency of a random classifier and the maximum value representing the efficiency of a perfect classifier. Therefore, the greater the area beneath the curve, the better the test distinguishes between individuals with and without disease [49, 50].
Notably, hypovitaminosis D3 was found in the majority of PCOS patients and healthy controls, with patients having significantly lower vitamin D levels than controls. The negative correlations of both antibodies against FSH and LH with vitamin D3 were observed in PCOS patients. Several studies also reported lower serum 25OHD levels in patients [51-53]. Vitamin D deficiency is prevalent in patients with PCOS, this might be attributed to metabolic and hormonal disorders in PCOS [54]. Furthermore, an indoor-lifestyle, reduced sunlight exposure, clothing style and sun block use, poor dietary vitamin D intake, seasonal variations, and genetics all contribute to the prevalence of deficient in vitamin D among women in our society. Vitamin D is well-known as a powerful immune system regulator and its deficiency is thought to be connected to B-cell hyperactivity [52, 55, 56]. Thus, it is considered as a risk factor for the development of auto-antibodies [57].
CONCLUSIONS
The HLA-B*0702 allele is regarded as an immunogenetic marker for PCOS among Kurdish women. Such findings will provide data on the immunogenetic basis of PCOS development and the clinical characteristics in our geographical area, potentially paving the way for large-scale population studies to clarify the relationship between the HLA-B*0702 allele and PCOS. Antibodies against both FSH and LH were greater in primary infertile women with PCOS than those of healthy controls and secondary infertile PCOS patients; however, there is still much knowledge to be acquired to fully understand the pathophysiological role played by both anti-FSH and anti-LH in the PCOS. Finally, while hypovitaminosis D3 is common in both patients and controls, women with PCOS are more likely to develop vitamin D deficiency.
ACKNOWLEDGEMENTS
All authors are grateful to the Erbil Health Directorate, Erbil Maternity Hospital, Scientific Research Center of Erbil Polytechnic University, Selar private lab, and Nobel private lab for providing support during sample collection and research work.
AUTHOR CONTRIBUTIONS
The main conception methodology, administration and writing-review was made by the supervisor Assistant Prof. Dr. NJAB and the design of the experiments, sample collections, lab practical work and data statistical analysis was done by the M.Sc. student RMA. Article drafting was done by RMA while NJAB revised it critically for significant intellectual content and gave final approval of the manuscript to be published. The published version of the manuscript has been read and approved by all authors.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Sattler L-M, Schniewind HA, Minich WB, Haudum CW, Niklowitz P, Münzker J, et al. Natural autoantibodies to the gonadotropin-releasing hormone receptor in polycystic ovarian syndrome. Plos one. 2021; 16(4): e0249639.
- [2]Bulsara JP, Patel P, Soni A and Acharya S. A review on brief insight into Polycystic Ovarian syndrome. Endocrine and Metabolic Science. 2021: 1-7.
- [3]Sanchez-Mazas A. A review of HLA allele and SNP associations with highly prevalent infectious diseases in human populations. Swiss Med Wkly. 2020; 150: w20214.
- [4]Ad’hiah AH. HLA-DQB1 Genotyping in Infertile Iraqi Patients. Iraqi Journal of Cancer and Medical Genetics. 2018; 5(1): 46-52.
- [5]Aajil AH. The association between HLA-class I antigens and polycystic ovary syndrome in a sample of Iraqi patients. Iraqi Journal of Cancer and Medical Genetics. 2018; 4(1): 52-56.
- [6]Haller-Kikkatalo K, Salumets A and Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clinical and Developmental Immunology. 2012; 2012: 1-15.
- [7]Mobeen H, Afzal N and Kashif M. Polycystic Ovary Syndrome May Be an Autoimmune Disorder. Scientifica (Cairo). 2016; 2016: 1-7.
- [8]Haller K, Salumets A, Grigorova M, Talja I, Salur L, Béné MC, et al. Putative predictors of antibodies against follicle-stimulating hormone in female infertility: a study based on in vitro fertilization patients. Am J Reprod Immunol. 2007; 57(3): 193-200.
- [9]Kara E, Dupuy L, Bouillon C, Casteret S and Maurel MC. Modulation of Gonadotropins Activity by Antibodies. Front Endocrinol (Lausanne). 2019; 10: 1-15.
- [10]Hussein S, Al-Saimary I and Sherif M. Level of Anti-FSH and Anti-LH Antibody in PCOS Women and Comparing it with Normal Control Group. Immunochemistry & Immunopathology. 2018; 4(01): 1-4.
- [11]Jabbar Ahmed N, Salih BA and Othman BS. Relation of Anti FSH Antibodies and Polycystic Ovarian Syndrome in Women. EXECUTIVE EDITOR. 2020; 11(01): 1954-1959.
- [12]Abood RM and Hathal HD. Study of Anti-Ovarian Antibody, Anti-FSH and Anti-LH Antibodies Along with Their Receptors in Polycystic Ovarian Syndrome. Indian Journal of Forensic Medicine & Toxicology. 2021; 15(2): 3250-3257.
- [13]Arslan E, Gorkem U and Togrul C. Is There a Relationship Between Vitamin D Deficiency Status and PCOS in Infertile Women? Geburtshilfe Frauenheilkd. 2019; 79(7): 723-730. doi: 10.1055/a-0871-6831.
- [14]Eftekhar M, Mirhashemi ES, Molaei B and Pourmasumi S. Is there any association between vitamin D levels and polycystic ovary syndrome (PCOS) phenotypes? Arch Endocrinol Metab. 2020; 64(1): 11-16.
- [15]group TREAsPcw. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction. 2004; 19(1): 41-47.
- [16]Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013; 98(12): 4565-92.
- [17]Law SC, Haigh OL, Walpole CM, Keane C, Miles JJ, Gandhi MK, et al. Simple, rapid and inexpensive typing of common HLA class I alleles for immunological studies. J Immunol Methods. 2019; 465: 72-76.
- [18]Mahajan VK, Chauhan PS, Chandel M, Mehta KS, Singh VK, Sharma A, et al. Clinico-investigative attributes of 122 patients with hirsutism: A 5-year retrospective study from India. International Journal of Women’s Dermatology. 2021; 7(3): 237-242. doi: 10.1016/j.ijwd.2020.11.007.
- [19]Xu Y and Qiao J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. Journal of Healthcare Engineering. 2022; 2022: 1-13.
- [20]Nawar AS, Obaid ZH and Sheikh QI. Oxidant and antioxidant status of erythrocytes and plasma samples in polycystic ovary syndrome patients. Journal of Advanced Biotechnology and Experimental Therapeutics. 2022; 6(1): 17-24.
- [21]Rowlands IJ, Teede H, Lucke J, Dobson AJ and Mishra GD. Young women’s psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Hum Reprod. 2016; 31(9): 2072-81.
- [22]Ganie MA, Rashid A, Sahu D, Nisar S, Wani IA and Khan J. Prevalence of polycystic ovary syndrome (PCOS) among reproductive age women from Kashmir valley: A cross-sectional study. Int J Gynaecol Obstet. 2020; 149(2): 231-236.
- [23]Tabassum F, Jyoti C, Sinha HH, Dhar K and Akhtar MS. Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage. PLoS One. 2021; 16(3): e0247486.
- [24]Hsu MI. Changes in the PCOS phenotype with age. Steroids. 2013; 78(8): 761-6.
- [25]Falcetta P, Benelli E, Molinaro A, Di Cosmo C, Bagattini B, Del Ghianda S, et al. Effect of aging on clinical features and metabolic complications of women with polycystic ovary syndrome. J Endocrinol Invest. 2021; 44(12): 2725-2733.
- [26]Khan MJ, Ullah A and Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. The application of clinical genetics. 2019; 12: 249-260.
- [27]Sachdeva G, Gainder S, Suri V, Sachdeva N and Chopra S. Obese and non-obese polycystic ovarian syndrome: Comparison of clinical, metabolic, hormonal parameters, and their differential response to clomiphene. Indian journal of endocrinology and metabolism. 2019; 23(2): 257-262.
- [28]Al-Shattawi SS, Al-Jumili EF and Al-Azzam MA. The relationship between obesity and polycystic ovary syndrome in a sample of Iraqi infertile women. Iraqi journal of biotechnology. 2018; 17(3): 40-46.
- [29]Al-Juaifari BJ and Al-Jumaili EF. Correlation of Body Mass Index and Some Hormones (Estradiol, Luteinizing, Follicle Stimulating Hormones) with Polycystic Ovary Syndrome among Young Females [20 to 35 Years]. Biomedical and Pharmacology Journal. 2020; 13(1): 193-198.
- [30]Uçkan K, Demir H, Turan K, Sarıkaya E and Demir C. Role of Oxidative Stress in Obese and Nonobese PCOS Patients. International Journal of Clinical Practice. 2022; 2022: 1-9.
- [31]Kamrul-Hasan A, Aalpona FTZ, Mustari M, Akter F, Chanda PK, Rahman MM, et al. Prevalence of thyroid dysfunction and thyroid autoimmunity in polycystic ovary syndrome: A multicenter study from Bangladesh. Thyroid Research and Practice. 2020; 17(2): 76-81.
- [32]Sidra S, Tariq MH, Farrukh MJ and Mohsin M. Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PLoS One. 2019; 14(10): e0223329.
- [33]Neubronner SA, Indran IR, Chan YH, Thu AWP and Yong EL. Effect of body mass index (BMI) on phenotypic features of polycystic ovary syndrome (PCOS) in Singapore women: a prospective cross-sectional study. BMC Womens Health. 2021; 21(1): 135.
- [34]Al-Musawi NJT, Qaysi SAA- and Witwit SJ. Effect of KISS1 gene variants (rs372790354 G>A and rs4889 G>A) on kisspeptin in patients with polycystic ovary syndrome in Iraq. Journal of Advanced Biotechnology and Experimental Therapeutics. 2022; 5(3): 562-576.
- [35]De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G and Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016; 14(1): 1-17.
- [36]Nath CK, Barman B, Das A, Rajkhowa P, Baruah P, Baruah M, et al. Prolactin and thyroid stimulating hormone affecting the pattern of LH/FSH secretion in patients with polycystic ovary syndrome: A hospital-based study from North East India. J Family Med Prim Care. 2019; 8(1): 256-260.
- [37]Malini NA and Roy George K. Evaluation of different ranges of LH:FSH ratios in polycystic ovarian syndrome (PCOS) – Clinical based case control study. Gen Comp Endocrinol. 2018; 260: 51-57.
- [38]Mitrašinović-Brulić M, Buljan M and Suljević D. Association of LH/FSH ratio with menstrual cycle regularity and clinical features of patients with polycystic ovary syndrome. Middle East Fertility Society Journal. 2021; 26(1): 1-9.
- [39]Nettleton D. Selection of variables and factor derivation. In: Commercial Data Mining, 2014, pp 79-104.
- [40]Morte C, Celma C, De Geyter C, Urbancsek J, Coroleu Lletget B and Cometti B. Assessment of the immunogenicity of gonadotrophins during controlled ovarian stimulation. American Journal of Reproductive Immunology. 2017; 78(3): e12675.
- [41]Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015; 76(7): e857-61.
- [42]Kim JJ, Hwang KR, Shin S, Yoon JH, Kim BJ, Choi YM, et al. Association of polycystic ovarian syndrome with human leukocyte antigen polymorphism in Korean women. Apmis. 2011; 119(9): 618-625.
- [43]Ahn S, Choi HB and Kim TG. HLA and Disease Associations in Koreans. Immune Netw. 2011; 11(6): 324-35.
- [44]Kaibe M, Takakuwa K, Murakawa H, Ishii K, Tamura M and Tanaka K. Studies on the human leukocyte antigens in patients with polycystic ovary syndrome in a Japanese population–possible susceptibility of HLA-A11 and -DRB1*0403 to patient population with polycystic ovary syndrome. Am J Reprod Immunol. 2006; 55(4): 301-6.
- [45]Haller K, Salumets A and Uibo R. Anti-FSH antibodies associate with poor outcome of ovarian stimulation in IVF. Reprod Biomed Online. 2008; 16(3): 350-5.
- [46]Thomas JW. Antigen-specific responses in autoimmunity and tolerance. Immunol Res. 2001; 23(2-3): 235-44.
- [47]Haller K, Mathieu C, Rull K, Matt K, Béné MC and Uibo R. IgG, IgA and IgM antibodies against FSH: serological markers of pathogenic autoimmunity or of normal immunoregulation? Am J Reprod Immunol. 2005; 54(5): 262-9.
- [48]Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Frontiers in immunology. 2017; 8: 603.
- [49]Doi SA and Williams GM. Methods of clinical epidemiology. Springer Series on Epidemiology and Public Health, Springer, 2013.
- [50]Habibzadeh F, Habibzadeh P and Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochemia medica. 2016; 26(3): 297-307.
- [51]Elkholy SS, Mostafa RA, Riad AA and AbouZaghla HM. Assessment of vitamin D levels in women with polycystic ovarian syndrome. The Egyptian Journal of Hospital Medicine. 2018; 70(4): 594-600.
- [52]Khalifa OM, ELgarhy ET, Alomda FA-e and Yehia MB. Assessment of serum vitamin D level in women with polycystic ovary syndrome. Al-Azhar International Medical Journal. 2021; 2(11): 43-48.
- [53]Shan C, Zhu YC, Yu J, Zhang Y, Wang YY, Lu N, et al. Low Serum 25-Hydroxyvitamin D Levels Are Associated With Hyperandrogenemia in Polycystic Ovary Syndrome: A Cross-Sectional Study. Front Endocrinol (Lausanne). 2022; 13: 894935.
- [54]Lin MW and Wu MH. The role of vitamin D in polycystic ovary syndrome. Indian J Med Res. 2015; 142(3): 238-40.
- [55]Trummer C, Pilz S, Schwetz V, Obermayer-Pietsch B and Lerchbaum E. Vitamin D, PCOS and androgens in men: a systematic review. Endocrine Connections. 2018; 7(3): R95-R113.
- [56]Miao CY, Fang XJ, Chen Y and Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. 2020; 19(4): 2641-2649.
- [57]Colaris MJ, van der Hulst RR and Tervaert JWC. Vitamin D deficiency as a risk factor for the development of autoantibodies in patients with ASIA and silicone breast implants: a cohort study and review of the literature. Clinical rheumatology. 2017; 36(5): 981-993.