Inhibitory effects of synbiotics on biofilm of uropathogenic Escherichia coli during urinary tract infection
Abstract
Uropathogenic Escherichia coli (UPEC) is a fundamental cause of nosocomial urinary tract infection (UTI). UPEC can form biofilms on the mucosa of the urinary bladder and the surface of urethral catheters, thus causing clinical problems. Therefore, the current study aimed at the potential of synbiotics to manage biofilm-associated UTIs by analyzing biofilm inhibition UPEC with cell-free supernatant (CFS) of synbiotics. Biofilm inhibition was accomplished by inoculating each microbial suspension into 96-well microplates on tryptic soy broth medium at 37°C for 48 h and a microtiter plate reader was used at 595 nm to read the OD value. The outcome (%) was calculated from the OD value of CFS-treated with UPEC. The result of this research was that each CFS of synbiotic treatment displayed significantly different (P<0.05) results and was able to inhibit UPEC biofilm. The highest percentage of biofilm inhibition of UPEC was shown in CFS 8 treatment with a value of 41.51 ± 0.687, where CFS of synbiotic from Lactobacillus rhamnosus bacteria with 2% inulin. The lowest percentage of biofilm inhibition UPEC was shown in CFS 1 treatment with a value of 36.56 ± 1.987, where CFS from Lactobacillus plantarum bacteria with 0% inulin. It could be concluded that the higher the concentration of inulin in the CFS of synbiotics, the higher the percentage value of biofilm inhibition on UPEC, which indicates the potentials to manage or prevent UPEC-induced biofilm infection.
INTRODUCTION
Urinary tract infection (UTI)-associated microbes are one of the most prevalent matters in young and older women, and infections that connect to the kidneys, ureters, bladder, and urethra. That may be infected within its system [1]. Uropathogenic Escherichia coli (UPEC) is a fundamental cause of UTI both in a community setting and in a hospital and represents significant morbidity and mortality worldwide [2]. UPEC forms microcolonies, so-called biofilms, on the surface catheter of the urethral and the bladder mucosa [3]. The biofilm formation significantly interferes with UTI treatment by guarding encased microbes against antimicrobial therapy and host immune responses. Moreover, the close association of microbes allows easy transfer of resistance determinants between the microbes in the biofilm [3,4]. Biofilm defends microbes by placing itself to beneficially use available nutrients and block access to antimicrobial agents [5], increasing resistance to a drug, then UTIs becoming more challenging to treat [3,4].
Non-antibiotic treatments have gained recognition lately due to the advent and spread of new antibiotic-resistant isolates [3]. Recently, probiotics with probiotic-based functional foods have been overgrowing, mainly because of their enormous health potential. Inulin is one of the prebiotics, a non-digestible food ingredient related to probiotics, as they positively influence the host by selectively stimulating the growth of beneficial microbe and inhibiting the growth of harmful microbe [6].
Synbiotics are a combination of probiotics and prebiotics that enhances the extant of living microbes by fastidiously stimulating the growth and initiating the metabolism of one or various beneficial microbe, thus boosting host welfare [7]. In previous studies, Staphylococcus aureus and Escherichia coli are inhibited by synbiotics [6]. However, no reports described synbiotics as biofilm inhibition UPEC that causes UTI. This study aimed to evaluate the biofilm inhibition of UPEC with synbiotics.
MATERIAL AND METHODS
Ethical consent
This study earned ethical consent from Faculty of Dental Medicine Health Research Ethical Clearance Univeritas Airlangga, Commission on April 18, 2022, with authentication reference number: 171/HRECC.FODM/IV/2022.
Microbes and growth circumstance
Probiotics (Lactobacillus plantarum and Lactobacillus rhamnosus) were obtained from stock cultures from the University of Surabaya, which were previously isolated from kefir grains, and stored at -20ºC with containing 20% (v/v) glycerol in the Man Rogosa and Sharpe (MRS) Broth medium (HIMEDIA, India).
For re-culture probiotic strains were grown onto the medium of MRS Agar slant (HIMEDIA, India). Then the gram stain and litmus test were carried out for confirmation the species of microorganism. Probiotic strains were inoculated into 5mL medium of MRS Broth (HIMEDIA, India) and incubated at 37°C for 24 h. Afterward, the culture is ready to be used to make cell-free supernatant.
UPEC was obtained from samples (urine) of patients with urinary tract infections referred to Dr. Soetomo hospital, Indonesia. UPEC strains were cultured on the medium of MacConkey agar (OXOID, UK) and incubated at 37°C for 24 h. Biochemical tests were carried out to identify isolates. Escherichia coli ATCC 25922 was purchased from the microbiology laboratory of Dr. Soetomo hospital, Indonesia, and used as a positive control/reference strain.
Media arrangement
In the present study, the reconstituted media for probiotic strain (MRS Broth) were prepared according to their composition. MRS Broth (HIMEDIA, India) mixed with inulin (ORAFTI®GR: Beneo, Germany) at 0%; 05%; 1.0%; 2% before autoclaved at 121ºC for 15 minutes [8].
After UPEC and E. coli ATCC 25922 were cultured and incubated. All E. coli was suspended to get turbidity of 0.5 McFarland by inoculating each E. coli strain to a medium of Tryptic Soy Broth (TSB) (OXOID, UK) in 5mL and incubated with the aerobic condition [9].
Cell-free supernatant
106 CFU/mL of probiotics strains (L. plantarum and L. rhamnosus) were inoculated into 15 mL of MRS Broth medium (HIMEDIA, India) containing various concentrations of inulin (0%, 0.5%, 1%, and 2%) and incubated at 37°C for 24 h. Then it was centrifuged for 10 minutes at 3000 rpm and using syringe filters (0.22 mm pore size) to filter the cell-free supernatants (CFS) [6].
Biofilm formation by pathogenic microbes
Biofilm formation of each pathogen microbes was evaluated in a 96-well microtiter plate (NEST, China). Shortly, 100 μL of diluted suspension pathogenic microbes (UPEC isolates or E. coli ATCC 25922) was pipette into the well of the microtiter plate and then incubated at 37°C for 48 h. Discard the solution's filling in the microtiter plate, wash the microtiter plate three times with 300 μL of Phosphate-Buffer Saline (PBS) solution, then dry. Pipette 150 µL of the crystal violet (CV) solution into the well and wait 15 minutes for the solution to be absorbed into the cells. Pipette back the solution, rinse the remaining coloring using water and then leave it to dry. In the final step, pipette 150 methanol into the well, then read the OD value in the well with a microtiter plate reader at 595 nm (Thermo scientific TM Multiskan TM GO) [10–12]. The following formula [9,13] :
ODcut-off = W¯OD control + 3SD control,
OD isolate = W¯ OD treatment – ODc.
Interpretation of OD isolate value was categorized into 4 groups as follows:
OD isolate ≤ ODcut-off (0) NBF
OD cut-off < OD isolate ≤ 2 x ODc (+) WBF
2 x OD cut-off < OD isolate ≤ 4 x OD cut-off (++) MBF
4 x OD cut-off< OD isolate (+++) HBF
Where NBF (non-biofilm-forming), WBF (weak-biofilm-forming), MBF (moderate-biofilm-forming), HBF (high-biofilm-forming).
Biofilm inhibition by CFS
The activity to biofilm inhibition was performed by inoculating the suspension of each microbial into a 96-well microtiter plate (NEST, China). In this study, we used ten treatments, and each well of treatment was repeated four times. The first well was used for the control medium group (having a content of 100 μL medium of TSB). Three well were used for pathogen microbes (have content 100 μL of diluted suspension UPEC), four wells were used for the treatment of CFS (1-4) synbiotic (have content 100 μL of diluted suspension UPEC and 100 μL of CFS), four wells used for the treatment of CFS (5-8) synbiotic (have content 100 μL of diluted suspension UPEC and 100 μL of CFS). Afterward, enclose the microtiter plate with sealing wraps and incubate at 37°C for 48 h. The next step was determined, as explained earlier. The percentage of biofilm inhibition (%) was calculated concerning untreated control using the following formula reported previously [14].
Statistical analysis
In this research, each well of the microplate was treated ten times, and each treatment was repeated four times. Each data result is displayed as the mean ± standard deviation (SD). The results of the data acquired from biofilm formation (OD) and the percentage of biofilm inhibition (%) were analyzed with the SPSS software application version 26.0 for differences using one-way ANOVA with Tukey's post hoc follow-up test (P-value <0.05 considered significantly different) with a 95% confidence level.
RESULTS
Effect of treatments on biofilm
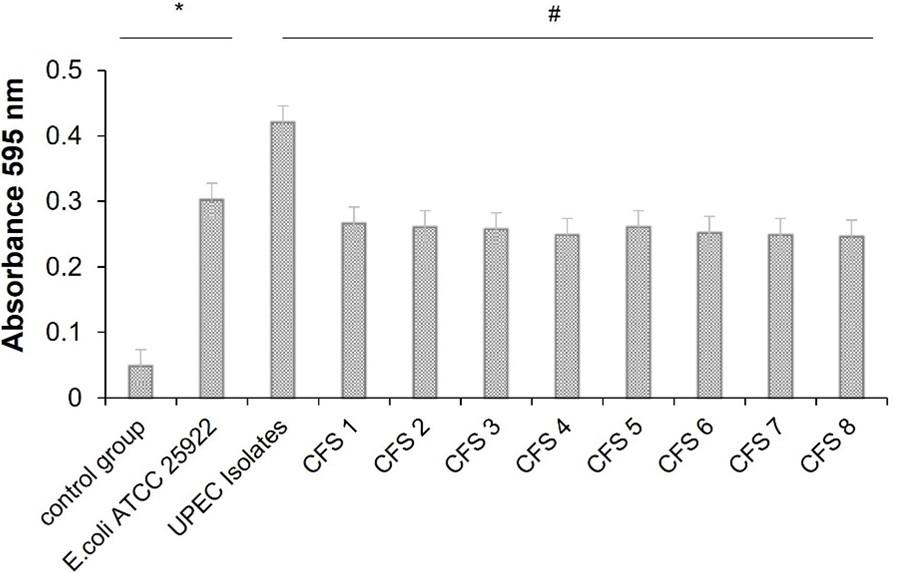
In this research, the biofilm formation test and biofilm inhibition test were carried out at the same time. Each treatment was performed by inoculating each microbe (UPEC or E. coli ATCC 25922) in each treatment in to 96-well microplates then incubating it for 48 and performing a microtiter plate reader reading at 595 nm by measuring total biomass (OD) after crystal violet staining. In addition, the biofilm inhibition test added inhibitory compounds from CFS of synbiotic or called CFS treatment. The OD value obtained was calculated as percentage biofilm inhibition (compared with untreated controls). The data showed that biofilm was formed in each treatment (UPEC, E. coli ATCC 25922, and CFS treatment), except in the control group.
According to the calculation of results, the OD of the isolates is four times higher and significant (0.057), indicating that the OD of the isolate is included in the high biofilm performing (HBF) category. Each treatment (UPEC, E. coli ATCC 25922, and CFS treatment) showed statistically significant (P<0.05) difference (Table 1). The formation of biofilm in the UPEC microbial treatment showed the highest value and CFS 8 treatment showed the lowest value (Table 1).
According to the data on the results of total biomass (Table 1), the difference in OD values from the treatments is shown in the graph (Figure 1). The one-way ANOVA statistical test displayed a significant difference (P<0.05) in biofilm treatments (Figure 1). There was a significant higher OD value in UPEC isolates while CFS treatments showed a significantly lower OD value (Figure 1).
Table 1. Effect of treatments on total biomass and percentage of biofilm inhibition.
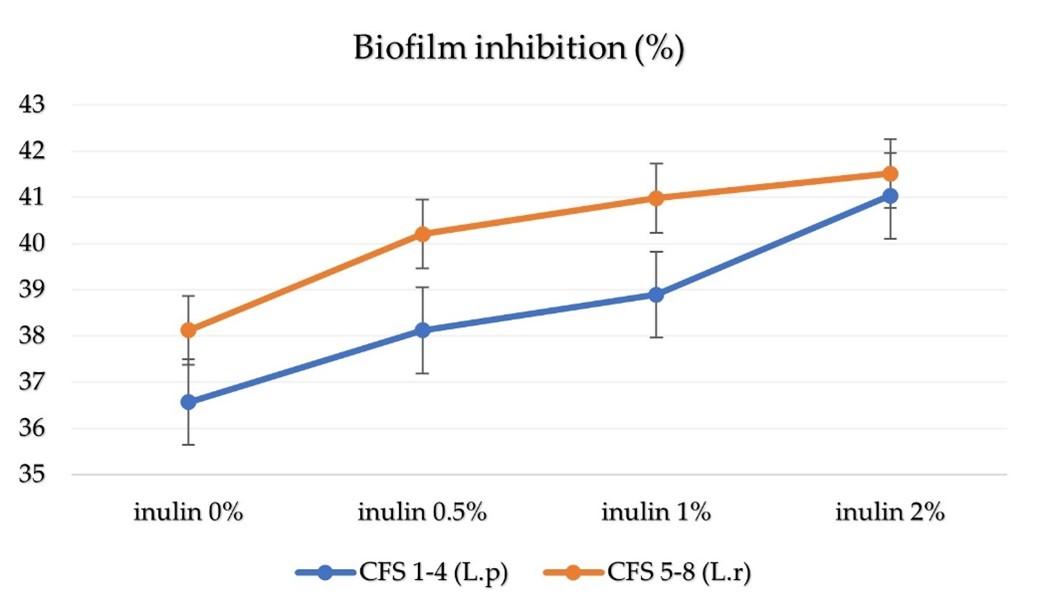
The data generated by each treatment on biofilm inhibition showed as mean ± SD and statistically significant (P<0.05). The higher the concentration of inulin in the CFS of synbiotics showed the higher the percentage of biofilm inhibition on UPEC. According on the data on the result of biofilm inhibition (Table 1), the percentage of the CFS treatment is a calculation of OD of the CFS treatment (treated sample) compared to the OD from UPEC isolates (untreated sample). The difference percentage in data value from each treatment is shown in the graph (Figure 2), with the highest data value CFS 8 treatment and the lowest data value CFS 1 treatment. The one-way ANOVA statistical test showed difference in biofilm inhibition in treatments (Figure 2).
DISCUSSION
In this research, in vitro studies for the treatment of biofilm formation and biofilm inhibition gave significant results. This research used the microtiter plate assay method with crystal violet (CV) solution to analyze biofilm formation and inhibition. CV binds to negatively charges surface molecules from living and dead cells and quantifies adherent biofilm biomass (exopolysaccharide) within the wells of the microtiter plate [15–17]. In addition, biofilm inhibition is assessed by adding a compound to be tested to inhibit biofilm formation at the same time as the bacterial suspension and then incubating within the specified time limit, followed by quantifying adhered biomass with CV can calculate percentage biofilm inhibition (compared with untreated controls) [17].
In research on in vitro biofilm formation, each treatment in this study showed the ability to form biofilms. This research showed that the treatment of UPEC and E.coli ATCC 25922 provides the highest value in biofilm formation. The ability of these bacteria to attach to surfaces is the cause of biofilm formation. Intrinsic factors such as fiber, adhesive proteins, and exopolysaccharide molecules can influence the adherence of these strains. Once attached, the bacterium produces an extracellular matrix (ECM) while replicating in a sessile form. ECM encases the microbes in a micro-colony (biofilm) and causes UPEC and E.coli ATCC 25922 biofilms to form strongly [4].
Biofilms consist of sessile microbial forms that adhere to surfaces as aggregates and are found in an extracellular matrix. Microbes that can form biofilms can avoid harsh conditions such as antimicrobial treatment, which can affect the host's immune response [18]. Additional studies showed critical steps in bacterial biofilm formation: attachment of the bacteria to the surface, adhesion, proliferation and growth, maturation, and dispersal of the cell or detachment. Different genetic and environmental factors can regulate biofilm formation, that suggests bacterial mobility (activated by flagella and fimbriae), extracellular polysaccharides, signaling molecules, and cell membrane proteins play essential roles [19].
Fimbriae-activated bacterial mobility is necessary for microcolony formation, and adhesin and specific cell membrane proteins maintain a stable association between the bacteria and the substrate surface [19]. For UPEC to colonize to the surface cause these strains encode several virulence genes; P fimbriae (pap), type1-fimbriae (fim-H), afimbrial-adhesin1 (afa1), S-fimbriae (sfa), hemolysin (hly), cytotoxic-necrotizing-factor (cfn1), aerobactin [5,20]. The virulence gene in UPEC that plays the most role at the urinary tract level is Type 1-fimbriae. Other studies have shown that UPEC can form intracellular bacterial communities and is mainly related to the colonization and invasion mechanisms of the bladder epithelium [21,22].
Due to the inadequacy of these approaches, therapies for biofilm inhibitory activity on microbial pathogens are of great interest. Many studies have suggested that probiotic products can be used to avert the growth of microbes that causes urinary tract infections [9]. Probiotics as antibiofilm agents against UPEC have been investigated previously [3]. Even so, no documented report regarding the inhibitory activity of synbiotics (Lactobacillus sp with inulin) isolates previously from kefir grains against UPEC isolates that causes urinary tract infection is available.
In research on in vitro biofilm inhibition, each CFS of synbiotics treatment in this study showed the ability to inhibit UPEC biofilm. This study showed that CFS 8 treatment gave the highest inhibitory activity biofilm formation of UPEC. This result is due to interference with the pathogen quorum sensing system caused by probiotic bacteria from Lactobacillus sp, which produce different exometabolites EPS, reactive oxygen species (ROS), biosurfactants, and bacteriocins [23,24]. Each probiotic strain has different effects and mechanisms on its host. Probiotic mechanisms include producing exometabolites which can lead to a physiologically restrictive environment for pathogenic bacteria, competition for nutrients, and inhibition of quorum sensing (QS) [25].
Prebiotics are described as non-digestible food ingredients or compounds in food that have a beneficial impact. In this research, we used inulin to perform a combination with probiotics. Inulin is a carbohydrate group that can stimulate lactic acid bacteria selectively. Animal and human studies have shown that consuming prebiotics can decrease the population of pathogen microbes by Lactobacillus sp and Bifidobacterium sp [26]. Inulin's rapid excretion from the body into the urine means it may be used for drug delivery into the urinary tract [27].
Inulin stimulates growth activity and also be fermented by probiotics. Inulin has benefits as a food source for probiotic bacteria. Short-chain fatty acids (SCFA) such as propionate, butyrate, and acetate are produced from inulin fermented by probiotic bacteria. Inulin creates an acidic environment to inhibit infection from acid-sensitive pathogens. Other studies have shown that inulin supplementation increased the abundance of probiotic bacteria (Lactobacillus sp). Inulin-type fructan can increase the abundance of Lactobacillus sp and inhibit pathogen proliferation (Salmonella sp, E.coli) by producing SCFA, creating an acidic environment (low pH), increasing the immune response, and suppressing pathogenic microbial colonization [28]. Other research also mentions that the indirect antimicrobial effect of inulin can be caused by the production of discontinued products, such as bacteriocins and short fatty acid chains, which can reduce pathogens through a decrease in pH [8]. This research showed that synbiotics of CFS 1 and CFS 5 without the addition of inulin or 0% inulin were proven to inhibit UPEC biofilms. However, CFS of synbiotics with the addition of inulin concentration can further inhibit UPEC biofilms.
CONCLUSIONS
In the present study, CFS of synbiotics containing two probiotics (L. plantarum and L .rhamnosus) previously isolated from kefir grain was added to the prebiotic inulin with various concentrations. This study obtained the results of biofilm growth (EPEC, ATCC, and CFS treatment) and inhibition of biofilm by CFS treatment showed inhibitory activity on UPEC biofilms. The results obtained were calculated from the OD value of CFS treatment with UPEC. Each treatment of CFS showed a significantly different result. The data suggest that synbiotics may be a promising strategy for preventing and treating urinary tract infections caused by UPEC biofilms. However, identified at the molecular level for specific pathophysiological states and in vivo studies are necessary for future applications of these as synbiotics. However, further research is needed, such as molecular identification and in vivo tests for future applications as a synbiotic.
ACKNOWLEDGEMENT
We acknowledge to Universitas Airlangga Surabaya, Indonesia and Institute of Tropical Disease Surabaya, Indonesia.
AUTHOR CONTRIBUTIONS
MICP drafted the manuscript and conducted the research. MRW and RJS provided direction for this research idea, and ADWW, NW, and MVA helped revise this manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ziyadi S, Bastani P, Homayouni A, Mohammad-Alizadeh-Charandabi S, Mallah F. Probiotics and Usage in Urinary Tract Infection. Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion, Elsevier Inc.; 2016, p. 827–30.
- [2]Tabasi M, Asadi Karam MR, Habibi M, Yekaninejad MS, Bouzari S. Phenotypic Assays to Determine Virulence Factors of Uropathogenic Escherichia coli (UPEC) Isolates and their Correlation with Antibiotic Resistance Pattern. Osong Public Health Res Perspect 2015;6:261–8.
- [3]Ghane M, Babaeekhou L, Ketabi SS. Antibiofilm Activity of Kefir Probiotic Lactobacilli Against Uropathogenic Escherichia coli (UPEC). Avicenna J Med Biotechnol 2020;12:221–9.
- [4]Eberly AR, Floyd KA, Beebout CJ, Colling SJ, Fitzgerald MJ, Stratton CW, et al. Biofilm formation by uropathogenic escherichia coli is favored under oxygen conditions that mimic the bladder environment. Int J Mol Sci 2017;18.
- [5]Katongole P; Nalubega F; Florence NC; Asiimwe B; Andia I. Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infectious Disease 2020:20.
- [6]Stefania DM, Miranda P, Diana M, Claudia Z, Rita P, Donatella P. Antibiofilm and Antiadhesive Activities of Different Synbiotics. J Probiotics Health 2017;05.
- [7]Markowiak P, Ślizewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog 2018;10:1–20.
- [8]Kareem KY, Ling FH, Chwen LT, Foong OM, Anjas Asmara S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog 2014;6:1–7.
- [9]Gümüş D, Yüksek FK, Bilgin M, Camadan FD, Küçüker MA. In Vitro Effects of Various Probiotic Products on Growth and Biofilm Formation of Clinical UPEC Strains. Acta Biol Marisiensis 2020;3:5–14.
- [10]Stepanovic S, Vukovic D, Hola V, Bonaventura G di, Djukic S, Ruzicka F, et al. The Authors Printed in Denmark. All Rights Reserved Journal Compilation C 2007;115:891–900.
- [11]Leonhard M, Zatorska B, Moser D, Schneider-Stickler B. Growth Media for Mixed Multispecies Oropharyngeal Biofilm Compositions on Silicone. Biomed Res Int 2019;2019.
- [12]Bhandari S, Khadayat K, Poudel S, Shrestha S, Shrestha R, Devkota P, et al. Phytochemical analysis of medicinal plants of Nepal and their antibacterial and antibiofilm activities against uropathogenic Escherichia coli. BMC Complement Med Ther 2021;21.
- [13]Kırmusaoğlu S. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. IntechOpen 2019.
- [14]Terzić J, Stanković M, Stefanović O. Antibiofilm activity of selected plant species. Book of Proceedings: 1st International Conference on Chemo and BioInformatics, Institute for Information Technologies, University of Kragujevac; 2021, p. 280–3.
- [15]Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 2008;72:157–65.
- [16]Cruz CD, Shah S, Tammela P. Defining conditions for biofilm inhibition and eradication assays for Gram-positive clinical reference strains. BMC Microbiol 2018;18.
- [17]Haney EF, Trimble MJ, Hancock REW. Microtiter plate assays to assess antibiofilm activity against bacteria. Nat Protoc 2021;16:2615–32.
- [18]Mekky AF, Hassanein WA, Reda FM, Elsayed HM. Anti-biofilm potential of Lactobacillus plantarum Y3 culture and its cell-free supernatant against multidrug-resistant uropathogen Escherichia coli U12. Saudi J Biol Sci 2022;29:2989–97.
- [19]Nazir R, Zaffar MR, Amin I. Bacterial biofilms: The remarkable heterogeneous biological communities and nitrogen fixing microorganisms in lakes. Freshwater Microbiology: Perspectives of Bacterial Dynamics in Lake Ecosystems, Elsevier; 2019, p. 307–40.
- [20]Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol 2019;19.
- [21]Minardi D, d’Anzeo, Cantoro, Conti, Muzzonigro. Urinary tract infections in women: etiology and treatment options. Int J Gen Med 2011:333.
- [22]Spurbeck RR, Mobley HLT. Uropathogenic Escherichia coli. Escherichia coli: Pathotypes and Principles of Pathogenesis: Second Edition, Elsevier Inc.; 2013, p. 275–304.
- [23]Fijan S, Frauwallner A, Langerholc T, Krebs B, ter Haar JA, Heschl A, et al. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed Res Int 2019;2019.
- [24]Barzegari A, Kheyrolahzadeh K, Mahdi S, Khatibi H, Sharifi S, Memar MY, et al. The battle of probiotics and their derivatives against biofilms. Infect Drug Resist 2020;13:659–72.
- [25]Carvalho FM, Teixeira-Santos R, Mergulhão FJM, Gomes LC. Effect of lactobacillus plantarum biofilms on the adhesion of escherichia coli to urinary tract devices. Antibiotics 2021;10.
- [26]Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019;8
- [27]Barclay T, Ginic-Markovic M, Cooper P, Petrovsky N. Inulin-a versatile polysaccharide with multiple pharmaceutical and food chemical uses. J Excipients and Food Chem 2010;1:27–50.
- [28]Song J, Li Q, Everaert N, Liu R, Zheng M, Zhao G, et al. Dietary Inulin Supplementation Modulates Short-Chain Fatty Acid Levels and Cecum Microbiota Composition and Function in Chickens Infected with Salmonella. Front Microbiol 2020;11.