Path of organotin complexes: synthetic factors, mechanisms, and broad-spectrum biological influences
Abstract
The review of the literature demonstrated that the diverse properties of the organotin (IV) attributed to the various moieties contained inside the molecule account for the functions and utility of the organotin (IV) complexes. Furthermore, the capacity of organometallic compounds to stabilize complexes with unique stereochemistry is well documented. Due to their robust coordination chemistry, consistency, and varied molecular structures, these complexes exhibit a wide spectrum of biological activity. This article provides an overview of complexes’ arrangement and geometry, spectroscopic research, and physical, chemical, and biological properties. This review also focuses on recent developments in conventional chemistry, practical synthesis methods, and the diverse functions of organotin (IV) complexes.
INTRODUCTION
Organotin complexes comprise one of the most important sections of organometallic chemistry as they are able to exist in different forms regarding the structure and geometries [1]. Now it is generally accepted that when tin (IV) bound to suitable ligands, it architects the tetra, penta, hexa and hepta coordinated structures and distorted trigonal bipyramidal, tetrahedral, octahedral, and distorted octahedral geometries respectively [2–7]. However, it is also observed that the one oxidation state can be changed to other by varying and changing different reaction conditions. Thus, tin (II) compounds can be converted to tin (IV) compounds by thermal decomposition [8]. Besides the theoretical and structural interests, Organotin complexes are important and could be exploited to develop novel metal-based medications. [9–13]. Similarly, these compounds have an important function in a variety of fields and are widely utilized for their natal qualities like antibacterial, antitumor, antifungal, and antiviral [9,14,23,15–22]. Furthermore, organotin carboxylates were well established for their biological effects (more than organic compounds of any other metal). Sedaghat et al. developed various organotin (IV) compounds against various hematologic and solid malignancies in vitro [24]. Literature showed that organotin (IV) compounds with carboxylato and dithiocarbamato ligands have also been studied extensively because they showed high antileishmanial, antifungal antiurease, and antibacterial activities. It was also reported that some complexes exhibit tremendous activity even then the standard drug like di-n-octyl and di-tert-butyl derivatives of N’-(2-hydroxy-3-methoxybenzylidene)formohydrazide and its complexes showed great activity for antileishmanial then the Amphotericin B [25–27], Organotin (IV) carboxylates have been found to have the highest cytotoxic activity in these experiments. [28]. Similarly, Deya et al. prepared the other derivatives of Organotin (IV) compounds possessing high-promising anticancer activities against human cancer cell lines in vitro [29]. Several studies are accessible on the activeness of Organotin (IV) compounds for Intercalative mode of interaction with DNA, heterobimettalic DNA (deoxyribo nucleic acid) receptors, DNA condensation, binding, cleaving activity, and cytostatic activity. Ultimately these activities give the evidence for their anticancer activity along with other important biological applications [30–35]. Furthermore, extensive study on the redox activity and catalytic behavior towards the glutathione S-transferase in Zebrafish, ring opening polymerization and transesterification reactions has been done on large extent which provides improvement in characteristics of new material [34,36–40]. Numerous studies on certain amino acids as readily accessible bioactive and as a mediocre in peptide combination are now underway. Amino acids can function as bidentate ligands and build the Organotin (IV) compounds, which further enables the interchange and transport machineries to sense metal ions in the humanoid frame, as demonstrated by Garcia-Lopez et al. [37]. Tin (IV) oxides could be used as light emitting diodes, solar cell, transmetallating agents and can be converted to nanoparticles or cross-linked nanocomposite [36,41,42]. Similarly, organotin compounds derived from Schiff bases are also known to exhibit optical properties and known to enhance biological activities [43–45]. Beside all these, Organotin compounds exhibit some other tremendous properties viz. PVC stabilizes, protection coatings for ships, antifouling paint [46]. Organotins can also pollute the ecosystem [47–49]. This review will be useful in determining the structure as well physicochemical properties of organotin complexes, moreover the review will emphasize in relating to its abundant properties.
SYNTHESIS AND CHARACTERIZATION OF ORGANOTINS
Organotin complexes catch the eyes of many researchers due to their broad range applications in various fields since its pioneer [50–52]. They synthesize the number of complexes and established their structure at the hand of various advance spectroscopic techniques [53–57]. In 2020, Du et al. synthesized the many organotin complexes having formula [(R2Sn)2L(μ3-O)] n, [(R3Sn)2L]n, [(Ph3Sn)2L], [R2SnL(1,10-phen)]n, [(R2SnCl)2L(1,10-phen)2] (R = Me, n-Bu) from 1,4-naphthalenedicarboxylic acid. Further, they used X-ray crystallography, FT-IR (Fourier-transform infrared spectroscopy), NMR (Nuclear magnetic resonance), PXRD (Powder X-ray diffraction), and elemental analysis to determine the structure of the produced complexes. The presence of absorption band at 456–495 and 556–588 cm–1 confirmed the Sn–O and Sn–C bonding. Similarly, 119Sn NMR signal at -108.6 ppm affirmed the tetra–coordination which was also explained by X-ray crystallography. Observations that some compounds exhibit 2D network structure containing tetranuclear 26-membered macrocycles and tetraorganodistannoxane unit while other complexes exhibit the dinuclear tin monomer unit which constructed the 1D infinite chain via C-H···π and C-H···O interaction. These further can architect the 2D supramolecular structure. Further, authors can perform the mass spectroscopy fragmentation which will gave the more information about the structure of synthesized compounds [58].
Similarly, Antonenko et al. in 2020 prepared the two strings of organotin (IV) carboxylates which were based on the natural bile acids (cholanic) and phenolic antioxidants having formula (cholate)SnMe3, (lithocholate)SnPh3, (deoxycholate)SnPh3, (cholate)SnPh3, (R(CH2)2COO)2SnBu2, (R(CH2)2COO)2SnMe2, (RCOO)2SnBu2, (RCOO)2SnMe2, RCOOH and R(CH2)2COOH (R= 3,5-di-tert-butyl-4-hydroxyphenyl), respectively. Further, the authors build the structure using 13C, 1H X-ray diffraction analysis, NMR, elemental, and IR spectra. In the IR spectra the valent vibrations due to C=O of carboxyl group bonded to the tin atom appeared at the 1597-1706 cm_1. It was reported that in the solid state they exist as monomer while the carboxylate unit was coordinated via O-atom bidentately. Furthermore, it was revealed that in the monocrystals, the Sn atom exhibit the distorted octahedral geometry. Further. the 119Sn NMR can also be studied of this published work which will uncover the environment of tin [59].
In another study, Naz et al. formed the tri and diorganotin subordinates from 2,4-dichlorophenoxyacetic acid with formula R2SnL2 and R3SnL where R = Me, Bu, Oct and L = 2,4-dichlorophenoxyacetate. They characterized the synthesized complexes in solid state using 1H, 13C-NMR, X-ray crystallography, IR, and elemental analysis. Further, the bidenticity of the ligand having penta and hexa-coordinated arrangements encircling the Sn (IV) and the Sn-O and Sn-C bonding was confirmed by the FT-IR spectroscopy appeared at 440-485 and 540-575 cm-1. While the Lockart’s equation was applied to calculate the C-Sn-C angle values from the NMR data and reported at 114.7° and 144.8° which were in the agreement with 5-coordinated and 6-coordinated geometry. Further, the mass fragmentation can also be studied of this published article which can be more informative regarding the structures [60].
Similarly, Tarassoli et al. synthesized the organotin (IV) compounds by treating 2-amino-1-cyclopentene-1-carbodithioic acid (ACDA) with organotin (IV) chlorides (RnSnCl4−n, where n = 3, R = Ph and n = 2, R = Ph or nBu). The synthesized complexes were framed in formula [Bu2Sn (ACDA)2], [Ph3Sn (ACDA)], [Ph2Sn (ACDA)2] and [Ph2SnCl (ACDA)]. Further, they characterized all the complexes by using various advance spectroscopic techniques. In the IR spectra of complexes, the absence of absorption band in the range of 2550–2430 cm−1 due to S-H bond showed the coordination of ligand with Sn atom via this site. 119Sn NMR was used to determine the coordination number of Sn atom ligand-Sn-ligand angle which was five and 359.5(7) ° respectively. Furthermore, the X-ray crystallography confirmed the environment of the tin which was distorted trigonal bipyramid in some complexes whereas in other irregular octahedral. The coordination of ACDA as an anisobidentate ligand was also observed and it oriented itself such that it can make the NH···S intramolecular hydrogen bonding. Further, the spectroscopic study of this work can extend by exploring the 13C NMR of the synthesized compounds which will reveal the neighboring and coordination environment of the C-atom [61].
In a separate study, Dey et al. reacted the ligand viz. 3-(2-hydroxyphenylimino)-1-phenylbutan-1-one and diorganotin (IV) dichlorides to form the tin complexes with general formula R2Sn[Ph(O)C=CH-C(Me)=N–C6H4(O)] where R = Ph, Me using methanol as solvent in the presence of triethylamine. Various spectroscopic techniques were adopted to establish the structure of synthesized complexes like IR, 13C, 1H, 119Sn, 15N NMR spectra and elemental analyses. Further, single crystal X-ray diffraction confirms the structure of complexes and free ligand. It was observed that in monomeric tin atom adopted distorted trigonal–bipyramidal whereas distorted octahedral in case of dimeric when consisting of the intermolecular Sn-O bond (phenolic). Whereas. 119Sn NMR data revealed that tin atom was penta coordinated in solution. The authors, in future can also study the mass fragmentation pattern of these prepared compounds which will provide the more knowledge about structures of complexes [62].
Similarly, Tariq et al. synthesized the library of organotin (IV) carboxylate complexes with formula [Ph3SnL], [Me2SnL2], [n-Oct2SnL2], [n-Bu2SnL2], [n-Bu3SnL] and [Me3SnL] where L= 3-(4-fluorophenyl) acrylic acid. Further, all the complexes were attributed by NMR (1H, 13C), FT-IR, and single crystal analysis. IR spectra showed the absorption band at 443–467 cm–1 that confirmed the deprotonation had occurred when the ligand coordinated via the COO– group with di and triorganotin (IV) component. Further, the Δν value of νsym(COO)– and νasym(COO)– showed the binding mode of COO- i.e. bidentate. Furthermore, some complexes exhibit the octahedral geometry while some trigonal bipyramidal geometry which was revealed by the 2J[119/117Sn-1H] coupling constant value found at 86/82 and 66/62 Hz respectively. Further, it was assisted by the C-Sn-C bond angle measured from 1J[119Sn-13C] value by employing Lockhart equation and observed at 138.9° and 116.4° which was for the 6 and 5-coordinated geometry. All the findings were supported by single crystal analysis. This study further can be hold out by exploring their mass fragmentation pattern and molecular docking also which will be more useful [63].
In another study, Win et al. described the preparation of 4-coordinated mono- and tetranuclear organotin (IV) carboxylate complexes and characterized them by using micro- and gravimetric analysis, 1H, 13C, 119Sn-NMR, FTIR and X-ray crystallography. IR spectra showed the absence of HL absorption band in the case of complexes which indicated that the coordination occur via COO- component. 119Sn NMR signal resolved that triphenyltin (IV) complexes exhibit distorted trigonal bipyramid geometry and five-coordinated by showing signal at –180 to –260 ppm. While another complex showed a signal at –260.88 ppm confirming the penta-coordination of tin (IV) atom and its trans-trigonal bipyramid geometry. Exceptionally, X-ray crystallographic and NMR spectroscopic study manifested that in the crystallization acetone molecule had also participated. The authors can also use the UV-Vis spectroscopy which will be helpful in describing the electronic transition and exact geometry of the complexes [64].
Furthermore, Sirajuddin et al. prepared the fourteen organotin (IV) carboxylate complexes having peptide linkages in 2-(4-methoxy-2-nitrophenylcarbamoyl) benzoic acid). The structure was recognized by using single crystal X-ray techniques, FT-IR, and NMR (1H, 13C and 119Sn) elemental analyses. The coordination occurred by the oxygen atom of the carboxylate group, according to FT-IR measurements. 2J(119Sn-1H), 1J(119Sn-13C) NMR and θ values obtained from Lockhart's equation revealed the octahedral and trigonal bipyramidal geometry of the diorganotin (IV) and triorganotin (IV) derivatives. Whereas the Crystallographic data of triorganotin (IV) compounds uncovered that the tin possessed the distorted trigonal bipyramidal geometry. The IR data confirmed the bidentate nature of ligand by providing the ∆ν value which is <200cm-1. Further, single crystal XRD data was also in support with the IR data for ∆ν values by using the equation, ∆ν= 1818.1δr + 16.47(θOCO-120) + 66.8. Further, the mass spectrometry will be more helpful in elucidating the structure [65].
Similarly, Sirajuddin et al. in 2018 prepared the Organotin (IV) compounds of ligand and portrayed by CHN analysis, NMR, FTIR, and other spectrometry tools. X-ray crystallography data of single crystal showed that the three-alkyl group occupied the equatorial position and distorted trigonal bipyramidal arrangement around Sn. Further, the oxygen atom of carboxylate and amide were behaved as bridge linking the adjacent tin atoms with the trigonal plane defined three phenyl ipso-C atoms. Similarly, the trigonal bipyramidal geometry was confirmed by the τ-values i.e., 0.78, 0.77, 0.82 and 0.92. FT-IR spectra showed the unidentate nature of ligand by observing ∆ν at 265 cm-1. Mass spectral data was also in agreement with the proposed structure by giving m/z peak for M+ ion near to calculated. The study of the Mossbauer spectroscopy and magnetic susceptibility further will expand the area of published work [66].
In another study, Vieira et al. reacted 2-aminobenzoic acid with 1,3-cyclohexadione to prepare 2-(3-oxocyclohex-1-enyl) benzoic acid (HOBz). Further, these ligand treated with organotin chlorides to synthesize the [Ph3Sn (OBz)], [Bu3Sn (OBz)], [Me3Sn (OBz)], [Ph2Sn (OBz)O]2, [Bu2Sn(OBz)O]2 , and [Me2Sn(OBz)O]2 . Furthermore, the structure of all synthesized compounds was constructed using a variety of elemental and spectroscopic techniques. The FT-IR spectra revealed that the vibrations due to intramolecular hydrogen bonding between –C=O…. HOOC– was disappeared in complexes and a new band found in the range of 1492–1360 cm-1 and 1630–1590 cm-1 assigned to the symmetric and asymmetric vibrations of COO group. The difference value of the symmetric and asymmetric vibrations proved the monodentate nature of ligand. Further, the polymeric chain arrangement of the complexes were established by moving down the frequency range at 1658, 1652 and 1658 cm-1 because of –C=O..…..Sn intermolecular interaction. It was also supported by the X-ray crystallography. Further, crystallography study uncovers the perfect trigonal bipyramid geometry of the complexes by showing that tin atom was adjoined by two oxygen atoms and three methyl groups which occupied the axial and equatorial positions, respectively. The study of 1H NMR and Mossbauer spectra further can reveal the more structural information [67].
In separate study, Ólafsson et al. used the metathesis reaction of aliphatic organotin: R’nSnX4-n, and [R2P(O)CS2]-, where (R = Ph, Bz; R’ = Me, Et; X = Cl, Br) to synthesize a string of tin (IV) phosphinoyldithioformate complexes. The structure analysis was done by the combination of multinuclear NMR (1H, 13C, 31P and 119Sn), UV, and IR spectroscopy. IR spectroscopy revealed the bidentate mode of coordination via S, O atoms for the series of 2 and 3 complexes whereas, monodentate for first complex series via S-atom. Further, 13C NMR and 119Sn coupling constants determined the geometry of the complexes. In addition, Mossbauer spectra told the oxidation state of tin atom and showed the quadrupole splitting and comparable isomer shifts for the compounds in solid state. This published work further can be widen by studying the single crystal X-ray crystallography, mass spectrometry and magnetic susceptibility of the synthesized compounds [68].
In another study, Singh et al. prepared the novel streak of organotin (IV) complexes and schiff bases by using condensation reaction between 𝛼-amino acids (isoleucine, phenylalanine, glycine) and 1H-indole-2,3-dione, 5-chloro-1H-indole-2,3-dione. The values of spectral studies showed that the monobasic bidentate behavior of schiff base ligands which were coordinated with the dibutyltin (IV) in octahedral geometry having general formula [Bu2Sn(L)2]. Furthermore, the single crystal X-ray diffraction study can be done against this published report [69].
Recently, Abbas et al. reported novel Schiff base-derived organotin (IV) compounds by reacting 1, 3-bis [(1E)-1-(2-hydroxyphenyl) ethylidene] thiourea with diorganotin chlorides in methanol under stirring conditions. The structure of all the synthesized complexes along with Schiff base ligand was analyzed and they found that the ligand act as a tri-dentate in these complexes [53].
THERAPEUTIC POTENTIAL OF ORGANOTIN COMPLEXES
Organotin complexes are really interesting primarily due to maturing biological, pharmaceutical, and industrial properties [70,71]. They are cast-off as operative biological agent against several diseases. A survey has been done on the application part of organotin complexes and summed up as follow:
Anti-cancer effects
In 2020, Du et al. found that the complexes with phenyl and n-butyl groups had the highest anticancer activity than complexes with methyl groups. Further, it was also observed that the prepared complexes displayed less cytotoxicity activity then the analogous organotin (IV) precursors [triphenyltin chloride and trimethyltin chloride, bis(tri-n-butyltin) oxide] for HBL-100 cells. Moreover, some complexes adopted the ROS (Reactive oxygen species)-mediated pathway to show its apoptotic and cytostatic effect against the HepG-2 cells. Further, the reported work can be broadening by screening the anti-microbial activity of synthesized compounds [58].
In another study Win et al. prepared the two 4-coordinated mono and tetra nuclear organotin carboxylate complexes. The authors examined the prepared complexes against one non-cancerous (3T3-L1) and three cancerous (breast cancer MCF 7, leukemia K562, and colon cancer HCT 116) cell lines to measure their anti-cancer activity. Further, the IC50 values of both the complexes were figured out in nanomoles and found that complexes were more potent for K562 and MCF 7 cell lines even than the standard drug viz. cis-platin, betulinic acid, 5-FU and Tamoxifen. While the complexes were mild toxic against 3T3-L1 (normal cells). Whereas the IC50 value of both the complexes were same for HCT 116 while for the other K562, MCF 7 and normal cells, there was difference in results of both the complexes. Anti-microbial and DNA-photocleavage activity can be studying to broaden the reported work [64].
Similarly, Adeyemi et al. synthesized and characterized the many organotin (IV) compounds coming from N-butyl-N-phenyldithiocarbamate, represented as (C6H5)2SnL2], (C4H9)2SnL2], (CH3)2SnL2] and [C4H9SnL2], where L = N-butyl-N-phenyldithiocarbamate. The complexes possessed five and six coordination geometry, was uncovered by the Single crystal X-ray study. Further, cytotoxic activities of the complexes were evaluated towards human cervical carcinoma (HeLa) cells by using MTT assay in-vitro. The relationship between structure and activity has also been investigated in this reported study. The viability of cell and IC50 value of the complexes has been measured at different concentrations and it was found that the compound showed concentration dependent activity against the cell lines. Furthermore, it was confirmed that the di-phenyl tin (IV) complex exhibits the least value as compared to the other complexes as well as the stranded drug used (5-fluorouracil). Whereas the butyl and di-methyl tin (IV) complexes possessed good cytotoxicity against the HeLa cell lines even then the standard drug. However, di- butyl tin (IV) complex showed lesser cytotoxicity and apical IC50 value than the other complexes. In this study, researchers described that the complexes with large alkyl group exhibit greater cytotoxicity than with the shorter alkyl group. It was observed that, among all the complexes the butyl tin (IV) complex carried increased lipophilicity and easily inserted into the base pairs of DNAs of cell lines because of the planar geometry caused by the diphenyl group attached with tin center. The anti-cancer action of the synthesized compound further can be screened against other cell linings like breast cancer cells, hepatocellular carcinoma cell lines, leukemia, and colon cancer [72].
The key trait of the cancerous cells is the uncontrolled cell division. The organotin compounds can have ability to inhibit the function of cyclin dependent kinase and many other proteins which regulates the cell cycle. In human’s ovarian cancer cells, anticancer agents arrested the cell cycle at G0/G1 phase in OV90 and ES2 cell lines and lead the cell to apoptosis also. However, in HCT-116, the agents arrested the cell cycle at G0/G1 phase by downregulating the cyclin D1 and in HepG2 cancerous cells also. Similarly, in lung cancer cells, it arrests the cell cycle at G1 phase by altering the cyclin D, p21, and cyclin A expression. While in DU145 and PC-3 cells, these compounds have the ability to seize the cells at phase G0/G1, via decreasing the D1/CDK4 and AKT/cyclin expression. Furthermore, if the compounds are capable of arresting the cell cycle in G0 or G1 phase and whether the compounds are capable of arresting the cell cycle at S and G2/M phase may be determined. [73–76].
Regulating oxidative stress
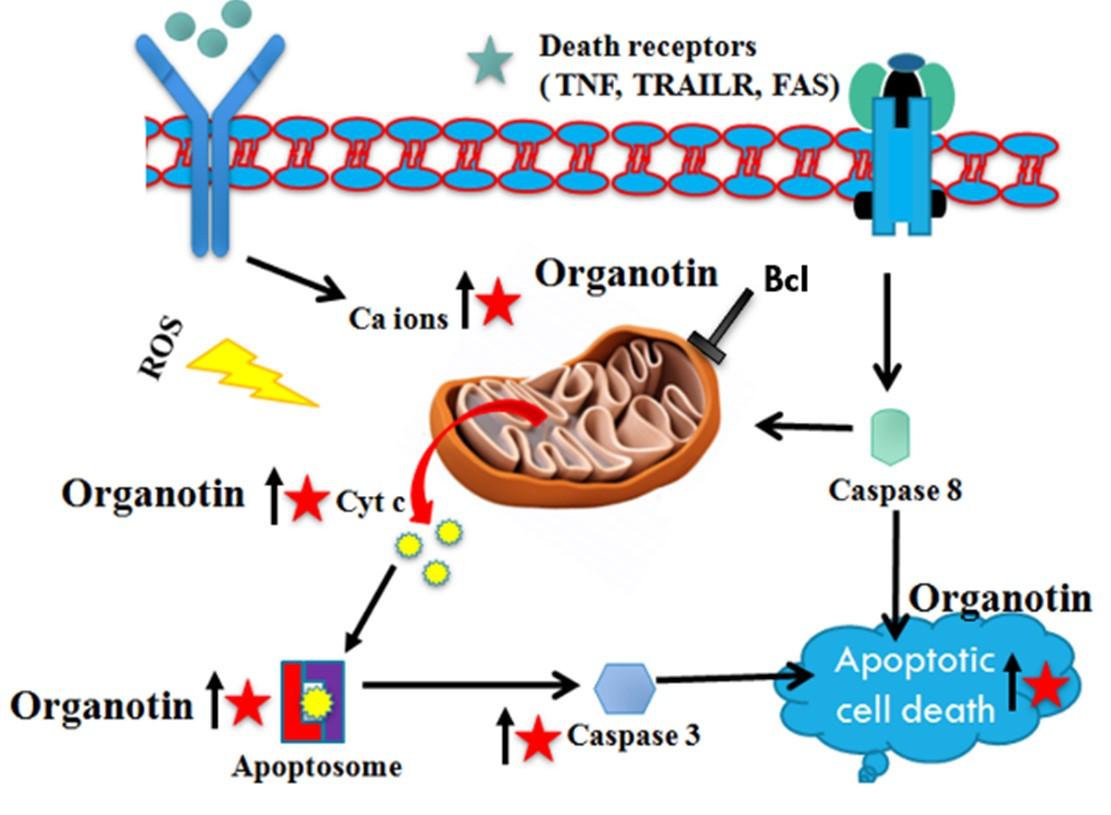
Oxidative stress enhanced the drug resistance, disrupts cell death signaling, angiogenesis, metastasis, survival, cell proliferation, and generation of reactive oxygen species [77,78]. ROS is playing a potential role in many cellular activities like in elimination of foreign pathogens and particles, enzyme regulation, differentiation, proliferation, cell survival and gene expression. Furthermore, ROS also helping the tumor cell to grow but this property of ROS nowadays is used in the cancer therapy as revealed by recent study. Many experiments uncovered that the exogenous ROS generation have been enhanced by the phytochemicals above the threshold level which further reduced the MMP and kills the cancerous cells selectively. The organotin compounds can induce the apoptosis and inhibit the proliferation in SMMC7721 cells via oxidative stress by MMP dissipation and ROS production. Organotin compounds can induce the apoptosis in human mental cell lymphoma (MCL) Z138,51, MDA-MB-231, SMMC7721,43 cell lines and HT-29 colon cancer cells via the intrinsic apoptosis pathway. This pathway considers the downregulation of Bcl-2 which did the Bax upregulation and enhance the mitochondrial permeability for apoptosis inducing factor (AIF) which was released in the cytosol by the mitochondria, caspase-3, -7 and -9 were activated by the AIF present in the cytosol which further target the DNA fragmentation and induce apoptosis ultimately as given in Figure 1 [79–81]. Oxidative stress also helps in the activation of intrinsic apoptosis pathway as given in Figure 1.
Further, metal mediated generation of free radicals increased the lipid peroxidation and alteration in the DNA bases [82]. The antioxidant enzymes and non-enzymatic antioxidants reduced the effect of ROS and reactive nitrogen species (RNS). Further, ROS were inactivated under the specific low concentration by these antioxidants which cause the inhibition in oxidative processes by hindering the radical chain reaction at both molecular and cellular level. Chelation of metal ion with the antioxidant was accountable to produce ROS which have the potential to operate in both aquas as well as membrane domains. This chelated complex has ability to penetrate the cell and make the complex with metallothionein and other proteins which enhances the elimination of metals without undergoing in other organs. However, this chelation therapy may cause various side effects mainly in the loss of essential elements because they showed the binding affinity towards all the positively charged ions. Therefore, it is necessity to develop the effective and safe chelating treatment having less side effect [83].
Anti-bacterial effects
In a recent study, Abbas et al. [13] reported the anti-microbial (bacterial and fungal) inhibitory action of diorganotin complexes of quercetin and biguanide (Schiff base ligand). Serial dilution and the agar well diffusion technique was used to assess the activity. Gram-positive species like Bacillus subtills (MTCC 1133) and Staphylococcus aureus (MTCC 9760) as well as Gram-negative strains like Pseudomonas aeruginosa (MTCC 9048) and Escherichia coli (MTCC 589) were used to test the antibacterial activity. While Aspergillus niger (MTCC 9933) and Scopulariopsis canadensis (MTCC 567) were the targets of antifungal activity. According to the study, the double bonds between the bi-flavonoid moiety's 25th and 26th positions and 4th and 5th positions are what give these compounds their potent inhibitory effects.
In 2018, Naz et al. studied the anti-bacterial and DNA binding property of the synthesized complexes. The bacterial strains gram positive viz. Micrococcus Leuteus and Staphylococcus Aureus and gram-negative viz. Enterobacter aerogens and Escherichia coli were selected to determine the anti-bacterial action of the synthesized complexes using disc diffusion method. It was observed that all the compounds were active against all the strains and Kanamycin was used as standard. Out of all the series Bu3SnL had shown the highest activity against Micrococcus luteus having MIC 53.0 mm. Further, the anti-angiogenesis, anti-malarial and Antileishmanial activity of the prepared compounds can be studied [60].
Similarly, Tariq et al. used the well diffusion method to investigate the antibacterial activity of all the produced compounds. The two-gram positive and six-gram negative bacterial strains were selected to determine the activity viz Staphylococcus aureus, Micrococcus luteus, and Klebsiella pneumoniae, Bordetella bronchiseptica, Enterobacter aerogenes, and Escherichia coli, respectively. Roxyithromycin and Cefixime were given as standard drug and it was found that all the compounds were active against the selected strains. The criteria used by authors to measure the anti-bacterial activity were based on the Zone of Inhibition in mm. Further, some compounds showed the activity more than 20 mm and were considered as excellent agent even more than the selected standard drug. While the compounds showed below 20 mm were considered as good anti-bacterial agent. The other biological activities of the synthesized compounds like anti-viral, anti-inflammatory and anti-malarial further can be explored [63].
In another study, Mahato et al. synthesized the mononuclear organotin (IV) compounds using two dithiocarbamato ligands (L1 and L2) which are thiomorpholine-4-carbodithiolate and 2,6-dimethylmorpholine-4-carbodithiolate respectively. Further, the structure of all the prepared ligand as well as complexes were analyzed by using FT-IR, 1H, 13C, 1H, 119Sn{1H} NMR, UV-visible spectroscopy, elemental and single-crystal X-ray diffraction analyses. Furthermore, the in vitro antibacterial properties of the complexes were screened against E. coli XL-1 Blue cells using agar disc-diffusion method. This biological activity was appraised by gauging the inhibition zones diameter neighboring the filter paper discs and DMSO (5%) was used as negative control. It was observed that the ligands get decomposed during incubation period at 37℃ for 24hrs, so the activity of ligands were not recorded but the complexes showed considerable activity against selected strain. Further, it was recorded that the activity was increased as the concentration of the dosage enhanced however the zone of inhibition of both ligand’s complexes was found nearly same at same concentration viz. at 130 µM the activity was ranges from 10.1 ± 0.14 to 10.75 ± 0.21 mm. The complexes possessed the lesser activity than the standard drug used (Kanamycin and Ampicillin) towards the selected microorganism. The anti-bacterial activity of the synthesized compounds further can be expanding against other species such as Staphylococcus aureus, Micrococcus luteus, Klebsiella pneumoniae, Bordetella bronchiseptica, and Enterobacter aerogenes [84].
Peptidoglycans with long sugar polymers make up the bacterial cell wall. Transglycosidase connected peptidoglycans to glycan strands and lengthened peptide chains from sugar in polymers from one peptide to the next. The presence of penicillin binding proteins (PBPs) causes cross-linking between the D-alanyl-alanine and glycine residues in the peptide chain. This cross-bonding provide strength to the cell wall and the glycopeptides and β‑lactams stops the cell wall synthesis [85–87].
Transcription is a process in which m-RNA is synthesized by using the information of bacterial DNA. Then the macromolecular structure of ribosome worked to synthesize the protein presented in m-RNA and the process is known as translation. Cytoplasmic factors and ribosomes are responsible for the biosynthesis of proteins. Both 30S and 50S ribonucleoprotein subunits collectively composed the 70S bacterial ribosome. Therefore, antibacterial agents target the synthesis of both ribonucleoprotein subunits and inhibit the protein biosynthesis [86].
Antileishmanial activity
Sirajuddin et al. in 2018, prepared a series of organotin (IV) carboxylate complexes and screened for antileishmanial property and reported that all the compounds significantly reduced the viable promastigotes and used amphotericin B as the standard drug having IC50 = 0.50 mg/mL and the maximum compounds possessed the IC50 = 1.26 μg/mL which was the less than half of standard drug. Whereas some compounds showed comparable activity to the selected standard drug. It was also found that the compounds exhibited activity by interfering function of parasite mitochondria. In this study, authors proved the synthesized compounds as the novel drug for medication of leishmaniasis. Further, the biological part of this reported study can be extended by investigating anti-angiogenesis, anti-oxidant and anti-diabetes property [65].
In another study, Sirajuddin et al. in 2019, synthesized the organotin (IV) compounds of carboxylate ligand. They screened all the complexes for Antileishmanial activity against the leishmanial major strain kwh 23 and significant reduction of viable promastigotes done by all the complexes were observed. Furthermore, it was found that NaL showed the very less antileishmanial activity in comparison to complexes and proved that the activity intensified after chelation with Sn atom. However, some complexes were more potent even than the standard drug which is amphotericin B. Authors reported that the complexes interfused the mitochondrial function of parasites to show their activity. Therefore, they can be used as a good candidate to treat the leishmaniasis. In future, the anti-viral, anti-oxidant and anti-inflammatory properties of the reported compounds can be examined [66].
The organotin compounds caused ultrastructural alterations in L. amazonensis promastigotes, mainly in plasma membrane and mitochondria after the treatment of 24h. The mitochondrial swelling, plasma membrane and storage-lipic body’s alterations were observed after 48h. After 72h, the mitochondrial swelling and flagellar pockets having vacuole were found with alterations. It was concluded from all the treatment hours that the organotin compounds induced the variation in cell division cycle and began the energy storage process (because of the stress) which led cells to the death. It was observed that from the SEM (Scanning Electron Microscopy) the treatment of 24, 48 and 72h provoked the reduction in cell volume and cell rounding [85,88,89].
Anti-fungal activity
Vieira et al. prepared the six organotin complexes by reacting 2-(3-oxocyclohex-1-enyl) benzoic acid (HOBz) and organotin chlorides. Further, all the complexes were screened for their anti-fungal activity against Cryptococcus neoformans and Candida albicans. Beside all these, it was observed that the total molecule surface (TSA) also affects the toxicity of the complexes hence the complexes of n-pentyl, n-butyl, n-propyl, etc, will be more toxic for the selected microorganisms than that of the ethyl, methyl. Therefore, the biological activity order suggested a close relation between lipophilicity and activity. Further, these reported compounds can be screened for their anti-bacterial, anti-diabetes and DNA-binding activities [67].
Similarly, Shaheen et al. synthesized the ligand by using diorganotin (IV) dichlorides and triorganotin (IV) chlorides with 4-(benzo[d] [1,3] dioxol-5-ylmethyl) piperazine-1-carbodithioate and a series of its derivatives having molecular formula R3SnL, R2SnLCl and R2SnL2 (where R = Ph, Bu, Me). Further, the characterization of the structure was compassed by 119Sn 13C and 1H NMR and FT-IR spectroscopy. Turbinafine (200 lg/mL) and DMSO were used as the positive and negative control while estimating antifungal activities. It was published that the activity of all the compounds were approximately equal to the reference drug used but the derivative is more active than the ligand against the selected fungal strains. Furthermore, it was reported that the phenyl and butyl derivatives were more potent than the respective methyl derivative. The activity related to the interaction of drug with cell or mitochondrial membrane has been also reported in this published study. Moreover, this study further can be explored by evaluating the DNA-photocleavage, anti-angiogenesis and anti-malarial activities [22].
The integrity, asymmetry and fluidity of the fungal membrane is regulated by ergosterol. The anti-fungal agents target the heme protein to co-catalyzes the lanosterol’s cytochrome P-450-dependent 14α-demethylation. The inhibition of 14α-demethylase result into the sterol precursors accumulation along with 14α-methylated sterols and ergosterol’s depletion which lead to the synthesis of plasma membrane having altered function and structure [90,91].
DNA binding effects
Mridula et al. synthesized the complexes of diorganotin (IV) having general formula [R2Sn(L)]2O and [R2Sn(L)]2Cl2 where R=Me, n-Bu, n-Oct, Ph and L=anion of mandelic acid using microwave-assisted reactions and conventional thermal method. Further, the distorted trigonal bipyramidal geometry of the complexes having Sn-O-Sn bridge were revealed by the various established spectral studies. The authors found that the complexes bound to DNA through the intercalative mode. The 10% DMSO/Tris-HCl buffer solution was used in the UV–Visible spectrophotometer to record the binding affinity of complexes with CT-DNA while the concentration of the complexes was kept constant and DNA concentration was varied from 0–28.83 μM. The reported compounds in future can be examined for their anti-microbial and Antileishmanial activity [92].
Similarly, Yin et al. prepared the six complexes of organotin (IV) by reacting the organotin (IV) chlorides and Schiff base ligands obtained from 4-aminobenzoic acid (or 3-aminobenzoic acid) and 2-hydroxy-1-naphthaldehyde. The stern-Volmer equation confirmed the intercalative manner of binding of DNA with complexes. The anti-microbial, and anti-oxidant properties of the synthesized compounds can be interpreted in future [93].
In a separate study, Nath et al. used microwave assisted method to synthesize the triphenyltin (IV) and diorganotin (IV) derivatives of L-proline and mixed ligand with 1,10-phenanthroline. The mixed ligands were binding more strongly as compared to the di- and triorganotin (IV) derivatives of L- proline. Further, the partial intercalative mode of binding has been confirmed by the change in viscosity and intensity of negative and positive bands in CD spectra as well as melting point of DNA. The authors can expand this study by investigating the anti-microbial properties [94].
There are different methods of interaction of drug molecules with DNA. There is no direct interaction between drug and DNA, but drug interact with the DNA binding proteins and alter the functioning. The drug bind to the RNA which leads to its binding with the single stranded DNA to form the DNA-RNA hybrid. This hybrid interferes the transcription activity. The small size aromatic ligands directly bound to the DNA double helix which is of different type like intercalative and groove binding.
The insertion of drug in between the base pairs of DNA molecule result into the opening of helix and lengthen the chain and stabilized by the π- π interaction between drug and DNA base pairs. This interaction results into the lengthening of the DNA chain to the 3 Å per molecule of drug binding and unwinding of DNA. The transcription and replication of DNA is altered by this binding and topoisomerase. The site and the arrangement of intercalation will measure the degree of unwinding of DNA. The topoisomerase, DNA and intercalated drug together create the ternary complex which is toxic for the proliferating cells. Hence, the intercalators are more fatal for the cancer cells rather than the normal cells [95].
Anti-inflammatory activity
Nath et al. synthesized the novel derivatives of non-electrolytic di- and tri-organotin (IV) compounds with general formula Ph3Sn (HL/H2L’), and R2Sn(L/HL’) where R = Ph, n-Bu and L/HL = dianion/monoanion of L-carnosine, and D-penicillamine and HL’/H2L’ = dianion/monoanion of triglycine. Further, the percentage of inhibition (anti-inflammatory activity) was evaluated using the dosage of 50mg/kg which was given orally against carrageenin induced edema on the adult albino rats (body weight 120-160 g) of Froster Charles species while the acute toxicity (ALD50) was investigated on albino mice with body weight 20-25 g of either sex. Anti-inflammatory activity of the complexes was compared with the standard drug phenyl butazone and it was observed that Ph3Sn (IV) derivatives showed the highest activity then the Ph2Sn (IV) derivatives against specific ligand. The study revealed that the presence of extra phenyl group in the equatorial position to the tin and lesser number of coordination number has facilitated the production of Ph3Sn+(IV) moiety as a part of inhibition. Whereas the di-n-butyltin (IV) complex showed the lowest activity because of the stronger interaction with tin which regulate the evolution of R2Sn2+(IV) moiety. This study can be expanded in future by exploring the anti-cancer, anti-tumor activities [96].
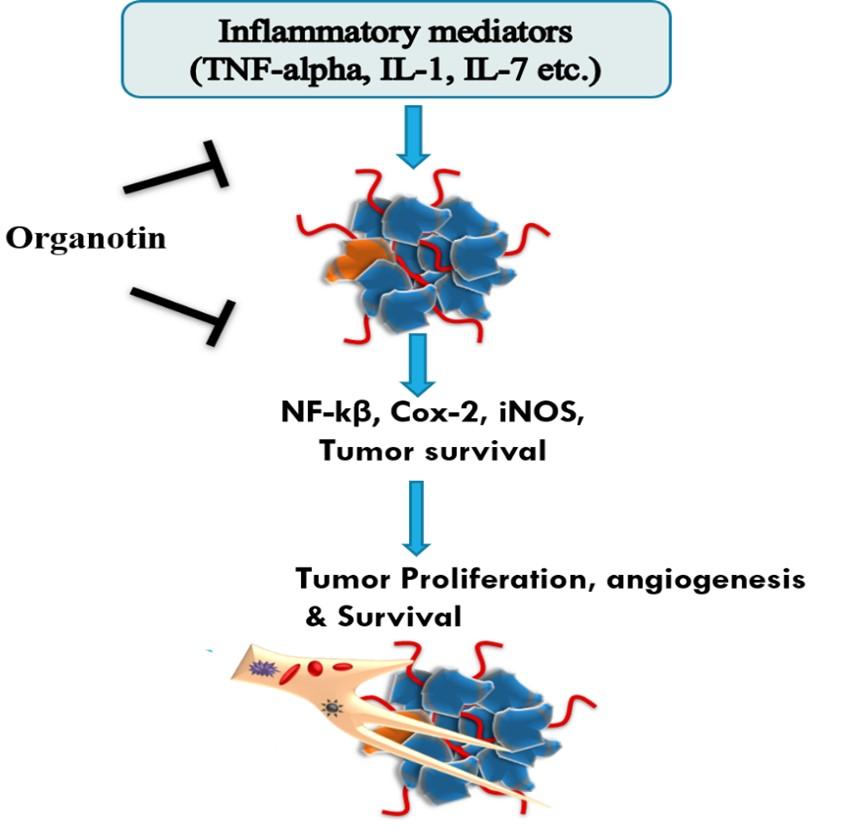
Non-inflammatory drugs act in various events of cell [97]. Some participated in inhibition of chemotaxis, suppression of superoxide and free radicals, calcium-mediated intracellular response, and downregulation of the production of IL-1. These drugs also inhibit the Arachidonic acid metabolism and regulate the Rho/Rho kinase pathway. Further, some drugs bound to the family member of peroxisome proliferator-activated receptor (PPAR) and some other intracellular receptors after that activate them. The activation of PPAR is believed to be cause for anti-inflammatory activities. It was also observed that these drugs enhance the heat shock protein (HSP) response and show the effect on COX pathway. The evidence of these mechanism is shown by the inhibition of nuclear factor-kappa beta (NF-κB) as shown in Figure 2 [97–100].
CONCLUSIONS
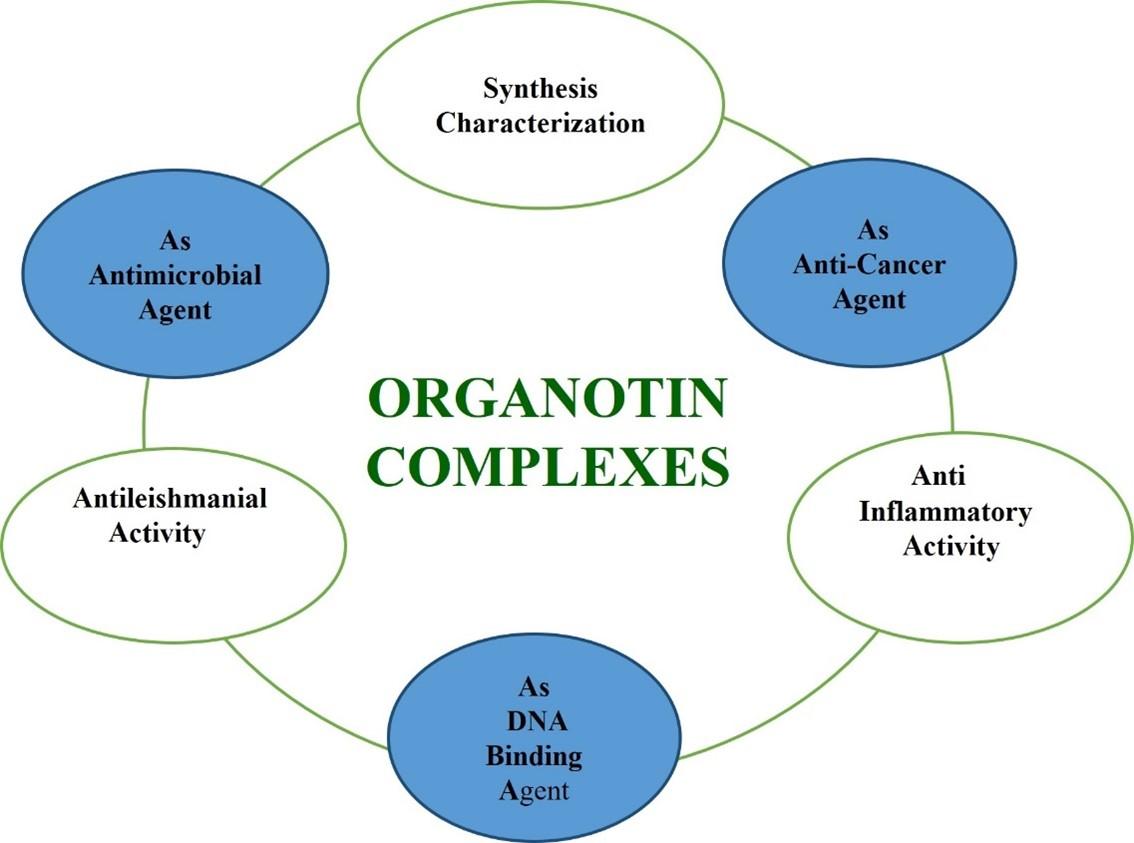
Organotin complexes are known to be therapeutically active mediators with promising worldwide recognition. The above deliberated text has proposed its antibacterial, antifungal, antineoplastic, and anti-inflammatory action. Therefore, organotin complexes have promising role in pharmaceutical sector to formulate novel formulations with broad spectrum medicinal applicability (Figure 3). These complexes' biological activity was demonstrated by their antifungal, antibacterial, and antileishmanial properties, and novel metal-based medications may be developed in the future.
In the future, derivatives of organotin with diverse ligands may open new doors to minimize death rate of dreadful malignancies. Further clinical trials are welcome to explore their wide use in near future. In addition, role of nanotherapeutic approaches should also be investigated by the scientific community to lower down the require dosages as well targeted delivery at the required site.
ACKNOWLEDGEMENT
We would like to acknowledge department of Biotechnology, Maharishi Markandeshwar (Deemed to be University), Mullana for the required support.
AUTHOR CONTRIBUTIONS
ZA, P, JS, MK: conceived the study; AR, SS, PA, and VKG: collected the data; HST: wrote the paper. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Ariza-Roldán A, López-Cardoso M, Tlahuext H, Vargas-Pineda G, Román-Bravo P, Acevedo-Quiroz M, et al. Synthesis, characterization, and biological evaluation of eight new organotin (IV) complexes derived from (1R, 2S) ephedrinedithiocarbamate ligand. Inorganica Chim Acta 2022;534:120810.
- [2]Lei B, Jiang J, Jia Y, Liu L, Chang W, Li J. Novel organotin-containing diblock copolymer with tunable nanostructures: Synthesis, self-assembly and morphological change. J Organomet Chem 2011;696:1416–24.
- [3]Rotar A, Varga RA, Jurkschat K, Silvestru C. Diorganotin(IV) compounds containing 2-(Et2NCH2)C6H4 moieties: Configurational stability in solution and solid state structures. J Organomet Chem 2009;694:1385–92.
- [4]Wen ZK, Xie YF, Zhao S Bin, Tan RY, Tang LF. Functionalized bis(pyrazol-1-yl)methanes by organotin halide on the methine carbon atom and their related reactions. J Organomet Chem 2008;693:1359–66.
- [5]Zima T, Bataev I. Morphology and phase transformations of tin oxide nanostructures synthesized by the hydrothermal method in the presence of dicarboxylic acids. J Solid State Chem 2016;243:282–9.
- [6]Yao M, Ding Y, Ma X, Deng Z, Zhong M, Yang Z. Synthesis and crystal structures of antimony(III) and tin(IV) compounds with an amino-amido-silane ligand. Inorganica Chim Acta 2017;455:271–5.
- [7]Shujah S, Zia-Ur-Rehman, Muhammad N, Ali S, Khalid N, Tahir MN. New dimeric and supramolecular organotin(IV) complexes with a tridentate schiff base as potential biocidal agents. J Organomet Chem 2011;696:2772–81.
- [8]Hong M, Yin HD, Chen SW, Wang DQ. Synthesis and structural characterization of organotin(IV) compounds derived from the self-assembly of hydrazone Schiff base series and various alkyltin salts. J Organomet Chem 2010;695:653–62.
- [9]Milaeva ER, Tyurin VY. Hybrid metal complexes with opposed biological modes of action - Promising selective drug candidates. Pure Appl. Chem., vol. 89, Walter de Gruyter GmbH; 2017, p. 1065–88.
- [10]Meyer N, Sivanathan S, Mohr F. Transfer of organic groups to gold using organotin compounds. J. Organomet. Chem., vol. 696, Elsevier; 2011, p. 1244–7.
- [11]Aggarwal P, Singh Tuli H, Kumar M. Novel Cyclic Schiff Base and Its Transition Metal Complexes: Synthesis, Spectral and Biological Investigations. Iran J Chem Chem Eng 2022;41:417–30.
- [12]Kumar M, Aggarwal P, Varol M, Sharma S, Rani A, Abbas Z, et al. Synthesis, Spectral Investigations, Biological Potential and Molecular Docking Study of Novel Schiff Base and its Transition Metal Complexes. Anti-Infective Agents 2021;20.
- [13]Abbas Z, Kumar M, Tuli HS, Janahi EM, Haque S, Harakeh S, et al. Synthesis, Structural Investigations, and In Vitro/In Silico Bioactivities of Flavonoid Substituted Biguanide: A Novel Schiff Base and Its Diorganotin (IV) Complexes. Molecules 2022;27:8874.
- [14]Zúñiga AE, Fidelibus PM, Mandolesi SD, Podestá JC. Synthesis of dineophyltin dihydride and stereoselective hydrostannation of alkynes and (E)-trisubstituted alkenes. J Organomet Chem 2011;696:1547–55.
- [15]Ruan B, Tian Y, Zhou H, Wu J, Hu R, Zhu C, et al. Synthesis, characterization and in vitro antitumor activity of three organotin(IV) complexes with carbazole ligand. Inorganica Chim Acta 2011;365:302–8.
- [16]Howie RA, De Lima GM, Wardell JL, Wardell SMSV, Harrison WTA. Solid state study of sulfoxide adducts of (2-amidoethyl-C,O) trihalostannanes: Supramolecular networks constructed from hydrogen-bonds involving the amido units. J Organomet Chem 2012;716:62–9.
- [17]Siqueira GO, Porto ADO, De Lima GM, Matencio T. Phase and morphology dependence on the annealing temperature of tin sulfides and oxides prepared by thermal decomposition of organotin precursors. J Organomet Chem 2012;715:48–53.
- [18]Tabassum S, Chandra Sharma G, Asim A, Azam A, Khan RA. Chiral nano heterobimetallic DNA receptors: In vitro binding studies, cleavage activity and DNA condensation studies (TEM and AFM imaging). J Organomet Chem 2012;713:123–33.
- [19]Devi J, Yadav J. Recent Advancements in Organotin(IV) Complexes as Potential Anticancer Agents. Anticancer Agents Med Chem 2018;18:335–53.
- [20]Devi J, Pachwania S. Recent advancements in DNA interaction studies of organotin(IV) complexes. Inorg Chem Commun 2018;91:44–62
- [21]Joshi R, Pandey N, Tilak R, Yadav SK, Mishra H, Pokharia S. New triorganotin(IV) complexes of quinolone antibacterial drug sparfloxacin: Synthesis, structural characterization, DFT studies and biological activity. Appl Organomet Chem 2018;32:e4324.
- [22]Shaheen F, Sirajuddin M, Ali S, Zia-ur-Rehman, Dyson PJ, Shah NA, et al. Organotin(IV) 4- (benzo[d][1,3]dioxol-5-ylmethyl)piperazine-1-carbodithioates: Synthesis, characterization and biological activities. J Organomet Chem 2018;856:13–22.
- [23]Jiang W, Qin Q, Xiao X, Tan Y. Diorganotin(IV) complexes based on tridentate ONO ligands as potential anticancer agents. J Inorg Biochem 2022;232:111808.
- [24]Sedaghat T, Aminian M, Bruno G, Amiri Rudbari H. Binuclear organotin(IV) complexes with adipic dihydrazones: Synthesis, spectral characterization, crystal structures and antibacterial activity. J Organomet Chem 2013;737:26–31.
- [25]Shujah S, Zia-Ur-Rehman, Muhammad N, Shah A, Ali S, Khalid N, et al. Bioactive hepta- and pentacoordinated supramolecular diorganotin(IV) Schiff bases. J Organomet Chem 2013;741–742:59–66.
- [26]Rehman W, Haq S, Muhammad B, Hassan SF, Badshah A, Waseem M, et al. Organotin (IV) based complexes as promiscuous antibacterials: Synthesis, in vitro, in silico pharmacokinetic and docking studies. J Organomet Chem 2014;767:91–100.
- [27]Pourayoubi M, Bayraq SS, Tarahhomi A, Nečas M, Fejfarová K, Dušek M. Hirshfeld surface analysis of new organotin(IV)-phosphoramide complexes. J Organomet Chem 2014;751:508–18.
- [28]Shujah S, Zia-Ur-Rehman, Muhammad N, Shah A, Ali S, Meetsma A, et al. Homobimetallic organotin(IV) complexes with hexadentate Schiff base: Synthesis, crystal structure and antimicrobial studies. J Organomet Chem 2014;759:19–26.
- [29]Dey DK, Saha MK, Das MK, Bhartiya N, Bansal RK, Rosair G, et al. Synthesis and characterization of diorganotin(IV) complexes of tetradentate Schiff bases: Crystal structure of n-Bu2Sn(Vanophen). Polyhedron 1999;18:2687–96.
- [30]Kovala-Demertzi D, Wiecek J, Ciunik Z, Wietrzyk J, Zervou M, Demertzis MA. Organotin compound derived from 3-hydroxy-2-formylpyridine semicarbazone: Synthesis, crystal structure, and antiproliferative activity. Bioinorg Chem Appl 2010;2010.
- [31]Wen GH, Zhang RF, Li QL, Zhang SL, Ru J, Du JY, et al. Synthesis, structure and in vitro cytostatic activity study of the novel organotin(IV) derivatives of p-aminobenzenesulfonic acid. J Organomet Chem 2018;861:151– 8.
- [32]Švec P, Leinweber P, Erben M, Růžičková Z, Růžička A. Employing a C,N-chelate makes organotin(IV) nitrates and nitrites exceptionally stable. J Organomet Chem 2017;845:90–7.
- [33]Gholivand K, Ebrahimivalmoozi AA, Gholami A, Dusek M, Eigner V, Abolghasemi S. Synthesis, characterization, crystal structures, QSAR study and antibacterial activities of organotin bisphosphoramidates. J Organomet Chem 2016;806:33–44.
- [34]Muñoz-Flores BM, Santillán R, Farfán N, Álvarez-Venicio V, Jiménez-Pérez VM, Rodríguez M, et al. Synthesis, X-ray diffraction analysis and nonlinear optical properties of hexacoordinated organotin compounds derived from Schiff bases. J Organomet Chem 2014;769:64–71.
- [35]Devi J, Boora A, Rani M, Arora T. Recent Advancements in Organotin(IV) Complexes as Potent Cytotoxic Agents. Anticancer Agents Med Chem 2022;22.
- [36]Yang Y, Hong M, Xu L, Cui J, Chang G, Li D, et al. Organotin(IV) complexes derived from Schiff base N’-[(1E)- (2-hydroxy-3-methoxyphenyl)methylidene]pyridine-3-carbohydrazone: Synthesis, in vitro cytotoxicities and DNA/BSA interaction. J Organomet Chem 2016;804:48–58.
- [37]García-López MC, Muñoz-Flores BM, Chan-Navarro R, Jiménez-Pérez VM, Moggio I, Arias E, et al. Microwave-assisted synthesis, third-order nonlinear optical properties, voltammetry cyclic and theoretical calculations of organotin compounds bearing push-pull Schiff bases. J Organomet Chem 2016;806:68–76.
- [38]Turek J, Kampová H, Padělková Z, Růžička A. Preparation and structure of tin(IV) catecholates by reactions of C,N-chelated tin(IV) compounds with a catechol or lithium catecholate, and various stannylenes with a quinone. J Organomet Chem 2013;745–746:25–33.
- [39]Salam MA, Hussein MA, Ramli I, Islam MS. Synthesis, structural characterization, and evaluation of biological activity of organotin(IV) complexes with 2-hydroxy-5-methoxybenzaldehyde-N(4)-methylthiosemicarbazone. J Organomet Chem 2016;813:71–7.
- [40]Gerbino DC, Fidelibus PM, Mandolesi SD, Ocampo RA, Scoccia J, Podestá JC. Sonochemical synthesis of bis(trin-butylstannyl) aromatic compounds via Barbier-like reactions. J Organomet Chem 2013;741–742:24–32.
- [41]Mihaljević I, Bašica B, Maraković N, Kovačević R, Smital T. Interaction of organotin compounds with three major glutathione S-transferases in zebrafish. Toxicol Vitr 2020;62.
- [42]Švec P, Padělková Z, Růžička A, Weidlich T, Dušek L, Plasseraud L. C,N-chelated organotin(IV) trifluoroacetates. Instability of the mono- and diorganotin(IV) derivatives. J Organomet Chem 2011;696:676–86.
- [43]Zhu C, Yang L, Li D, Zhang Q, Dou J, Wang D. Synthesis, characterization, crystal structure and antitumor activity of organotin(IV) compounds bearing ferrocenecarboxylic acid. Inorganica Chim Acta 2011;375:150–7.
- [44]Frankel M, Gertner D, Wagner D, Zilkha A. Organotin Esters of Amino Acids and Their Use In Peptide Syntheses. J Org Chem 1965;30:1596–9.
- [45]Basu Baul TS, Kehie P, Duthie A, Guchhait N, Raviprakash N, Mokhamatam RB, et al. Synthesis, photophysical properties and structures of organotin-Schiff bases utilizing aromatic amino acid from the chiral pool and evaluation of the biological perspective of a triphenyltin compound. J Inorg Biochem 2017;168:76–89.
- [46]Muhammad N, Shah A, Zia-ur-Rehman, Shuja S, Ali S, Qureshi R, et al. Organotin(IV) 4-nitrophenylethanoates: Synthesis, structural characteristics and intercalative mode of interaction with DNA. J Organomet Chem 2009;694:3431–7.
- [47]Metelkova L, Zhakovskaya Z, Kukhareva G, Voskoboinikov G, Zimina O. Organotin compounds (OTs) in surface sediments, bivalves and algae from the Russian coast of the Barents Sea (Kola Peninsula) and the Fram Strait (Svalbard Archipelago). Environ Sci Pollut Res 2022;29:34659–69.
- [48]Zhao A, Miao J, Liu L, Pan L. Potencies of organotin compounds in scallop RXRa responsive activity with a GAL4-based reconstituted yeast assay in vitro. Environ Sci Pollut Res 2022;29:19890–7.
- [49]Norén A, Lointier C, Modin O, Strömvall AM, Rauch S, Andersson-Sköld Y, et al. Removal of organotin compounds and metals from Swedish marine sediment using Fenton’s reagent and electrochemical treatment. Environ Sci Pollut Res 2022;29:27988–8004.
- [50]Dodokhova MA, Kotieva IM, Safronenko A V., Alkhusein-Kulyaginova MS, Sukhorukova N V., Kotieva VM, et al. Effect of Hybrid Organotin Compound on Activity of LPO and Antioxidant Protection of the Liver Tissues in Animals with Melanoma B16. Bull Exp Biol Med 2022;172:752–5.
- [51]Metelkova L, Zhakovskaya Z, Kukhareva G, Voskoboinikov G, Zimina O. Organotin compounds (OTs) in 401 www.bsmiab.org/jabet Kumar et al., J Adv Biotechnol Exp Ther. 2023 May; 6(2): 386-402 surface sediments, bivalves and algae from the Russian coast of the Barents Sea (Kola Peninsula) and the Fram Strait (Svalbard Archipelago). Environ Sci Pollut Res 2022;29:34659–69.
- [52]Auer M, Diab F, Eichele K, Schubert H, Wesemann L. Reactivity of organogermanium and organotin trihydrides. Dalt Trans 2022;51:5950–61.
- [53]Abbas Z, Tuli HS, Varol M, Sharma S, Sharma HK, Aggarwal P, et al. Organotin (IV) complexes derived from Schiff base 1,3-bis[(1E)-1-(2-hydroxyphenyl)ethylidene] thiourea: synthesis, spectral investigation and biological study to molecular docking. J Iran Chem Soc 2022;19:1923–35.
- [54]Kumar M, Abbas Z, Tuli HS, Rani A. Organotin Complexes with Promising Therapeutic Potential. Curr Pharmacol Reports 2020;6:167–81.
- [55]Jiang W, Zhang Z, Ni P, Tan Y. Self-assembly synthesis of diorganotin complexes based on arylformylhydrazone possessing ONO donor set: anticancer activity and mechanism. Metallomics 2022;14:mfac021.
- [56]Ma JH, Needham C, Wang H, Neureuther A, Prendergast D, Naulleau P. Mechanistic Advantages of Organotin Molecular EUV Photoresists. ACS Appl Mater Interfaces 2022;14:5514–24.
- [57]SINGH HL, Dhingra N, Singh J. Synthesis, spectroscopy, and density functional theory of organotin and organosilicon complexes of bioactive ligand containing nitrogen, sulfur donor atoms as antimicrobial agents: in-vitro and in-silico studies. Dalt Trans 2022;51:8821–31.
- [58]Du X, Zhang R, Li Q, Cheng S, Li Y, Ru J, et al. Organotin(IV) complexes derived from 1,4- naphthalenedicarboxylic acid: synthesis, structure, in vitro cytostatic activity. J Organomet Chem 2021;935:121654.
- [59]Antonenko TA, Shpakovsky DB, Berseneva D, Gracheva YA, Dubova LG, Shevtsov PN, et al. Cytotoxic activity of organotin carboxylates based on synthetic phenolic antioxidants and polycyclic bile acids. J Organomet Chem 2020;909:121089.
- [60]Naz N, Sirajuddin M, Haider A, Abbas SM, Ali S, Wadood A, et al. Synthesis, characterization, biological screenings and molecular docking study of Organotin(IV) derivatives of 2,4-dichlorophenoxyacetic acid. J Mol Struct 2019;1179:662–71.
- [61]Tarassoli A, Sedaghat T, Neumüller B, Ghassemzadeh M. Synthesis, spectroscopic investigations and crystal structures of organotin(IV) derivatives of 2-amino-1-cyclopentene-1-carbodithioic acid. Inorganica Chim Acta 2001;318:15–22.
- [62]Dey DK, Dey SP, Karan NK, Datta A, Lycka A, Rosair GM. Structural and spectral studies of 3-(2- hydroxyphenylimino)-1-phenylbutan-1-one and its diorganotin(IV) complexes. J Organomet Chem 2009;694:2434–41.
- [63]Tariq M, Sirajuddin M, Ali S, Khalid N, Tahir MN, Khan H, et al. Pharmacological investigations and Petra/Osiris/Molinspiration (POM) analyses of newly synthesized potentially bioactive organotin(IV) carboxylates. J Photochem Photobiol B Biol 2016;158:174–83.
- [64]Win YF, Choong CS, Dang JC, Iqbal MA, Quah CK, Kanuparth SR, et al. Synthesis, crystal structures and spectroscopic properties of two new organotin (IV) complexes and their antiproliferative effect against cancerous and non-cancerous cells. Comptes Rendus Chim 2015;18:137–48.
- [65]Sirajuddin M, McKee V, Tariq M, Ali S. Newly designed organotin(IV) carboxylates with peptide linkage: Synthesis, structural elucidation, physicochemical characterizations and pharmacological investigations. Eur J Med Chem 2018;143:1903–18.
- [66]Sirajuddin M, Ali S, McKee V, Wadood A, Ghufran M. Exploration of organotin(IV) derivatives for medicinal applications: Synthesis, spectroscopic characterization, structural elucidation and molecular docking study. J Mol Struct 2019;1181:93–108.
- [67]Vieira FT, de Lima GM, Maia JR d. S, Speziali NL, Ardisson JD, Rodrigues L, et al. Synthesis, characterization and biocidal activity of new organotin complexes of 2-(3-oxocyclohex-1-enyl)benzoic acid. Eur J Med Chem 2010;45:883–9.
- [68]Ólafsson SN, Bjornsson R, Helgason Ö, Jonsdottir S, Suman SG. Coordination geometry determination of stannane compounds with phosphinoyldithioformate ligands using multinuclear NMR, Sn Mössbauer and DFT methods. J Organomet Chem 2016;825–826:125–38.
- [69]Singh HL, Singh J. Synthesis, spectroscopic, molecular structure, and antibacterial studies of dibutyltin(iv) Schiff base complexes derived from phenylalanine, isoleucine, and glycine. Bioinorg Chem Appl 2014;2014.
- [70]Pellerito C, Emanuele S, Giuliano M, Fiore T. Organotin(IV) complexes with epigenetic modulator ligands: New promising candidates in cancer therapy. Inorganica Chim Acta 2022;536:120901.
- [71]Tuli HS, Rani A, Kumar M, Khare R. Schiff bases as an antimicrobial agent: A review. J Biol Chem Sci 2015;2:62–91.
- [72]Adeyemi JO, Onwudiwe DC, Ekennia AC, Okafor SN, Hosten EC. Organotin(IV)N-butyl-Nphenyldithiocarbamate complexes: Synthesis, characterization, biological evaluation and molecular docking studies. J Mol Struct 2019;1192:15–26.
- [73]Tan YX, Zhang ZJ, Liu Y, Yu JX, Zhu XM, Kuang DZ, et al. Synthesis, crystal structure and biological activity of the Schiff base organotin(IV) complexes based on salicylaldehyde-o-aminophenol. J Mol Struct 2017;1149:874– 81.
- [74]Pantelić ND, Zmejkovski BB, Žižak Ž, Banjac NR, Božić BD, Stanojković TP, et al. Design and in vitro biological evaluation of a novel organotin(IV) complex with 1-(4-carboxyphenyl)-3-ethyl-3-methylpyrrolidine-2,5-dione. J Chem 2019;2019.
- [75]Jiang D, Rasul A, Batool R, Sarfraz I, Hussain G, Mateen Tahir M, et al. Potential Anticancer Properties and Mechanisms of Action of Formononetin. Biomed Res Int 2019;2019.
- [76]Milaeva ER, Shpakovsky DB, Dyadchenko VP, Gryzlov AI, Gracheva YA, Antonenko TA, et al. Synthesis and biological activity of novel Au(I) complexes with a protective antioxidant 2,6-di-tert-butylphenol group. 402 www.bsmiab.org/jabet Kumar et al., J Adv Biotechnol Exp Ther. 2023 May; 6(2): 386-402 Polyhedron 2017;127:512–9.
- [77]Kolyada MN, Osipova VP, Berberova NT, Pimenov YT, Milaevac ER. Decline of prooxidant activity of butyl and phenyl derivatives of tin in the presence of meso-tetrakis(3,5-di-tert-butyl-4- hydroxyphenyl)porphyrin. Macroheterocycles 2017;10:57–61.
- [78]Nikitin EA, Shpakovsky DB, Tyurin VY, Kazak AA, Gracheva YA, Vasilichin VA, et al. Novel organotin complexes with phenol and imidazole moieties for optimized antitumor properties. J Organomet Chem 2022;959:122212.
- [79]Ahmad B, Khan S, Liu Y, Xue M, Nabi G, Kumar S, et al. Molecular Mechanisms of Anticancer Activities of Puerarin. Cancer Manag Res 2020;12:79–90.
- [80]Niu L, Li Y, Li Q. Medicinal properties of organotin compounds and their limitations caused by toxicity. Inorganica Chim Acta 2014;423:2–13.
- [81]Basu Baul TS, Dutta D, Duthie A, Prasad R, Rana NK, Koch B, et al. Triphenyltin(IV) benzoates with diazenyl/imino scaffold exhibiting remarkable apoptosis mediated by reactive oxygen species. J Inorg Biochem 2017;173:79–92.
- [82]Latsis GK, Banti CN, Kourkoumelis N, Papatriantafyllopoulou C, Panagiotou N, Tasiopoulos A, et al. Poly organotin acetates against DNA with possible implementation on human breast cancer. Int J Mol Sci 2018;19.
- [83]Flora SJS. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2009;2:191.
- [84]Mahato M, Mukherji S, Van Hecke K, Harms K, Ghosh A, Nayek HP. Mononuclear homoleptic organotin(IV) dithiocarbamates: Syntheses, structures and antimicrobial activities. J Organomet Chem 2017;853:27–34
- [85]Shujah S, Ali S, Khalid N, Alam MJ, Ahmad S, Meetsma A. Synthesis, spectroscopic characterization, X-ray structure, DFT calculations, and antimicrobial studies of diorganotin (IV) complexes of monotopic oxygen nitrogen donor Schiff base. Chem Pap 2018;72:903–19.
- [86]Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol 2017;33:300.
- [87]Devi J, Yadav J, Singh N. Synthesis, characterisation, in vitro antimicrobial, antioxidant and anti-inflammatory activities of diorganotin(IV) complexes derived from salicylaldehyde Schiff bases. Res Chem Intermed 2019;45:3943–68.
- [88]Baréa P, Barbosa VA, Bidóia DL, de Paula JC, Stefanello TF, da Costa WF, et al. Synthesis, antileishmanial activity and mechanism of action studies of novel β-carboline-1,3,5-triazine hybrids. Eur J Med Chem 2018;150:579–90.
- [89]Waseem D, Butt AF, Haq I ul, Bhatti MH, Khan GM. Carboxylate derivatives of tributyltin (IV) complexes as anticancer and antileishmanial agents. DARU, J Pharm Sci 2017;25.
- [90]Borgers M. Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev Infect Dis 1980;2:520–34.
- [91]Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12:501–17.
- [92]Mridula, Nath M. Conventional and microwave-assisted synthesis, characterization, DFT calculations, in vitro DNA binding and cleavage studies of potential chemotherapeutic diorganotin(IV) mandelates. J Photochem Photobiol B Biol 2016;162:348–60.
- [93]Yin H, Liu H, Hong M. Synthesis, structural characterization and DNA-binding properties of organotin(IV) complexes based on Schiff base ligands derived from 2-hydroxy-1-naphthaldy and 3- or 4-aminobenzoic acid. J Organomet Chem 2012;713:11–9.
- [94]Nath M, Mridula, Kumari R. Microwave-assisted synthesis of mixed ligands organotin(IV) complexes of 1,10- phenanthroline and L-proline: Physicochemical characterization, DFT calculations, chemotherapeutic potential validation by in vitro DNA binding and nuclease activity. J Photochem Photobiol B Biol 2017;174:182–94
- [95]Sangeetha Gowda K.R., Blessy Baby Mathew, C.N. Sudhamani HSBN. Mechanism of DNA Binding and Cleavage, Biomedicine and Biotechnology. Biomed Biotechnol 2014;Vol. 2 No.:1–9.
- [96]Nath M, Pokharia S, Eng G, Song X, Kumar A. Comparative study of structure-activity relationship of di- and tri- organotin(IV) derivatives of amino acid and peptides. J Organomet Chem 2003;669:109–23.
- [97]Antonenko TA, Gracheva YA, Shpakovsky DB, Vorobyev MA, Tafeenko VA, Mazur DM, et al. Cytotoxic activity of organotin compounds containing non-steroidal anti-inflammatory drugs. J Organomet Chem 2022;960:122191.
- [98]Romero-Chávez MM, Pineda-Urbina K, Pérez DJ, Obledo-Benicio F, Flores-Parra A, Gómez-Sandoval Z, et al. Organotin(IV) compounds derived from ibuprofen and cinnamic acids, an alternative into design of antiinflammatory by the cyclooxygenases (COX-1 and COX-2) pathway. J Organomet Chem 2018;862:58–70.
- [99]Adeyemi JO, Onwudiwe DC, Ekennia AC, Anokwuru CP, Nundkumar N, Singh M, et al. Synthesis, characterization and biological activities of organotin(IV) diallyldithiocarbamate complexes. Inorganica Chim Acta 2019;485:64–72
- [100]Gunaydin C, Bilge SS. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J Med 2018;50:116–21.