Role of plasma calcium, phosphorus, alkaline phosphatase, and parathyroid hormone in the prediction of vitamin D deficiency
Abstract
Vitamin D deficiency is a highly prevalent medical condition associated with various clinical and biochemical outcomes although these outcomes might be absent in a significant percentage of patients. Altered serum calcium, phosphorus (PO4), parathyroid hormone (PTH) and alkaline phosphatase (ALP) activity are expected but not proven by various research. The current study aims to investigate the possibility of these biomarkers to be used as indicators of vitamin D deficiency or insufficiency. The study enrolled 150 randomly selected participants who have no acute or chronic medical condition with various levels of serum vitamin D ranging from normal to severely deficient. They were investigated for serum total calcium, PO4, PTH, and ALP activity. The results were statistically compared among the studied groups and correlated with serum vitamin D levels. There was no significant difference in mean serum calcium, PO4, and ALP activity among the groups with poor correlation with vitamin D. Mean serum PTH has shown a significant difference among the studied groups with a strong negative correlation with vitamin D. This difference was apparent even in patients with vitamin D insufficiency. Serum calcium, PO4 and ALP activity seem to have a poor correlation with vitamin D concentration. In addition, serum PTH might be considered a sensitive marker of both vitamin D deficiency and insufficiency.
INTRODUCTION
Vitamin D is a well-known example of fat-soluble vitamin that has a major role in physiological bone growth due to its direct relation to calcium, magnesium, and phosphorus homeostasis. Its deficiency is a commonly encountered medical condition affecting around one billion individuals of various ages leading to serious clinical consequences, other than bone disorders, that should be managed properly. Examples of these medical conditions that have been linked to vitamin D deficiency include autoimmune diseases, preeclampsia, cardiovascular disease and serious cancers [1].
The endogenous synthesis of cholecalciferol by subcutaneous hydroxylation of 25-hydroxyvitamin D represents the main source of vitamin D in the human body. This synthetic process is stimulated by the UVB component of sunlight. This process is affected by various factors including age, duration of exposure, type of clothes, application of sunscreen and the condition of the weather in addition to many other conditions [2]. However, the dietary content of vitamin D has a grave role in maintaining both circulating and stored vitamin D [3], but unfortunately, most of the common foods contain no amount of vitamin D [4]. The presence of these multiple factors related to optimizing the plasma level of vitamin D explains the long list of causes of its deficiency or insufficiency although underestimating the role of sun exposure in preventing vitamin D deficiency represents the major cause globally [5].
The definition of vitamin D deficiency has been repeatedly updated during the last decades. Depending on various factors such as the correlation of plasma parathyroid hormone and bone biopsy, plasma vitamin D of > 30 ng/ml is regarded as sufficient while it is regarded as deficient if it was < 20 ng/ml. Plasma vitamin D of 21-29 ng/ml is regarded to be insufficient [6]. Certain groups of the population are thought to have a higher risk for vitamin D deficiency; these groups include pregnant women, obese individuals, blacks, and Hispanics [7]. In most cases of vitamin D deficiency, the patients are free of symptoms which will only appear in case of severe and prolonged deficiency where the patients might develop osteomalacia in adults and rickets in children [8]. Surprisingly, early stages of osteomalacia might be associated with a high plasma concentration of vitamin D mediated by high circulating PTH. The level of vitamin D will decrease in the following stages of osteomalacia [9]. Putting in mind these facts, in addition to the relatively high cost of frequent estimation of plasma level of vitamin D, it would be justified to look for a sensitive and specific biomarker of vitamin D deficiency or insufficiency sparing the need for frequent estimation of vitamin unless there is a preceded alteration in these specific biomarkers. Hypocalcemia and hypophosphatemia increased plasma PTH and ALP activity are expected in patients with vitamin D deficiency. However, the onset of the alteration of these biomarkers in patients with various degrees of vitamin D deficiency needs to be established. The aim of this study is to investigate the use of hypocalcemia and hypophosphatemia, increased plasma PTH and ALP activity as predictors of vitamin D deficiency.
MATERIALS AND METHODS
Subjects
The current study was performed in Baghdad during February and March 2022. It enrolled 150 participants who were attending a private clinical laboratory for routine checking that included serum vitamin D. They were equally classified according to their serum vitamin D level into 3 groups. Those with vitamin D of ≥30 ng/ml were regarded as a control group while those with serum vitamin D of < 20 ng/ml were regarded as a deficient group. Those with vitamin D of 20-29 ng/ml were regarded as an insufficient group. Full medical history was taken from each participant including past medical and drug history in addition to reviewing previous medical records when present. Both demographic and anthropometric data were recorded for each participant.
Inclusion and exclusion criteria
Adult subjects, with no acute or chronic illness and on no current medications were included in the study. Others with the following criteria were excluded:
- Age < 18 years
- Patients with diabetes mellitus, hypertension, or any chronic illness.
- Subjects on any current medications including supplements.
- Subjects with hypoalbuminemia regardless of its cause.
- Subjects who refused to share their data.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethical approval was obtained from the ethical approval committee at Al-Rasheed University College, Department of Pharmacy, approval number 4 on 15.1.2022.
Data collection and sample analysis
For all participants, serum vitamin D, calcium, PO4 and PTH were measured. To ensure that all participants fulfil the inclusion criteria, the following laboratory tests were estimated as well:
- Complete blood count (by CBC Analyzer Sysmex XP300)
- Blood urea (by Kinetic test with urease and glutamate dehydrogenase)
- Serum creatinine (by Jaffé method)
- Serum albumin (by Immunoturbidimetric assay)
- Serum ALP activity (by measuring the catalytic activity of the enzyme)
- Erythrocyte sedimentation rate (by automated method)
A data collection form was filled out during the interview with each participant after obtaining written informed consent. Blood samples were collected by a highly skilled laboratory technician for analysis where 10 ml of whole blood was collected from each participant. The blood sample of each participant was kept in a vacuum tube for 15 minutes for full clotting before being centrifuged for 10 minutes at 4000 rpm. The separated serum was collected in a plain tube and stored at -20°C till the day of measurement.
Calcium ions react with 5 nitro 5’ methyl BAPTA (NM-BAPTA) under alkaline conditions to form a complex. This complex reacts in the second step with EDTA. However, calcium concentration is directly proportional to the change in absorbance and is measured photometrically.
Inorganic phosphate forms an ammonium phosphomolybdate complex having the formula (NH4)3[PO4(MoO3)12] with ammonium molybdate in the presence of sulfuric acid. However, the concentration of inorganic phosphate is directly proportional to the phosphomolybdate formed and is measured photometrically.
In the presence of magnesium and zinc ions, p nitrophenyl phosphate is cleaved by phosphatases into phosphate and p nitrophenol. The p nitrophenol released is directly proportional to the catalytic ALP activity. It is determined by measuring the increase in absorbance.
Serum vitamin D and PTH were measured using a Mybiosure ELISA kit with a fully automated device while serum PO4, calcium and ALP were measured using a fully automated spectrophotometer (Roche C111). Also, all laboratory works were performed on the same day. Noteworthy, the quality control steps were strictly applied to all procedures using both high and normal values.
Statistical analysis
Statistical analysis was performed using SPSS version 26. In the first place, normality testing was performed using Cronbach's alpha test. Demographic data were analysed next, followed by applying the ANOVA test to find out any significant difference among groups while Pearson's test was applied to measure the correlations.
RESULTS
Demographic characteristic of all participants stratified by vitamin D
The demographic characteristics of the participants are listed in Table 1. The mean age of the control, insufficient and deficient groups was 48.3, 51.1 and 54.3 years, respectively. The BMI of the participants was calculated, and it was 27.4 kg/m2 for the control group, 29.3 kg/m2 for the insufficient group and 28.8 kg/m2 for the deficient group. Males represent 42% of the total number of participants while the remaining 58% were females. Mean serum vitamin D was 36.7 ng/ml in the control group, 23.5 ng/ml in the insufficient group and 8.3 ng/ml in the deficient group (Table 2). Mean serum PTH (ng/l) was 38.3 (±14.6) in the control group, 79.2 (± 17.1) in the insufficient group and 92.5 (±25.3) in the deficient
group where the reference range was 10-65 ng/l (Table 2). Mean serum calcium (mg/dl) was 9.8 (±2.1) in the control group 9.6 (±3.1) in the insufficient group and 10.1 (±2.9) in the deficient group where the reference range was 8.4-10.4 mg/dl (Table 2). Mean serum PO4 (mg/dl) was 4.1 (±1.9) in the control group, 4.5(±1.4) in the insufficient group and 4.2 (±1.6) in the deficient group where the reference range was 2.5-4.5 mg/dl (Table 2). Mean serum ALP activity (IU/l) was 85 (±23) in the control group, 112 (± 33) in the insufficient group and 109 (±25) in the deficient group where the reference range was 30-129 IU/L as shown in Table 2.
Table 1. The demographic characteristic of all participants stratified by vitamin D status.
Table 2. A comparison of the measured variables among the studied groups.
Comparisons and correlations between vitamin D and other biomarkers
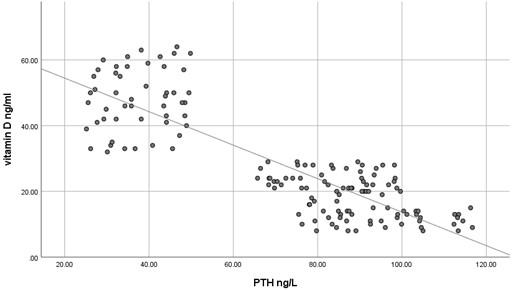
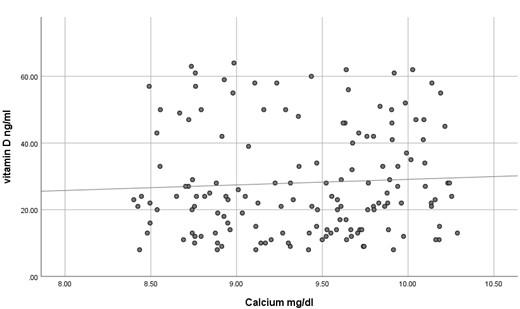
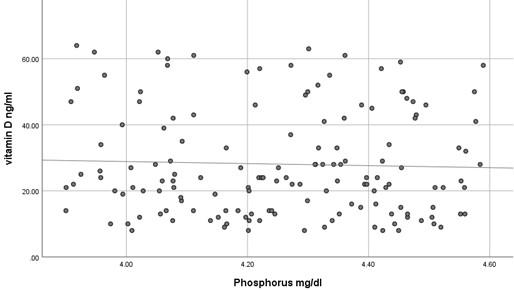
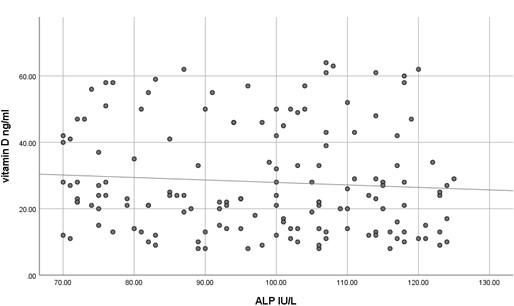
The current study has shown no significant difference in mean serum calcium, PO4 and ALP among the three studied groups (P >0.05) as shown in Table 2 and all the results of all participants were within the reference range. In contrast, mean serum PTH has shown a significant difference among the studied groups (P<0.05) where it was significantly higher in participants with vitamin D deficiency compared to participants with normal or insufficient vitamin D. At the same time, mean serum PTH was significantly higher in participants with insufficient vitamin D compared to those with normal vitamin D as shown in Table 2. Using Pearson's test, there was a strong negative correlation between serum PTH and vitamin D where the r value was – 0.787 as shown in Table 3. On the other hand, serum calcium, PO4 and ALP activity have shown a very weak correlation with serum vitamin D, 0.055, - 0.037 and -0.076, respectively. These correlations are shown as scatter plots in Figures (1-4) where it was so apparent that the results of calcium, phosphorus and ALP were scattered randomly with respect to vitamin D levels in contrast to that of PTH.
Table 3. Correlation between serum vitamin D and PTH in the studied groups.
DISCUSSION
Vitamin D deficiency is a common condition of various severity affecting about 18% of the population, although this percentage is quite variable according to the definition of vitamin D deficiency [10]. Nevertheless, insufficient data are present to assess the balance of benefit versus harm of screening serum vitamin D in asymptomatic individuals [11]. Moreover, many studies have confirmed inadequate evidence of the benefit of treating patients with asymptomatic vitamin D deficiency [12]. Taking previously mentioned factors into account, it is noteworthy to screen for the laboratory findings that are expected to be present in patients with vitamin D deficiency to avoid unnecessary treatment, diagnose asymptomatic patients and decrease the cost of unnecessary screening for vitamin D deficiency. The current study has shown that the most concurrent laboratory alteration in patients with vitamin D deficiency is the increase in serum PTH while serum calcium, phosphorus and ALP activity were surprisingly within the reference range even in patients with severe vitamin D deficiency. It seems that it requires a prolonged duration of deficiency for the expected laboratory alterations to occur. This makes the use of hypocalcemia, hypophosphatemia and increased ALP activity questionable for initial screening of vitamin D deficiency because of their misleading results. At the same time, using serum PTH as a screening test for vitamin D deficiency makes no sense because the cost is higher, and the specificity is relatively low despite its high sensitivity. In addition to the relatively small sample size, an important limitation of the current study is that we have measured serum total calcium instead of serum free calcium, which might better reflect the calcium status although Srinath Rajaraman et al. has shown a strong correlation between total and free calcium in different vitamin D levels [13]. Numerous studies have shown various levels of sensitivity of serum calcium, PO4 and ALP activity in detecting vitamin D deficiency [14-17]. No single biochemical marker is substantiated to be strongly correlated with vitamin D deficiency. This should be always kept in mind in the management of a patient with vitamin D deficiency. Serum PTH has a negative strong correlation with vitamin D as it seems to be increased even in patients with insufficient vitamin D making bone resorption a possibility even at this level of vitamin D. This strong negative correlation was repeatedly confirmed by many studies[18-20] but what is remarkably of additional clinical importance is that certain researchers have found an increased serum PTH concentration even at what is supposed to be normal serum vitamin D level (<38 ng/ml) [21, 22]. These findings highlight the importance of assessment of serum PTH in the management of vitamin D deficiency and even in patients with lower normal vitamin D levels yet have clinical features suggestive of bone resorption. Until now, serum PTH has not been considered an indicator of response to vitamin D replacement, but as far as we can tell, it is worthy to investigate such a role as it is not guaranteed to return to its reference range with the correction of vitamin D unless vitamin D reaches a specific serum concentration.
CONCLUSIONS
In conclusion, serum calcium, PO4 and ALP activity seem to have a poor correlation with vitamin D concentration and might have misleading results if used to assess vitamin D status. However, serum PTH seems to be a sensitive marker of vitamin D deficiency and insufficiency.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONS
The main part of the writing and research design was made by RDH and IN. EG and TMH were responsible for sample collection and laboratory work including direct measurement of the studied tests. The statistical analysis of the results was done by MHM. The final revision of the whole work was performed by IAT. The published version of the manuscript has been read and approved by all authors.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153-65.
- [2]Webb AR. Who, what, where and when—influences on cutaneous vitamin D synthesis. Progress in biophysics and molecular biology. 2006;92:17-25.
- [3]Lachowicz K, Stachoń M. Determinants of Dietary Vitamin D Intake in Population-Based Cohort Sample of Polish Female Adolescents. International Journal of Environmental Research and Public Health. 2022;19:12184.
- [4]Ahmed N, Araf Y, Ullah MA. Prospects of vitamin D in the treatment of COVID-19 patient and improving maternal and child health during pandemic. J Adv Biotechnol Exp Ther. 2021;4:133-48.
- [5]Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Reviews in Endocrine and Metabolic Disorders. 2017;18:153-65.
- [6]Valcour A, Blocki F, Hawkins D, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. The Journal of Clinical Endocrinology & Metabolism. 2012;97:3989-95.
- [7]Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720- 55.
- [8]Kreutz JM, Adriaanse MP, van der Ploeg EM, Vreugdenhil AC. Narrative review: nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients. 2020;12:500.
- [9]Minisola S, Colangelo L, Pepe J, Diacinti D, Cipriani C, Rao SD. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR Plus. 2021;5.
- [10]Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, et al. Vitamin D status in the United States, 2011–2014. The American journal of clinical nutrition. 2019;110:150-7.
- [11]LeFevre ML, Force* UPST. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2015;162:133-40.
- [12]Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for Vitamin D Deficiency in Adults. JAMA. 2021;325:1436.
- [13]Rajaraman S, Selvanayagam S, Chidambaram R. Correlation study between total calcium, ionized calcium, serum albumin and their significance with Vitamin D. Journal of Experimental Sciences. 2012;2.
- [14]Hashemipour S, Larijani B, Adibi H, Sedaghat M, Pajouhi M, Bastan-Hagh MH, et al. The status of biochemical parameters in varying degrees of vitamin D deficiency. Journal of bone and mineral metabolism. 2006;24:213-8.
- [15]Mobarki AA, Dobie G, Saboor M, Madkhali AM, Habibullah MM, Nahari MH, et al. Prevalence and Correlation of Vitamin D Levels with Hematological and Biochemical Parameters in Young Adults. Annals of Clinical & Laboratory Science. 2022;52:815-24.
- [16]Niedziela N, Pierzchała K, Zalejska-Fiolka J, Niedziela JT, Romuk E, Torbus-Paluszczak M, et al. Assessment of Biochemical and Densitometric Markers of Calcium-Phosphate Metabolism in the Groups of Patients with Multiple Sclerosis Selected due to the Serum Level of Vitamin D<sub>3</sub>. Biomed Res Int. 2018;2018:9329123.
- [17]Kiran B, Prema A, Thilagavathi R, Rani RJ. Serum 25-Hydroxy vitamin D, calcium, phosphorus and alkaline phosphatase levels in healthy adults above the age of 20 living in Potheri Village of Kancheepuram District, Tamilnadu. Journal of Applied Pharmaceutical Science. 2014;4:030-4.
- [18]Kilicarslan A, ASLAN AC, Gezgen G. The role of vitamin D deficiency in parathyroid hormone levels. Turkish Journal of Medical Sciences. 2013;43:368-72.
- [19]Tsugawa N, Uenishi K, Ishida H, Ozaki R, Takase T, Minekami T, et al. Association between vitamin D status and serum parathyroid hormone concentration and calcaneal stiffness in Japanese adolescents: sex differences in susceptibility to vitamin D deficiency. Journal of bone and mineral metabolism. 2016;34:464-74.
- [20]Martins JS, Palhares MDO, Teixeira OCM, Gontijo Ramos M. Vitamin D Status and Its Association with Parathyroid Hormone Concentration in Brazilians. Journal of Nutrition and Metabolism. 2017;2017:1-5.
- [21]Quaggiotto P, Tran H, Bhanugopan M. Vitamin D deficiency remains prevalent despite increased laboratory testing in New South Wales, Australia. Singapore medical journal. 2014;55:271.
- [22]Lejnieks A, Slaidina A, Zvaigzne A, Soboleva U, Eivazova G, Daukste I, et al. Vitamin D status and its seasonal variations and association with parathyroid hormone concentration in healthy women in Riga. Medicina. 2013;49:51.