Association of serum level of interleukin-33 and insulin resistance in overt and subclinical hypothyroidism patients
Abstract
Hypothyroidism is a condition that occurs when the thyroid gland fails to produce an adequate amount of the hormone thyroid, as a result of various factors such as hypothalamic or pituitary gland disease, common tissue resistance to thyroid hormones, and thyroid gland diseases. Hypothyroidism is the most prevalent thyroid disorder. Interleukin (IL)-33 is a nuclear cytokine of the IL-1 family members that is abundantly expressed during homeostasis and inflammation in endothelial, epithelial, and fibroblast-like cells. It is worth noting that higher IL-33 levels have been associated with insulin resistance. The purpose of the study was to examine the correlation between insulin resistance and IL-33 in patients with hypothyroidism. In this case-control study, 180 people were recruited and split into three categories: those with overt hypothyroidism (60), those with subclinical hypothyroidism (60), and those who were otherwise healthy control (60). There were of a similar age range to the patient, with 55.5% female participants and 44.5% male participants. Standard ELISA kits assess insulin, IL-33, thyroid stimulating hormone (TSH), thyroxine (T4), and triiodothyronine (T3). The healthy control group showed lower serum IL-33 levels than individuals with both overt and subclinical hypothyroidism. Compared to the healthy control group, all hypothyroidism groups had higher BMI, serum TSH, fasting glycemic glucose (FSG), insulin, triglyceride (TG), cholesterol levels (Chol), very low-density lipoprotein (VLDL), bad cholesterol (LDL), IL-33, and homeostatic model assessment of insulin resistance (HOMA-IR). In hypothyroid patients, IL-33 levels were negatively correlated with HOMA-IR and HDL-C and positively correlated with body mass index, TSH, FSG, insulin, HOMA-IR, total cholesterol, LDL, and total cholesterol. Increased levels of circulating IL-33 in the serum of patients with hypothyroidism are linked to alterations in lipid profiles and HOMA-IR. Hypothyroid patients, especially those with obvious symptoms showed elevated levels of IL-33, suggesting a possible role for this cytokine in detecting the metabolic shifts that precede these complications.
INTRODUCTION
Hypothyroidism is defined as any condition that results in a lack of thyroid hormones, which may due to hypothalamic or pituitary disease, widespread tissue resistance to thyroid hormones, or thyroid gland problems [1]. Approximately 5% of the population has hypothyroidism, with an additional 5% estimated to be undiagnosed. Primary hypothyroidism affects more than 99.99 percent of patients [2]. Subclinical hypothyroidism is a form of primary hypothyroidism characterized by an increased thyroid stimulating hormone (TSH) level and normal levels of free thyroxine (T4) and triiodothyronine (T3) in the blood. The transition from subclinical to overt hypothyroidism occurs in about 2-5% of cases annually [3]. If untreated, hypothyroidism can cause high blood pressure, abnormal lipid profiles, infertility, mental decline, and muscle weakness.
Aging is a significant factor in hypothyroidism, and this disease is more common in women than in men [4]. Thyroid dysfunction alters the composition and transport of lipoproteins [5]. Thyroid hormones also stimulate the catabolism of low-density lipoprotein (LDL) particles by activating LDL receptors. This is because the promoter of the LDL receptor gene contains a thyroid hormone responsive region that allows T3 to up-regulate LDL receptor gene expression [6]. Thyroid hormones influence glucose metabolism and the development of insulin resistance. According to the evidence, insulin resistance of peripheral tissues predominates in hypothyroidism [7]. Thyroid gland autoimmune diseases are thought to be the most common cause of thyroid gland problems. There are two kinds of autoimmune thyroid diseases: autoimmune thyroiditis (AIT) and Graves' disease. The AIT includes the most T cells, which can cause thyrotoxicosis at first and hypothyroidism later due to thyroid gland destruction [8]. Autoimmune disease develops when the immune system incorrectly targets a person's tissue, resulting in organ destruction or dysfunction. Autoimmune diseases are the most common reasons for death in women between the ages of 15 and 44, according to reports. Autoimmune diseases are frequently caused by a combination of genetic factors, environmental influences, and abnormal immunity, which may include infections [9].
Interleukin-33 (IL-33) is a nuclear cytokine that belongs to the IL-1 family and has been shown to be highly expressed in endothelial, epithelial, and fibroblast-like cells during homeostasis as well as inflammation [10]. IL-33 production promotes the progression of inflammation following damage to cells or death, and it is capable of stimulating several immune system effectors and regulating a wide range of immune responses [11]. The release of biologically active IL-33 occurs when a cell undergoes either apoptosis or necrosis [12, 13]. Autoimmune diseases are characterized as self-reactive immune disorders that cause the body to attack its own tissues. The most prevalent autoimmune diseases include rheumatoid arthritis (RA), inflammatory bowel disease (IBD), multiple sclerosis (MS), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), psoriasis, type 1 diabetes (T1D), uveitis, and autoimmune thyroid disease (AITD) [8]. These diseases are a concern for public health because they are long-lasting and incurable, causing not only significant individual suffering but also significant costs to society. Thus, the purpose of the study was to examine the correlation between insulin resistance and IL-33 in patients with hypothyroidism.
MATERIALS AND METHODS
Selection of patients
A total of 180 participants were included in the case-control study. 120 patients with hypothyroidism, of which 20 were male and 100 were female. Since laboratory testing is the primary factor in diagnosing hypothyroidism, the patients with the condition were separated into two distinct subgroups: overt hypothyroidism (OH, 60 patients), and subclinical hypothyroidism (SCH, 60 patients). Patients with overt hypothyroidism are diagnosed with the condition when their serum TSH levels are elevated while their T4 and T3 levels are deficient. Milder forms of hypothyroidism patients who have a normal serum T4 and T3 concentration but a high serum TSH concentration (greater than 4.5 IU/ml), may have a mild form of hypothyroidism. Also, the study was carried out with the formation of histories of patients including anthropometric measurements (ages, sex, body mass index, weight, and height) within age 18-55 years, compared with 60 individuals who served as a healthy control (HC) group and were matching with patients in age and sex. Patients who had a thyroidectomy because of cancer, patients who were anemic, patients who had any kind of obvious systemic disease or chronic disease, and patients who were taking any kind of medication that could potentially affect lipid metabolism were not allowed to participate in this study. The study was carried out in a specialized center for Al-Hakeem hospital in the Iraqi province of Al-Najaf between the months of October 2022 and February 202. The research was approved by the University of Kufa's institutional ethics board (8298/2022). All controls and patients as well as their guardians (parents or other close family members) gave written informed consent prior to participation in this study.
Calculation of body mass index
According to body mass index (BMI) was calculated by taking an individual's weight in kilograms and dividing it by their height in meters squared [14]. The formula for BMI is weight in kilograms divided by height in meters squared.
Serological assays
After fasting for a period of 12 hours, blood samples were collected via venipuncture and placed in gel tubes for later analysis. Separating the serum required 15 minutes of centrifugation at 3000 xg followed by storage at -20°C until use. Using the ELISA kit (Melsin Medical Co Company, China), levels of insulin, total triiodothyronine (T3), total tetraiodothyronine (T4), and thyroid stimulating hormone (TSH) were determined. While the enzymatic method (kits from BIOLABO, France) was used to determine HDL, total cholesterol, and triglyceride (TG) levels. The method was used to determine the levels of glucose in the serum while the subject was fasting. The following is how the insulin resistance index, also known as the homeostatic model assessment for insulin resistance (HOMA-IR), was calculated:
HOMA IR = [glucose (mg/dl) * insulin (U/ml) / 405
HOMA-β percentage =360 insulin/ (Glucose - 63) [15]
Low density lipoprotein cholesterol (LDL-C) was measured by the indirect method using Fried Ewald equation [16]
LDL-C= total cholesterol – (HDL-cholesterol + VLDL cholesterol).
LDL-C = total cholesterol - (HDL-cholesterol + TG/5)
Statistical analysis
A Kruskal-Wallis test was carried out on the data by using SPSS v27 software (which was developed by SPSS Inc. and is based in Chicago, Illinois, United States) in order to determine whether or not there were differences between the research variables. In the cases where the p-values were either less than 0.05 or less than 0.01, respectively. The coefficient of correlation developed by Pearson, which was used to assess the association between the two variables, was used to analyze the link between analyte levels within each research group to determine whether or not there was a correlation between the two. This was done in order to determine whether or not there was an association between the two variables. In addition, receiver operating characteristic curves (ROC curves) were generated through the use MedCalc software in order to evaluate the usefulness of biomarkers in the diagnosis and prognosis of hypothyroidism. The area under the curve (AUC) was computed so that it could be used as a method for determining how reliable the test was. The cutoff for statistical significance was set at P less than 0.05 and P less than 0.01, which indicates that differences with a probability of less than 5% or 1% were considered to be statistically significant. This threshold for statistical significance was established at the University of California, Berkeley.
RESULTS
Baseline characteristics of the patients
Table 1 and Figure 1 shows the study's baseline characteristics. This included data from study groups such as OH, SCH, and HC. Age and sex had no significance between study groups. The mean levels of BMI, serum TSH, FSG, insulin, TG, cholesterol, VLDL-C, LDL-C, and IL-33 and HOMA-IR were significantly higher in all hypothyroidism patient groups, while HOMA-β decreased significantly in patients compared with healthy control group. Also, SCH group had significantly higher levels of T3 and T4 as compared with OH group.
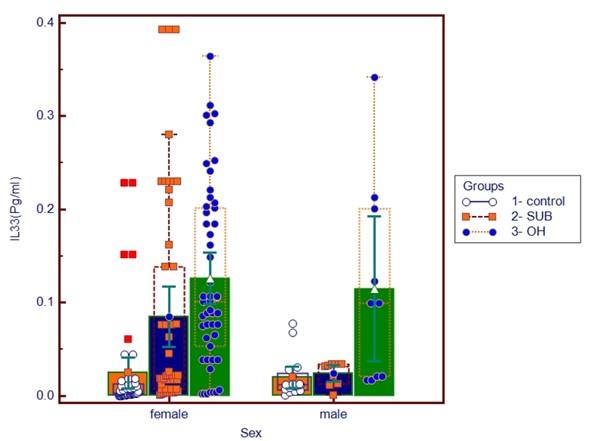
Table 1. Comparison of the means of clinical parameters between patients and a control group.
Correlation between serum IL-33 levels and other parameters in hypothyroidism patients
The results of the correlation analysis between serum IL-33 levels in overt hypothyroidism patients’ group and other parameters are summarized in Table 2 and Figure 1. There was a positive correlation between IL-33 with BMI, HOMA-IR and significant negative correlation was found between levels of IL-33 and T4. While the correlation of IL-33 in subclinical patients group revealed a significant positive correlation with TG, and VLDL-C and significant negative correlation with T3 level.
In Table 2, the following a positive correlation was found between serum IL-33 levels and other parameters such as FSG, TG, LDL-C and VLDL-C, while A negative correlation was found between IL-33 and serum level of TSH, HOMA-β, and HDL in participants.
Table 2. The univariate analysis of serum IL-33 level with the investigated parameters in the enrolled patients.
DISCUSSION
Hypothyroidism is caused by a thyroid that produces insufficient thyroid hormone is necessary for maintaining the body's normal functioning. Thyroid hormone deficiency causes the functions of the body to become less efficient, resulting in widespread symptoms such as fatigue and a loss of energy. It is also one of the more common diseases in women than in men, and it affects young women far more than young men. Hypothyroidism can develop at any age, and the risk increases with age [17, 18]. Thyroid dysfunction manifests in a non-specific and frequently variable manner, so biochemical abnormalities are used to make the diagnosis. T3 and T4, thyroid hormones, have a complex inverse relationship with the pituitary hormone, TSH [19]. TSH levels are the most accurate indicator of a person's thyroid health because of a negative feedback mechanism between TSH and thyroid hormones [20]. In the current study, results showed increase level of IL-33 in hypothyroidism patients especially in overt group which may be related to numerous inflammatory and autoimmune diseases, suggesting the crucial role of IL-33 as an active participant in contributing to aberrant local and systemic damage [21]. IL-33 display intricate traits that are relevant to both health and disease. IL-33 is expressed by many tissues, with endothelial cells being the main source of this molecule. Additionally, barrier tissues' epithelial cells are the main producers of IL-33 [22]. Fibrosis, autoimmune disorders, and the reaction to antigen-allergens have all been linked to the IL-33/tumorigenicity 2 (ST2)pathway, an intercellular signaling system [23]. IL-33 may play a critical role in regulating inflammatory processes and pathogeneses, suggests mounting evidence [24]. IL-33, which suppresses ST2, causes immune and non-immune cells to release it quickly in response to stress, activating inflammatory and immune responses. It is known that IL-33 exhibits pro- and anti-tumorigenic effects in a variety of diseases. These effects are likely dependent on the primary target cells, the level of IL-33/ ST2 expression, the cellular environment, and the cytokine microenvironment [25, 26]. It is interesting to note that patients who suffer from chronic heart failure have higher serum levels of IL-33. IL-33 has been demonstrated to have antioxidant effects, so this finding is particularly relevant. Because IL-33 plays such a significant role in both inflammatory conditions and oxidative stress, it is possible for individuals to take part in a wide variety of pathophysiological processes [27]. Previous research has tended to support the idea that heart failure (HF) is influenced by a variety of factors, including inflammation, eating habits, heredity, and others, among which inflammatory reactions are more prevalent. By combining with downstream ST2, IL-33 can start an inflammatory response that damages myocardial cells and even results in heart failure. Additionally, this study discovered that HF patients had higher peripheral blood levels of IL-33 and ST2 than the healthy control group. It might be because this study views the IL-33/ST2 signaling pathway as the cardiac dynamics' activation system, and since excessive myocardial stretch results in excessive ST2 secretion, which in turn activates the IL-33/ST2 signaling pathway and eventually speeds up ventricular remodeling and causes HF, this pathway may be to blame. This backs up earlier studies [28, 29]. Insulin resistance (IR), which is brought on by both hyperthyroidism and hypothyroidism, is well-known to be impacted by thyroid hormones [30]. Coronary artery disease risk has been linked to subclinical or overt hypothyroidism [31]. Results from the current study show that SCH and OH patients have higher serum insulin levels compared to the general population. Insulin resistance, defined as a reduced cellular response to insulin, is associated with increased danger of developing diabetes and cardiovascular disease. A higher insulin resistance index is associated with an increased risk of diabetes and metabolic syndrome in hypothyroid patients [32].
CONCLUSION
The present study revealed that there was a significantly higher circulating levels of IL-33 in hypothyroidism patients (especially in overt hypothyroidism) while lower level of IL-33 was detected in healthy control. The IL-33 was negatively correlated to T3, T4, and HDL-C levels and positively correlated with insulin resistance in hypothyroidism patients. This increased IL-33 may play an important role in the pathogenesis and progression of thyroid dysfunction and may exert antioxidant activity which may attenuate oxidative stress. More studies are required to confirm these findings in hypothyroidism patients.
ACKNOWLEDGEMENT
The authors would like to thank all the patients and medical staff at the AL-Hakeem hospital in Najaf province. This work would never have been completed without the patient’s role and cooperation in collecting the sample.
AUTHORS CONTRIBUTION
Material preparation, data collection, and analysis were performed by ZAJ. RAA written the first draft of the manuscript and helped to revise the manuscript. HAA who is the co-corresponding author, designed the study, and took responsibility for the integrity of data and the accuracy of data analysis; provided the administrative, technical, and material support. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Nallagonda S, Inusa A, et al. Thyroid disorders in neonates: A practical approach to congenital hypothyroidism and thyrotoxicosis. 2023.
- [2]Chiovato L, Magri F, et al. Hypothyroidism in context: Where we’ve been and where we’re going. Advances in Therapy. 2019;36:47-58.
- [3]Khandelwal D, Tandon NJD. Overt and subclinical hypothyroidism: Who to treat and how. 2012;72:17- 33.
- [4]Das D, Sahu D, et al. Ayurvedic approach to management of hypothyroidism-a case study”. 2021;11:43645-8
- [5]Rashed Ali Khan DJJoPNR. The spectrum of lipid disorders in hypothyroid patients; a cross-sectional study. 2022:2659-64.
- [6]Fiorucci S, Distrutti E, et al. Bile acids and their receptors in metabolic disorders. 2021;82:101094.
- [7]Al-Beltagi M, Bediwy AS, et al. Insulin-resistance in paediatric age: Its magnitude and implications. 2022;13:282.
- [8]Wu Z, Zhu Y, et al. Serum ratio of free triiodothyronine to thyroid-stimulating hormone: A novel index for distinguishing graves’ disease from autoimmune thyroiditis. 2021;11:620407.
- [9]Xiao ZX, Miller JS, et al. An updated advance of autoantibodies in autoimmune diseases. 2021;20:102743.
- [10]Cayrol C, Girard JPJIr. Interleukin‐33 (il‐33): A nuclear cytokine from the il‐1 family. 2018;281:154-68.
- [11]Allegra A, Innao V, et al. The st2/interleukin-33 axis in hematologic malignancies: The il-33 paradox. 2019;20:5226.
- [12]Liu X, Xiao Y, et al. The role of the il-33/st2 axis in autoimmune disorders: Friend or foe? 2019;50:60-74.
- [13]Calcagno TM, Mirsaeidi M. Pulmonary manifestations of autoimmune diseases. Translational autoimmunity: Elsevier; 2022. p. 265-94.
- [14]Nuttall FQJNt. Body mass index: Obesity, bmi, and health: A critical review. 2015;50:117.
- [15]DeUgarte CM, Bartolucci AA, et al. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. 2005;83:1454-60.
- [16]riedewald WT, Levy RI, et al. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. 1972;18:499-502.
- [17]Shahid MA, Ashraf MA, et al. Physiology, thyroid hormone. 2018.
- [18]Kaltsas G, Vgontzas A, et al. Fatigue, endocrinopathies, and metabolic disorders. 2010;2:393-8.
- [19]Caron P, Grunenwald S, et al. Factors influencing the levothyroxine dose in the hormone replacement therapy of primary hypothyroidism in adults. 2021:1-21.
- [20]Hoermann R, Pekker MJ, et al. The role of supporting and disruptive mechanisms of ft3 homeostasis in regulating the hypothalamic–pituitary–thyroid axis. 2023;14:20420188231158163.
- [21]Shakerian L, Kolahdooz H, et al. Il-33/st2 axis in autoimmune disease. 2022;158:156015.
- [22]Chan BC, Lam CW, et al. Il33: Roles in allergic inflammation and therapeutic perspectives. 2019;10:364.
- [23]Hong Y-S, Moon S-J, et al. Measurement of interleukin-33 (il-33) and il-33 receptors (sst2 and st2l) in patients with rheumatoid arthritis. 2011;26:1132-9.
- [24]Qiu H, Ni C, et al. Circrna7632 down-regulation alleviates endothelial cell dysfunction in kawasaki disease via regulating il-33 expression. 2023:1-12.
- [25]Guo H, Bossila E, et al. Dual immune regulatory roles of interleukin-33 in pathological conditions. Cells 2022, 11, 3237. s Note: MDPI stays neutral with regard to jurisdictional claims in published …; 2022.
- [26]Guo H, Bossila EA, et al. Dual immune regulatory roles of interleukin-33 in pathological conditions. 2022;11:3237.
- [27]Miao Y, Zhang Z-x, et al. Il-33 as a novel serum prognostic marker of intracerebral hemorrhage. 2021;2021.
- [28]Kotsiou OS, Gourgoulianis KI, et al. Il-33/st2 axis in organ fibrosis. Frontiers in immunology. 2018;9:2432.
- [29]Xiang N, Liao H, et al. Expression and significance of inflammatory reactions mediated by the il-33/st2 signaling pathway in the serum of heart failure patients. American journal of translational research. 2021;13:8247-52.
- [30]Teixeira P, Dos Santos PB, et al. The role of thyroid hormone in metabolism and metabolic syndrome. Therapeutic advances in endocrinology and metabolism. 2020;11:2042018820917869.
- [31]Sue LY, Leung AM. Levothyroxine for the treatment of subclinical hypothyroidism and cardiovascular disease. Frontiers in endocrinology. 2020;11:591588.
- [32]Ebrahimpour A, Vaghari-Tabari M, et al. Direct correlation between serum homocysteine level and insulin resistance index in patients with subclinical hypothyroidism: Does subclinical hypothyroidism increase the risk of diabetes and cardio vascular disease together? 2018;12:863-7