High-intensity interval training increases AMPK and GLUT4 expressions via FGF21 in skeletal muscles of diabetic rats
Abstract
In type 2 diabetes, a decrease in expression of glucose transporter type 4 (GLUT4) results in decreased glucose uptake while fibroblast growth factor 21 (FGF21) promotes glucose uptake. The liver and other organs, such as the muscles, produce FGF21. Physical activity increases FGF21 secretion from skeletal muscles. FGF21 is thought to promote glucose uptake during exercise by activating AMP-activated protein kinase (AMPK). Thus, the goal of this study is to determine how high-intensity interval training (HIIT) affects glucose uptake via FGF21 signaling in diabetic rats. Twenty-four eight-week-old male Wistar rats were randomly divided into four categories including Control (Con), DM intervention (ConDM), DM group undergoing continuous training (DM-CT), and DM group undergoing high-intensity interval training (DM-HIIT). The trainings in the exercise groups (DM-CT and DM-HIIT) were performed six days a week for six weeks. We analyzed FGF21 levels in serum and muscles by ELISA. Measurement of phosphorylated AMPK in muscles used ELISA, and that of mRNA GLUT4 expression used RT-PCR. Following HIIT interventions, the glucose level in plasma was closer to normal level. An increase in FGF21 levels aided in the reduction in blood glucose. The HIIT group had significantly higher FGF21 levels in serum and muscles. An increase in FGF21 was followed by an increase in AMPK phosphorylation and GLUT4 expression in the HIIT group. In diabetic rats, HIIT promotes glucose uptake via FGF21, which activates AMPK and increases GLUT4 expression.
INTRODUCTION
Insulin is a polypeptide hormone secreted by pancreatic β cells that binds to plasma membrane-bound receptors on target cells to regulate an integrated anabolic response to nutrient availability that maintains normal blood glucose levels by facilitating cellular glucose uptake and regulating cellular metabolism [1]. Insulin signaling is initiated by the phosphorylation of the tyrosine residue, which induces the insulin receptor substrate (IRS) protein, thereby triggering the phosphatidylinositol 3 kinases (PI3K) pathway, which is responsible for glucose uptake [2-4]. Defective action of insulin in tissues often precedes the development of systemic insulin resistance, progressively leading to type 2 diabetes mellitus (T2DM) [5]. The detailed mechanisms underlying insulin resistance remain unclear. However, previous research reports that insulin resistance occurs because of excessive secretion of counter-insulin hormones [1]. In addition, other mechanisms that may underlie insulin resistance include disruption of downstream signaling, such as IRS, PI3K, and abnormal function of glucose transporter type 4 (GLUT4). The primary glucose transporter that facilitates glucose absorption in the musculoskeletal system is GLUT4 [6].
The hormone fibroblast growth factor 21 (FGF21) promotes glucose uptake and is produced in the liver and several other organs, including the muscles. The binding of FGF21 with its receptor FGFRs and coreceptor βKlotho may increase insulin sensitivity due to the activation of the PI3K pathway, thereby increasing GLUT4 translocation in the muscles [7]. In addition, although the mechanism is unclear, FGF21 directly affects GLUT4 expression and translocation as insulin independent manner. Binding of FGF21 to the FGF receptor 1 and coreceptor βKlotho complex activates AMP-activated protein kinase (AMPK) via serine–threonine liver kinase B1 (LKB1) [8]. LKB1 itself is identified as an essential upstream AMPK during electrically induced muscle contractions [8].
AMPK may increase GLUT4 expression through response elements in the promoter bp 895 and the transcription factor such as guanine nucleotide exchange factors (GEF) and myocyte enhancer factor (MEF). Meanwhile, the GLUT4 translocation is mediated by Rab-GTPase activating protein [9, 10]. Physical exercise can activate AMPK, thereby increasing insulin-independent glucose uptake [11]. The previous study showed that rodents fed high-fat diets given regular exercise showed physiological changes by involving FGF21–AMPK signaling in skeletal muscles [11]. However, the previous study did not explain the activation of AMPK by physical exercise through FGF21 signaling in promoting glucose uptake.
Physical exercise increases FGF21 secretion from muscles as a myokine [12]. Therefore, physical exercise may induce the sympathetic nervous system to release norepinephrine, which stimulates FGF21 secretion by the skeletal muscles [13]. However, the sympathetic response during exercise depends on the intensity of the exercise. High–intensity during training in healthy subjects can increase FGF21 secretion [14]. In line with the previous study, FGF21 levels were increased by HIIT in skeletal muscles, greater than continuous moderate training in obese rats [15]. However, there has been no evidence of the outcome of HIIT on glucose uptake in DM, specifically through FGF21 signaling. Therefore, the goal of this research is to determine the impact of physical activity such as high-intensity interval training (HIIT) on glucose uptake via AMPK activation by FGF21 in diabetic condition.
MATERIALS AND METHODS
Study design
Twenty-four eight-week-old male Wistar rats (Rattus norvegicus) weighing 180–250 g were housed in six cages with a 12 h:12 h dark/light cycle. Ambient climate was kept constant at 25°C ±1°C. The Ethical Board of the Faculty of Medicine, Universitas Indonesia, approved all experiments, and Universitas Indonesia has supported this research. All procedures of ethical Approval were performed in accordance with the ICH-GCP standard procedure (KET-1345/UN2.F1/ETIK/PPM.00.02/2020).
Animals
Twenty-four eight-week-old male Wistar rats were randomly divided into four categories such as control (Con), DM intervention (ConDM), DM group undergoing continuous training (DM-CT), and DM group undergoing HIIT (DM-HIIT). Streptozotocin (STZ) was injected intraperitoneally at a dose of 30–40 mg/kg dissolved in 0.1 M cold citrate buffer (pH 4.5). After 72 h, blood was drawn from the tail vein and glucose levels were measured using a blood glucose meter test. Diabetic rats had blood glucose levels greater than 200 mg/dL. All rats developed diabetes 72 h after injection.
Exercise protocol
All rats went through an adaptation period before beginning exercise training to reduce the potential stress of the equipment. The Afzalpour protocol was used for the intervention of continuous raining (CT) and HIIT (Table 1) as described previously [16]. The training was held six days a week for six weeks. Warm-up, core, and cool-down exercises were included in the CT and HIIT sessions. Warm-up and cool-down sessions for both CT and HIIT were performed at a constant speed and duration, particularly, for 5 min at a speed of 16 m/min, which corresponded to a maximum oxygen absorption of 68% (VO2 max).
The speed of the core CT exercise was set at 27 m/min, and the length of each daily routine gradually increased, reaching 60 min in the last two weeks. The core HIIT exercise was made up of several cycles (intervals) of each process’s short rest period. The number of daily interval training sessions was gradually increased as the speed of exercise and rest sessions alternated between even and odd days at 40 m/min–30 s or 54 m/min–3 s. CT was 80% of one’s maximum VO2 max intensity, whereas HIIT was 90%–100% of one’s maximum VO2 max intensity. Following exercise group training (DM-CT and DM-HIIT), the mice in the Con and ConDM groups were displayed to the exercise room on a daily basis.
Table 1. HIIT and continuous training protocols.
Sampling
Xylazine (0.01 mL/kg) and ketamine (0.05 mL/kg) were injected intraperitoneally to deeply anesthetize the rats at 2 × 24 h after the last exercise regimen. The muscles were then dissected and detached from the bones to remove the gastrocnemius muscle tissue. The gastrocnemius muscle was immediately frozen at −80°C for further examination. The sample preparation protocol from the Enzyme-linked immunosorbent assay (ELISA) kit manual was followed for tissue homogenate preparation.
Measurement of blood glucose
Blood was taken from the tail vein and glucose levels were measured using a blood glucose meter test. Blood sugar measurements were carried out every week until the research was completed.
Measurement of serum FGF21
Blood was taken through retro-orbitally, then centrifuged for 10 min at 3000 rpm to obtain serum and stored at −80°C before analysis. Bradford assay (Thermo Fisher Scientific, USA) was used to determine the protein content of the serum. The ELISA kits (Rat FGF21 BZ-08182631-EB Bioenzy, Indonesia) were used to test FGF21 levels according to manufacturer’s recommendation. Samples were measured in duplicate using a microplate reader at 450 nm. FGF21 levels on serum were expressed in ng/mL.
Measurement of FGF21 and phosphorelated AMPK in skeletal muscles
Tissue samples (900–100 mg) were homogenized in 1000 𝜇L of PBS with homogenizer tools on ice. The samples were centrifuged at 12,000g under 4°C for 20 min. The supernatant was removed and divided into aliquots for storing at −80°C. Bradford assay (Thermo Fisher Scientific, USA) was used to determine the protein content of the homogenate.
FGF21 and phosphorelated AMPK were quantified in homogenate samples using ELISA kits (Rat FGF21 BZ-08182631-EB Bioenzy, Indonesia and phosphorylated-AMPK BZ-08186340-EB-Bioenzy, Indonesia) to test FGF21 and phosphorylated AMPK levels as directed by the manufacturer’s instructions. The absorbance of the samples was measured in duplicate using a microplate reader at 450 nm. The levels of FGF21 and phosphorylated AMPK levels were expressed in ng/mg.
Measurement of mRNA GLUT4
Total cellular RNA was extracted from the gastrocnemius muscle using the Quick-RNATM Miniprep Plus kit (Zymo Research, California). Reverse transcribed using ReveraTra Ace qPCR master RT master mix with gDNA remover (Toyobo, Japan) was used to create single-stranded cDNA.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and GLUT4 (Slc2a4) were quantified using the Applied BiosystemTM StepOne Real-Time PCR system with the SensiFASTTM SYBR HI-ROX kit (Bioline, Toronto).
The primers listed below were used:
|
|
GAPDH |
GLUT4 (Slc2a4) |
|
Forward primer |
5′-aagttcaacggcacagtcaagg-3′ |
5′-ggctgtgccatcttgatgac-3′ |
|
Reverse primer |
5′-catactcagcaccagcatcacc-3′ |
5′-cacgatggacacataactcatggat-3′ |
The 2−ΔΔCt method and relative quantification were used to analyze the data.
Statistical analysis
Prism software was used to conduct statistical analysis (Graphpad ver. 9). The normality test was conducted using Shapiro-Wilk to see the data distribution and Levene’s test for data homogeneity. FGF21, phosphorylated AMPK, and mRNA GLUT4 expression data were analyzed using a one-way ANOVA, followed by Tukey’s post-hoc test. All data were presented as mean ± SEM, with a Sig (2-tailed) p-value less than 0.05 considered statistically significant.
RESULTS
Physical exercise for six weeks decreased plasma blood sugar levels in rats
Before physical exercise, the mean blood sugar was obtained in the Con group 117 ± 2.082 mg/dL; the ConDM group 302.3 ± 24.11 mg/dL; the DM-CT group 295.3 ± 5.149 mg/dL; and the DM-HIIT group 287 ± 3.416 mg/dL (Figure 1A). After six weeks of physical exercise, blood sugar was obtained in the Con group 116 ± 1.826 mg/dL, ConDM group 282.2 ± 21.27 mg/dL, DM-CT group 258.2 ± 6.123 mg/dL, DM-HIIT group 216.5 ± 2.277 mg/dL (Figure 1B).
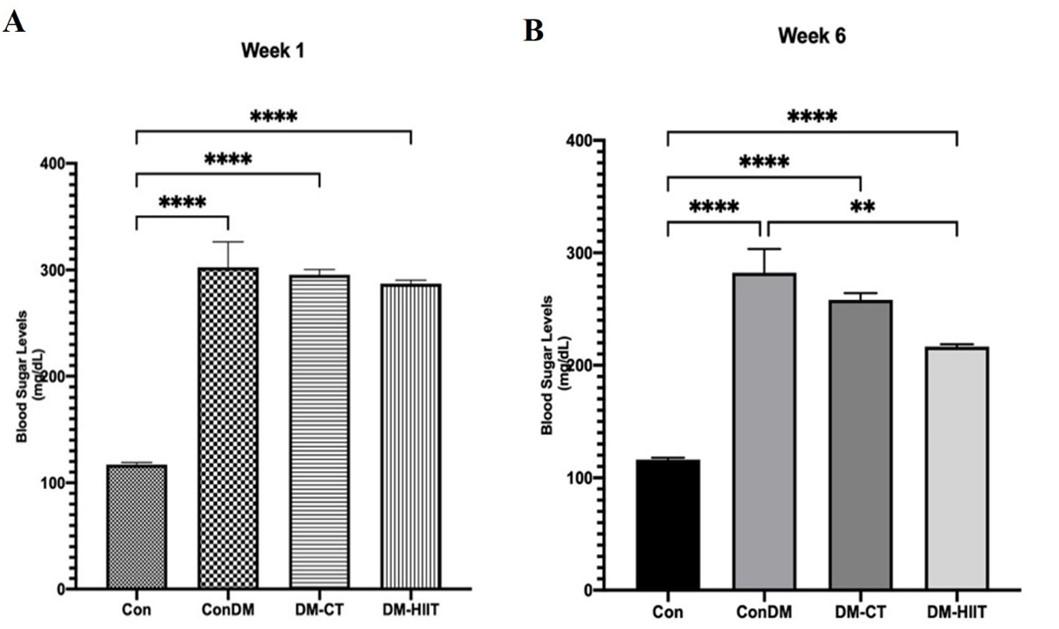
The results in Figure 1A showed that the blood sugar level in the first week was >200 mg/dL in the ConDM, DM-CT, and DM-HIIT groups. The provision of physical exercise CT for six weeks in Figure 1B decreased blood sugar levels reached 258 mg/dL. HIIT also significantly reduced blood sugar levels, close to the normal level (216 mg/dL).
High-intensity interval training increases GLUT4 expression in muscles
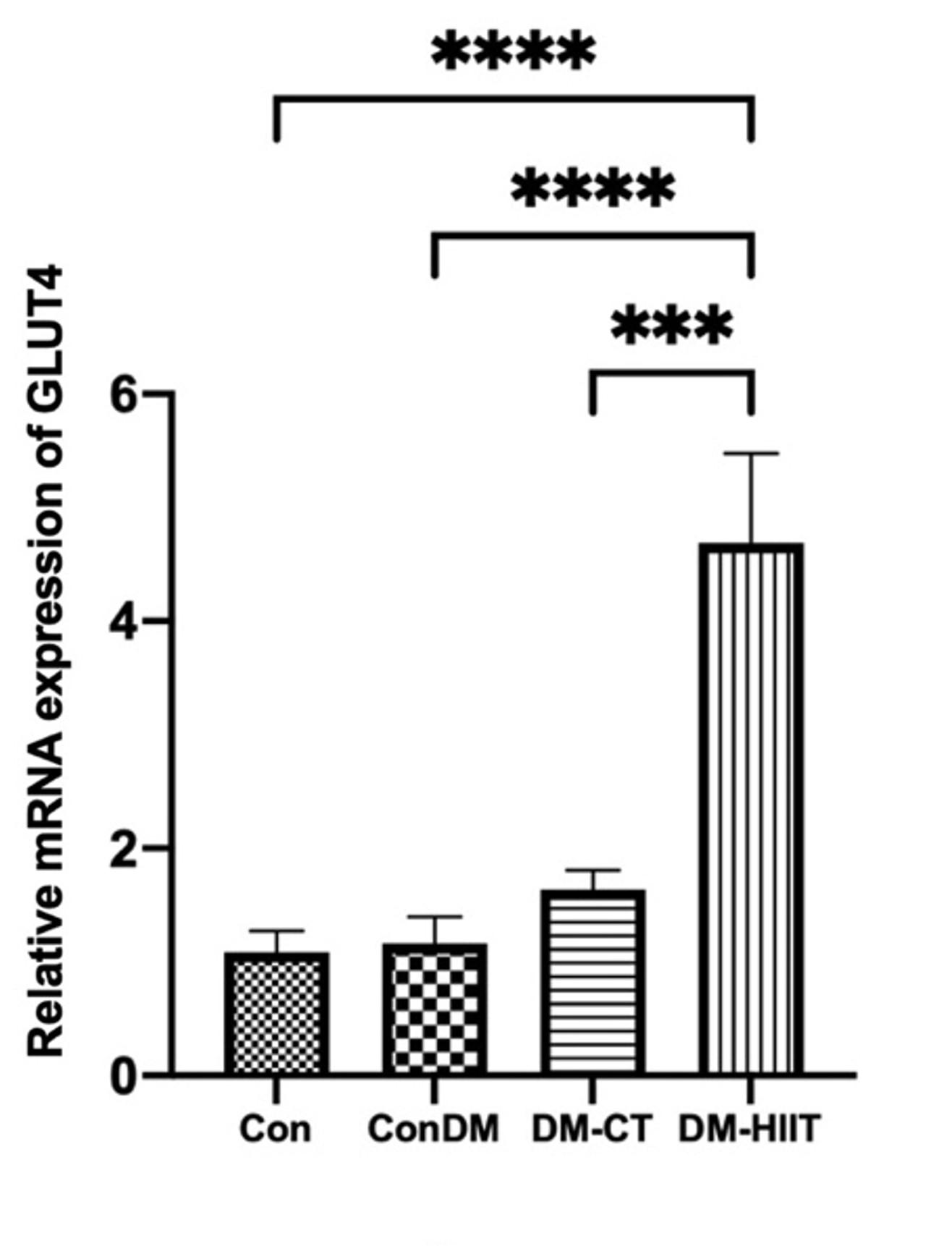
GLUT4 gene expression in the DM group was found to be insignificantly different from that in the Con group in this study (Figure 2). DM-CT slightly increased GLUT4 expression compared to the Con group. However, the provision of the DM-HIIT significantly increased GLUT4 expression even though its expression was more significant than that in the Con group (Figure 2).
High-intensity interval training increases phosphorylated AMPK in skeletal muscles
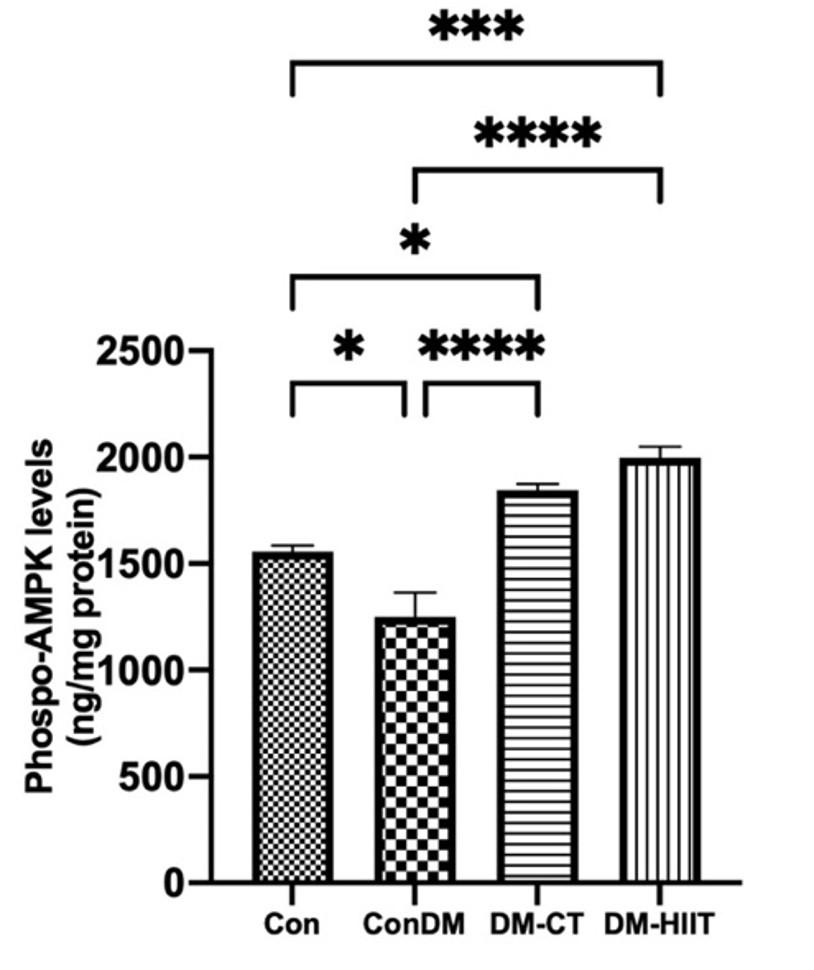
In this study, the AMPK phosphorylation levels in the DM group were lower than those in the Con group (Figure 3). Meanwhile, the provision of DM-CT and DM-HIIT increased AMPK phosphorylation significantly compared to the Con group. The highest AMPK phosphorylation levels were found in the DM-HIIT group. This indicates that physical exercise, especially DM-HIIT, has increased AMPK phosphorylation levels in DM conditions (Figure 3).
High-intensity interval training increases the FGF21 level in skeletal muscles
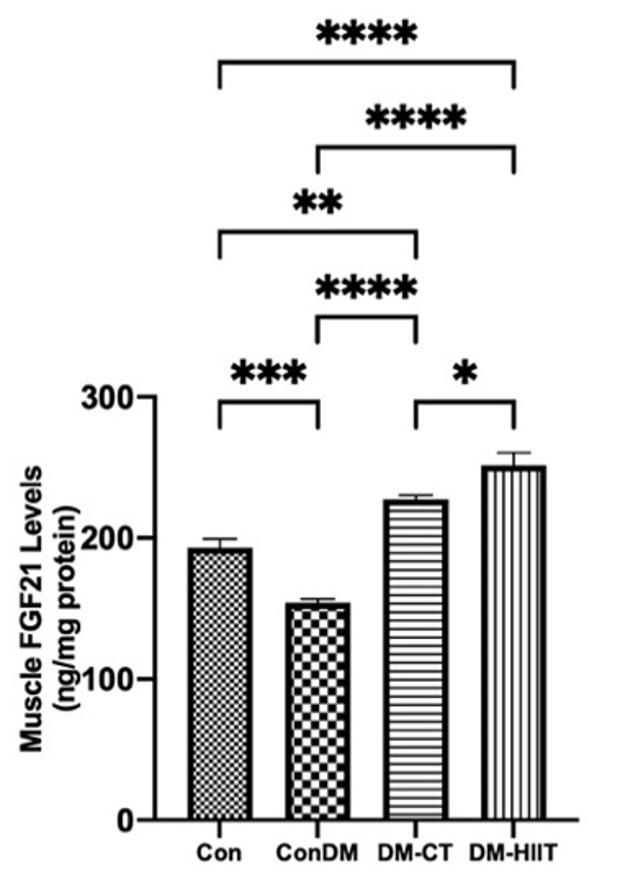
This study showed that the FGF21 level in skeletal muscles in the DM group was lower than that in the Con group (Figure 4). The provision of the DM-CT and DM-HIIT increased the FGF21 levels in skeletal muscles significantly, in contrast to the Con group. However, HIIT had significantly greater FGF21 levels in skeletal muscles than CT. This indicates that physical exercise, especially HIIT, has increased FGF21 levels in skeletal muscles in DM conditions (Figure 4).
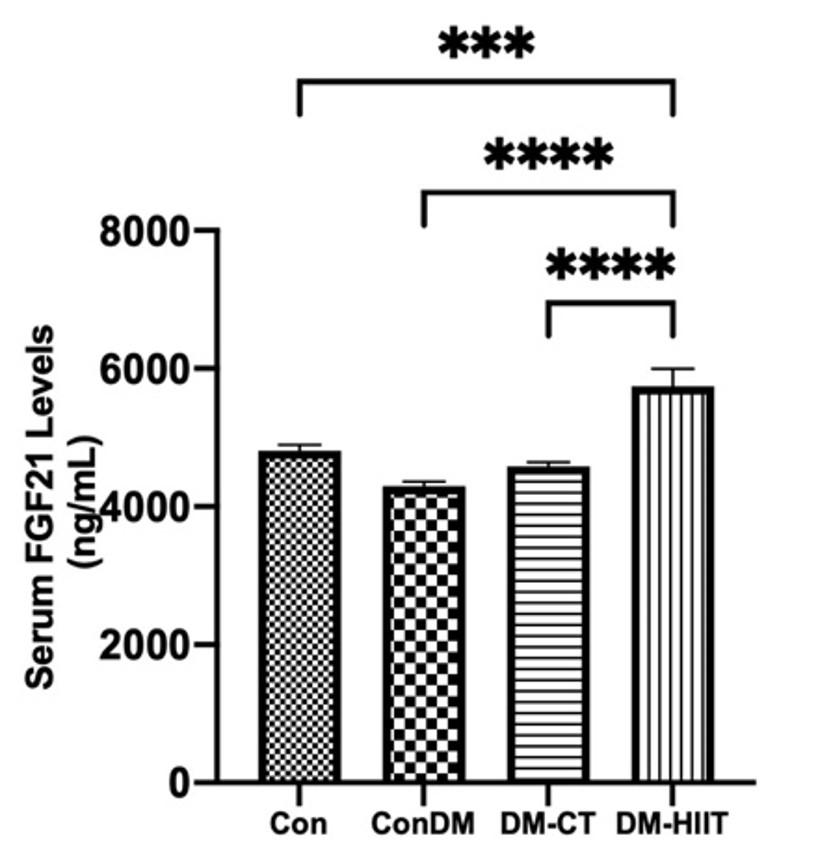
High-intensity interval training increases serum FGF21 levels
In this study, the DM group’s serum FGF21 level was discovered to be marginally lower than that in the Con group (Figure 5). In addition, FGF21 levels in serum were increased by DM-CT and DM-HIIT in comparison to the DM group. FGF21 levels in serum were higher in the DM-HIIT group than in the DM-CT group. The DM-HIIT group had the highest serum FGF21 levels, which were significantly higher than those in the Con group. This suggests that HIIT in diabetes may increase FGF21 levels in serum, even if they are higher than in normal conditions (Figure 5).
DISCUSSION
Blood glucose levels were found to be lower than baseline after six weeks of CT and HIIT. Even blood glucose levels were closer to normal level in the HIIT group. The decrease in blood glucose levels indicates an escalation in glucose absorption by the muscles. The primary glucose transporter that facilitates glucose absorption in skeletal muscles is GLUT4.
The decrease in blood levels following exercise is linked to increased GLUT4 expression in the muscles. This study found that GLUT4 expression was greater in the exercise groups than in the control groups. This study supports the findings that six weeks of physical exercise increased GLUT4 levels in skeletal muscles [17]. According to previous study reports, that the increase in GLUT4 transporter activity is due to muscle contraction [18]. The intensity of the exercise is related to muscle contraction. Cunha et al. demonstrated that high-intensity exercise and moderate-intensity exercise could increase GLUT4 protein in T2DM mice [19]. However, Chavenelle et al. found that HIIT for ten weeks increases GLUT4 [20]. According to this study, HIIT is more effective at increasing GLUT4 expression, even its expression is greater than normal group. This study discovered that increased GLUT4 expression resulted in increased phosphorylated AMPK.
AMPK activation increases GLUT4 exocytosis. When AMPK is activated, TBC1 domain family member 1 (TBC1D1) and TBC1 domain family member 4 (TBC1D4) are phosphorylated, performing GLUT4 translocation to the cell surface membrane [10, 21, 22]. Meanwhile, through phosphorylation of HDAC5, AMPK activation increases GLUT4 expression. AMPK regulates GLUT4 expression through interactions with histone deacetylase 4/5-myocyte enhancer factor 2 (HDAC4/5-MEF2) and myocyte enhancer factor 2- guanine nucleotide exchange factors (MEF2-GEF). Histone hyperacetylation at the GLUT4 promoter is caused by HDAC4/5 nuclear export, resulting in increased GLUT4 expression [21, 22].
AMPK levels increased after continuous training and HIIT, which was consistent with previous research. According to Chen et al., that increased AMPK alpha and beta activity after high-intensity cycling exercise increased AMPK phosphorylation in both subunits [23]. Conversely, an increase in phosphorylated AMPK was associated with exercise type and intensity. HIIT resulted in a greater increase in phosphorylated AMPK than continuous training. Richter et al. also discovered that high-intensity physical activity activated AMPK more than low-intensity physical exercise did [10]. Therefore, the intensity and type of exercise are related to the recruitment of muscle fiber types, especially type II fibers, and the AMPK phosphorylation. HIIT increased AMPK phosphorylation (Thr172) by 184% in type II fibers. Gibala et al. found that high-intensity cycling exercise showed increased phosphorylation of AMPK alpha and beta subunits [24]. Martinez-Huenchullan et al., reported that the proportion of phospho-AMPK Thr172/AMPK in skeletal muscles was also linked to HIIT exercise [25]. In line with these findings, Khalafi et al. reported that HIIT exercise increased AMPK phosphorylation in skeletal muscles more effectively [26].
Physical exercise can increase AMPK phosphorylation caused by a decrease in ATP. Energy expenditure during muscle contraction will reduce intracellular ATP levels and increase AMPs, thereby inducing AMPK phosphorylation/activation. Thus, the high energy expenditure of HIIT exercise increases intracellular ADP/ATP and AMP/ATP proportions, which has the ability to trigger AMPK. Hancock et al. reported that binding to AMP increases AMPK Thr172 phosphorylation and exertion by more than 100 times [27-29]. LKB1, an upstream of AMPK, will phosphorylate the Thr172 subunit in skeletal muscles. LKB1 is one of the phosphorylation targets of FGFR1, which is a receptor of FGF21 [8].
Thus, there is a relation between FGF21 and AMPK activation. Currently, the mechanism of FGF21 signaling is still not clearly understood. In adipose tissue, the role of FGF21 is to control mitochondrial function through AMPK activation. However, the role of FGF21 in increasing glucose uptake through AMPK activation remains unclear. In this study, the increase in phosphorylated AMPK after HIIT exercise was in line with FGF21 levels in both skeletal muscles and serum. The increase in FGF21 is due to sympathetic activity during physical exercise. This is evidenced by the use of nonselective-adrenergic receptor agonists in experimental animals that can increase mRNA and FGF21 secretion. When the sympathetic nervous system is activated, norepinephrine is released, which further modulates muscle contraction. Then, muscle contraction activates the PI3-kinase/Akt signaling pathway, stimulating the release of FGF21 from muscles and increasing serum FGF21 [30, 31]. Sympathetic activations are related to exercise intensity. Scalzo et al. showed that moderate-intensity exercise could not increase serum FGF21 [13]. By contrast, Tanimura et al, reported that high-intensity exercise increased FGF21 in the serum of experimental animals and humans [32]. An earlier study has demonstrated the outcome of HIIT increasing FGF21 in normal conditions. Meanwhile, Kartinah et al. discovered that HIIT exercise increased FGF21 levels in muscles more than moderate continuous exercise in HFD-induced mice [30]. Thus, few studies analyze the effect of HIIT in increasing FGF21 in DM rats [30].
This study shows that HIIT exercise can increase FGF21 in serum and skeletal muscles in DM rats. The role of FGF21 can be autocrine or endocrine. As an autocrine, FGF21 will bind to its receptors and coreceptors, namely, FGFR and βKlotho. This binding will activate the signaling pathway through LKB1 to activate AMPK. However, the drawback of this study is that it did not examine the FGFR and LKB1. However, this study shows an increase in FGF21, followed by an increase in AMPK, after HIIT exercise. Then, the increase in phosphorylated AMPK will increase GLUT4 expression, which is involved in glucose transfer. However, this increase in GLUT4 expression was followed by a decrease in blood glucose level; even the levels were close to normal levels. Moreover, the drawback of this study was that it did not examine GLUT4 translocations.
CONCLUSIONS
HIIT exercise promotes glucose uptake and reduces glucose levels in diabetic mice after six weeks of exercise, reaching close to normal levels. Physical exercise increases energy expenditure during muscle contraction and increases AMPs, with the escalation level of FGF21, which activates and increases AMPK levels, leading to a greater amount of GLUT4 expression in diabetic mice. GLUT4 expression facilitates glucose transport into muscle cells.
ACKNOWLEDGMENTS
The authors would like to express deepest gratitude to all colleagues at the Department of Physiology, University of Indonesia for their support and useful input. This work was funded by research PUTI DRPM-UI 2020 (Grant no.: NKB-1527/N2.RST/AKP.05.00/2020).
AUTHOR CONTRIBUTIONS
Conceptualization, formal analysis, funding acquisition, resources, supervisions, writing-original draft, and writing-review & editing: NTK. The investigation, methodology, resources, and validation: HR. Investigation: BCP. Validation: TA. Formal analysis, writing-original draft, and writing-review & editing: EII. Formal analysis, investigation, and resources: JM. Conceptualization, formal analysis: NP. Conceptualization, formal analysis, writing-original draft, and writing-review & editing: DISS. The authors declare that all data were generated in-house and that no paper mill was used.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Wilcox G. Insulin and insulin resistance. Clinical Biochemistry. 2005;26.
- [2]Ewa Świderska JS, Adam Wróblewski, Janusz Szemraj, Józef Drzewoski and Agnieszka Śliwińska. Role of pi3k/akt pathway in insulin-mediated glucose uptake. Intechopen. 2018.
- [3]Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850-67.
- [4]Laurie J.Goodyear FG, L. Adam Sherman, Julie Carey, Robert J.Smith, and G. Lynis Dohm. Insulin receptor phosporylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subject. The American Society for Clinical Investigation, Inc. 1995;Volume 95.
- [5]Rahman MS, Hossain KS, et al. Role of insulin in health and disease: An update. Int J Mol Sci. 2021;22.
- [6]Bradley H, Shaw CS, et al. Visualization and quantitation of glut 4 translocation in human skeletal muscle following glucose ingestion and exercise. Physiological reports. 2015;3:e12375.
- [7]Kruse R, Vienberg SG, et al. Effects of insulin and exercise training on fgf21, its receptors and target genes in obesity and type 2 diabetes. Diabetologia. 2017;60:2042-51.
- [8]Salminen A, Kauppinen A, et al. Fgf21 activates ampk signaling: Impact on metabolic regulation and the aging process. J Mol Med (Berl). 2017;95:123-31.
- [9]Richter EA, Ruderman NB. Ampk and the biochemistry of exercise: Implications for human health and disease. Biochemical Journal. 2009;418:261-75.
- [10]Richter EA, Hargreaves M. Exercise, glut4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993-1017.
- [11]Loyd C, Magrisso IJ, et al. Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding. Journal of applied physiology. 2016;121:687-98.
- [12]Oh KJ, Lee DS, et al. Metabolic adaptation in obesity and type ii diabetes: Myokines, adipokines and hepatokines. Int J Mol Sci. 2016;18.
- [13]Scalzo RL, Peltonen GL, et al. Regulators of human white adipose browning: Evidence for sympathetic control and sexual dimorphic responses to sprint interval training. PLoS One. 2014;9:e90696.
- [14]Kim KH, Kim SH, et al. Acute exercise induces fgf21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517.
- [15]Tine Kartinah N, Rosalyn Sianipar I, et al. The effects of exercise regimens on irisin levels in obese rats model: Comparing high-intensity intermittent with continuous moderate-intensity training. Biomed Res Int. 2018;2018:4708287.
- [16]Afzalpour ME, Chadorneshin HT, et al. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiology & behavior. 2015;147:78-83.
- [17]Kalhor H, Peeri M, et al. The effect of 6 weeks resistance training and high-intensity interval training on glut-4 gene expression of diabetic rats. Iranian Journal of Diabetes and Obesity. 2017;9:32-9.
- [18]Jensen TE, Sylow L, et al. Contraction-stimulated glucose transport in muscle is controlled by ampk and mechanical stress but not sarcoplasmatic reticulum ca2+ release. Molecular metabolism. 2014;3:742-53.
- [19]Cunha VN, de Paula Lima M, et al. Role of exercise intensity on glut4 content, aerobic fitness and fasting plasma glucose in type 2 diabetic mice. Cell biochemistry and function. 2015;33:435-42.
- [20]Chavanelle V, Boisseau N, et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Scientific reports. 2017;7:1-10.
- [21]Novikova D, Garabadzhiu A, et al. Amp-activated protein kinase: Structure, function, and role in pathological processes. Biochemistry (Moscow). 2015;80:127-44.
- [22]Okabe K, Yaku K, et al. Implications of altered nad metabolism in metabolic disorders. Journal of biomedical science. 2019;26:34.
- [23]Chen Z-P, Stephens TJ, et al. Effect of exercise intensity on skeletal muscle ampk signaling in humans. Diabetes. 2003;52:2205-12.
- [24]Gibala MJ, McGee SL, et al. Brief intense interval exercise activates ampk and p38 mapk signaling and increases the expression of pgc-1α in human skeletal muscle. Journal of applied physiology. 2009;106:929-34.
- [25]Martinez-Huenchullan SF, Ban LA, et al. Constant-moderate and high-intensity interval training have differential benefits on insulin sensitive tissues in high-fat fed mice. Frontiers in physiology. 2019;10:459.
- [26]Khalafi M, Mohebbi H, et al. The impact of moderate-intensity continuous or high-intensity interval training on adipogenesis and browning of subcutaneous adipose tissue in obese male rats. Nutrients. 2020;12:925.
- [27]Hancock CR, Janssen E, et al. Contraction-mediated phosphorylation of ampk is lower in skeletal muscle of adenylate kinase-deficient mice. Journal of Applied Physiology. 2006;100:406-13.
- [28]MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. The Journal of physiology. 2017;595:2915-30.
- [29]Suchankova G, Nelson LE, et al. Concurrent regulation of amp-activated protein kinase and sirt1 in mammalian cells. Biochemical and biophysical research communications. 2009;378:836-41.
- [30]Neng Tine Kartinah IRS, Rabia and Nafi’ah. High intermittent intensity training induces fgf21 secretion in obese rats. Journal of Obesity and Metabolism. 2018.
- [31]Panahi Y, Bonakdaran S, et al. Serum levels of fibroblast growth factor 21 in type 2 diabetic patients. Acta Endocrinol (Buchar). 2016;12:257-61.
- [32]Tanimura Y, Aoi W, et al. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol Rep. 2016;4.