Pomegranate fruit peel extract improves cardiac functions via suppressing oxidative stress, fibrosis, and myocardial infarction in Long-Evans rats
Abstract
The pomegranate fruit (locally known as Dalim) is a rich source of various bioactive metabolites. This study aimed to investigate the cardioprotective effect of polyphenol-rich pomegranate peel extract (PPE) in isoproterenol, a synthetic catecholamine-induced cardiac stress in rats. Twenty-four adult rats were taken to evaluate the effects. The selected rats were divided into four groups, such as control (Group I), Isoproterenol (ISO) administered (Group II), PPE with ISO treatment (Group III), and gallic acid (polyphenol) along with ISO administration (Group IV). As expected, ISO administration increased inflammatory cell infiltration, fibrosis, and lipid peroxidation. However, antioxidant enzyme activities were reduced in heart tissues of ISO-treated rats. Interestingly, PPE treatment effectively reduced ISO-induced various detrimental effects in heart tissues in rats. This protection was associated with several positive outcomes, including reduced inflammatory cell infiltration and fibrosis, reduction in free radicals by improving antioxidant enzymes activities, suppression of oxidative stress markers, and reduction in inflammatory marker. Besides, PPE treatment normalized the elevated levels of liver and cardiac injury markers. The cardioprotective effects shown by PPE may be due to the presence of polyphenolic compounds, as determined by HPLC analysis. Overall, these data suggest that polyphenol enriched PPE may protect cardiac tissues in ISO-administered rats by suppressing oxidative stress, fibrosis, and myocardial infarction as well as enhancing antioxidant enzymes.
INTRODUCTION
The ongoing increase of cardiovascular disorders epitomizes an alarming and concerning public health challenge nowadays. Many cardiovascular diseases are connected to acute myocardial infarction (MI) or atherosclerosis [1]. Genetic predisposition, smoking, hypertension, senility, anxiety, diabetes, and raised lipid parameters, especially low-density lipoprotein (LDL)-cholesterol in plasma etc. are the considerable factors responsible for MI. The pathophysiology of MI is attributed to the reduction or cessation of blood supply and hence reduces oxygen supply in the myocardium. Due to tissue injury, inflammation causes recruitment of neutrophils in the inflamed region that triggers the production of various endogenous pro-inflammatory cytokines, interleukins, bioactive amines, bradykinins, and prostaglandins, which in turn causes more damage to the myocardium. Moreover, these inflammatory mediators through autoxidation generate reactive oxygen species (ROS) and adversely influence the blood cell’s NADPH-oxidase enzyme system, which induces lipid peroxidation [2]. Isoproterenol (ISO), a synthetic catecholamine, an agonist of the β-adrenergic receptor, negatively affects the heart muscle. Myocardial infarction has been efficaciously prepared in animal model for preclinical screening of therapeutically promising drugs by introducing ISO [3]. Damage to the cardiomyocytes and free radical build-up has been suggested as a causative agent in the pathophysiology of MI in ISO-induced animals [4, 5]. ISO also stimulates the biosynthesis of ROS such as superoxide, peroxide, peroxynitrite, hydrogen peroxide, and hydroxyl radicals, are responsible for the damage to heart tissues [6].
Keeping the roles of oxidative stress in the pathogenesis of cardiovascular disease in consideration, the current study hypothesized that the use of antioxidants might be an effective therapeutic approach to prevent and cure cardiac damage. Recently, scientific interests in phenolic ingredients have emerged significantly due to their antioxidant properties. The fruit of pomegranate, locally known as dalim (Punica granatum L., Punicaceae family) is enriched with tannins and phenolic compounds which possess abundant health benefits [7, 8]. For example, ellagic acid, flavonoids, anthocyanidins, anthocyanins, and flavanols are reported to be the most promising biologically active and therapeutically beneficial constituents of pomegranate fruit [9]. Previous studies on the peel extract of pomegranate were found to possess numerous health benefits, including hepatoprotective, anti-hyperglycemic, and anti-hyperlipidemic effects [10]. However, very few data exist regarding the potential benefits of pomegranate peel extract on cardiac muscle. Therefore, recognizing the significance of oxidative stress in the development of MI and fibrosis, this study was attempted to assess the effectiveness of an ethanol extract of pomegranate peel to ameliorate ISO-induced myocardial infarction in rats.
MATERIALS AND METHODS
Chemicals and reagents
Thiobarbituric acid was obtained from Sigma-Aldrich USA. Trichloroacetic acid (TCA) was obtained from J.I. Baker (USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) assay kits were purchased from DCI Diatec diagnostics (Budapest, Hungary). Gallic acid was purchased from Lobe Chemicals Ltd, Mumbi, India. Remaining chemicals which were used in these experiments were analytical grade.
Pomegranate fruit peel extract (PPE) preparation
Pomegranate or dalim fruits were collected from the local market of Dhaka, Bangladesh. The peel of the fruits was separated and dried in the hot air oven at 45°C. Once dried, the peels were finely ground into a coarse powder and subsequently immersed in ethanol (70 %) for 7 days. After extraction, the filtrate was separated using the cotton filter in a funnel and filter paper, respectively. The rotary evaporator (at 45 ℃) was used to yield the desired extract.
HPLC-DAD analysis for polyphenolic compounds quantification
According to the method described earlier, HPLC-DAD analysis was performed for the identification and quantification of targeted phenolic compounds in the selected extract [5, 11]. Briefly, the chromatographic analysis was done by using a Dionex UltiMate 3000 system (Thermo-Scientific) assembled with a suitable quaternary pump (LPG-3400RS) and photodiode array detector (DAD-3000RS). The analysis process was carried out by injecting 20 µL extract in Acclaim® C18 (5 µm) Dionex column (4.6 x 250 mm) by maintaining the temperature (30°C) and flow rate at 1 mL/min.
Animal study
Twenty-four healthy adult Long-Evans male rats of 10-12 weeks old (180-225 g) were randomly collected from the animal care house of North South University, Bangladesh. They were housed in separate cages placed in a room maintained at a temperature of 22±3°C, relative humidity of 55%, and 12 h/12 h dark/light cycles. The use of animals and the study protocol related to this study was approved by the Ethical Committee, North South University, Bangladesh, for animal care and experimentation (2022/OR-NSU/IACUC/0303).
Rats were evenly separated into four groups (n=6). Group I received only normal food and water. Group II was administered with ISO (50 mg/kg S.C. twice a week for 14 days). Group III (ISO + PPE) was treated by PPE (200 mg/kg) orally (gavage), for 14 days daily and ISO 50 mg/kg S.C. twice a week for 14 days. Group IV (ISO + GA) was given gallic acid (100 mg/kg) orally (by gavage) every day for 14 days, and ISO 50 mg/kg S.C. twice a week for 14 days. The body weights were measured daily for 14 days.
After finishing the experimental period, all the rats were maintained at a fasting state for 13-15 h and taken water only during that fasting condition. Ketamine (80 mg/kg) was used to anesthetize the experimental rats for sacrifice and samples were immediately collected. The blood sample was collected in citrate buffer containing tubes and centrifuged at 3500 g (4 ℃) to separate the plasma samples and then stored in a freezer at −20 °C for further analysis. The organs like kidney and heart were weighed and preserved in 10% v/v neutral buffered formalin and stored for subsequent investigation.
Assessment of biochemical markers
The activities of several enzymes, including alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were estimated in plasma according to the manufacturer’s protocol using respective kits of DCI Diagnostics (Hungary).
Assessment of creatinine kinase-muscle brain (CK-MB) activity
CK-MB is a cardiac function marker. CK-MB kit which was purchased from DCI Diagnostics (Hungary) was used to analyze the CK-MB activities in the plasma sample. The experiment was performed as mentioned in the manufacturer’s protocol.
Assessment of myeloperoxidase (MPO) activity
The MPO is an inflammation marker, which was assessed using the previously described protocol [12]. Briefly, protein samples (10 mg) were added in triplicate to a reaction mixture containing 0.53 mM of o-dianisidine dihydrochloride and 0.15 mM of H2O2 in PBS and measured at 460 nm. The result of MPO was expressed as MPO/mg protein [12].
Estimation of oxidative stress markers
Homogenization of the collected kidney and heart tissues was performed using 10 volumes of phosphate buffer solution (pH 7.4). Then, the tissue homogenates were centrifuged at approximately 3500 g for 30 min at 4oC. The supernatant was separated carefully to measure the protein content and enzymatic activities. According to the previous method, the concentration of malondialdehyde (MDA), the final product of lipid peroxidation, is an oxidative stress indicator was quantified at 532 nm in both plasma and tissue homogenates [12, 13]. Nitric oxide (NO) level was checked using the previously described method [14, 15]. The absorbance was measured at 540 nm against the blank solutions and the result was expressed as nmol/g of tissue. Advanced protein oxidation product (APOP) concentration was measured at 340 nm according to previously reported protocol [16, 17]. APOP concentrations were calculated using the chloramine-T standard curve, which expressed in nmol/mL.
Assessment of superoxide dismutase (SOD) and catalase (CAT) activity
The SOD activity was assayed in plasma and tissue homogenates according to previous protocol [18, 19]. The method relies on the concept that the auto-oxidation of epinephrine within the sample solution generally decreases by 50% due to the enzymatic activity of each enzyme unit. The colorimetric approach as outlined previously [12, 20] was used for the determination of catalase activity. In this method, a change in absorbance of 0.01 units per minute at 240 nm is considered equivalent to one unit of enzyme activity.
For histopathological assessment, 10% neutral buffered formalin (NBF) solution was used to fix the kidney and heart tissues and then they underwent a series of treatments involving graded ethanol and xylene. These prepared tissues were then carefully embedded in paraffin blocks and microtome machine was used to slice these blocks into thin sections, approximately 5 µm thick. Freshly prepared blocks were collected on fresh slides and then stained with hematoxylin/eosin (H&E) for the purpose of evaluating any inflammatory cell infiltration within the tissues. Additionally, Picrosirius red was also used for staining to assess the presence and extent of fibrosis. After finishing the standard staining procedures, all slides with stained tissue sections were photographed and meticulously examined under a light microscope at 40X magnification using a Zeiss Axioscope.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). Using GraphPad Prism Software (version 6.01), the results obtained were compared among all groups with One-way ANOVA followed by Newman-Keuls post hoc test. In all cases of analysis, the statistical significance was considered as p < 0.05.
RESULTS
HPLC-DAD analysis showed the presence of polyphenolic compounds
HPLC-DAD was performed for the qualitative and quantitative analysis of gallic acid and rutin hydrate in the ethanol extract of pomegranate peel (Figure 1). Using a calibration curve, the quantity of abovementioned phenolic compounds was calculated, which were 561.47 mg/100 g of dry extract for gallic acid and 984 mg/100 g of dry extract for rutin hydrate (Table 1).
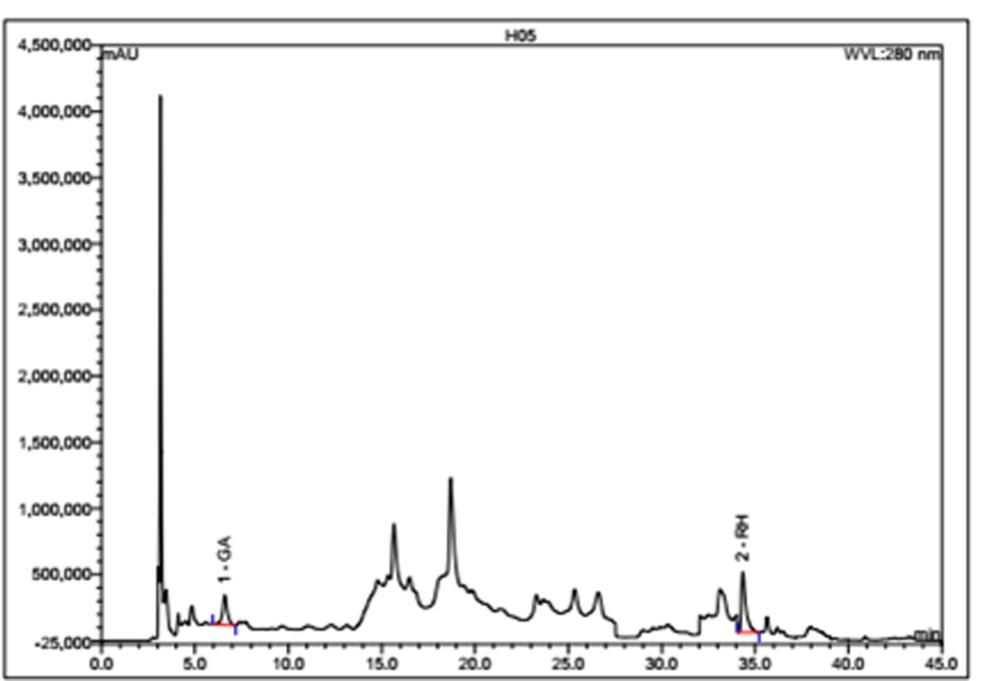
Table 1. Contents of polyphenolic compounds in the pomegranate peel.
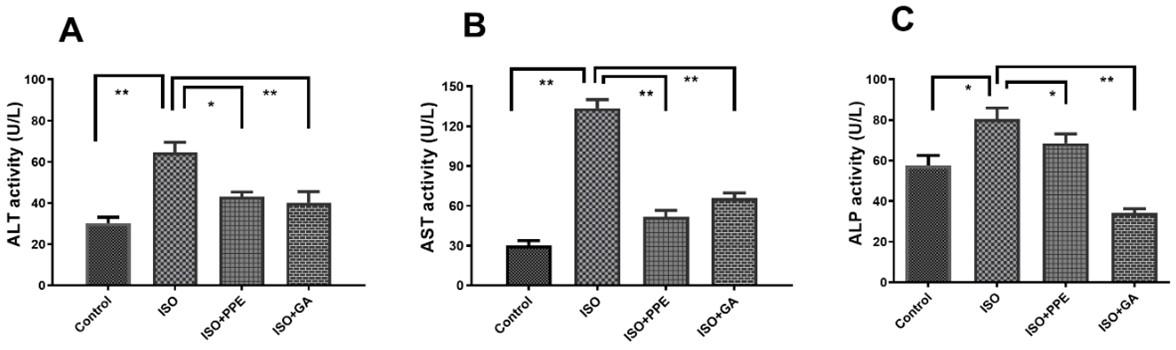
PPE treatment reduced ALT, AST, and ALP activities in ISO-administered rats
The activity of important liver biomarker was significantly (p<0.05) elevated due to 50 mg/kg subcutaneous injection of isoproterenol for twice a week (for 14 days) compared to control rats in which isoproterenol was not administered (Figure 2A). Pomegranate peel extract (PPE) (200 mg/kg orally for 14 days) significantly (p<0.05) reduced the ALT activity in isoproterenol-administered rats. Gallic acid treatment (100 mg/kg orally for 14 days) was also found to be effective in reducing the elevated ALT activity in comparison to the isoproterenol-administered rats (Figure 2A).
The AST activity was also markedly (p<0.05) increased due to isoproterenol administration, in comparison to the control group (Figure 2B). Treatment with PPE caused significant (p<0.05) reduction of AST activity in isoproterenol-administered rats in comparison to the isoproterenol-administered rats. Similarly, the treatment of isoproterenol-administered rats with gallic acid resulted in significant (p<0.05) reduction of AST activity.
In the isoproterenol-administered rats, the ALP activity was significantly (p<0.05) elevated than the control group (Figure 2C). Interestingly, treatment with PPE in isoproterenol-administered rats showed decreased ALP activity significantly (p<0.05) compared to the isoproterenol-administered rats (Figure 2C). Oral administration of gallic acid also ended up with a significant (p<0.05) reduction of ALP activity in isoproterenol-administered rats (Figure 2C).
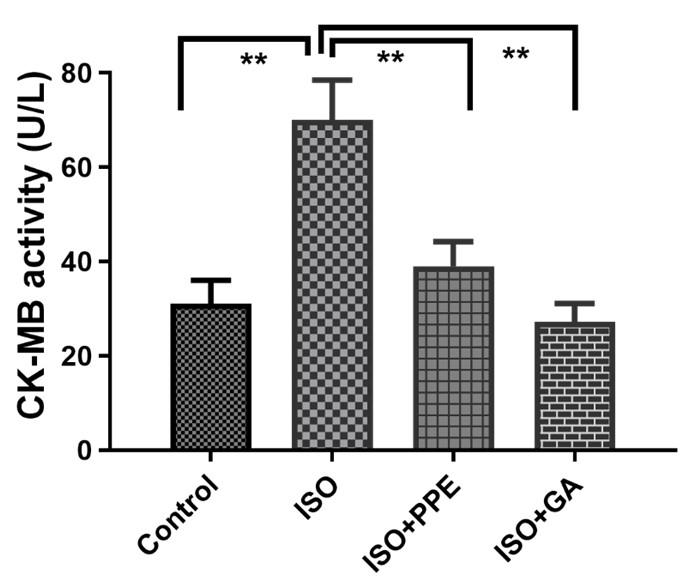
PPE treatment reduced creatinine kinase-MB in ISO-administered rats
This current study revealed that CK-MB activity was expressively (p<0.05) higher in the rats that received isoproterenol compared to the control group (Figure 3). PPE was able to normalize the CK-MB activity in plasma compared to the isoproterenol-administered rats (Figure 3). In addition, CK-MB activity was also reduced markedly (p<0.05) in gallic acid with isoproterenol-administered rats (Figure 3).
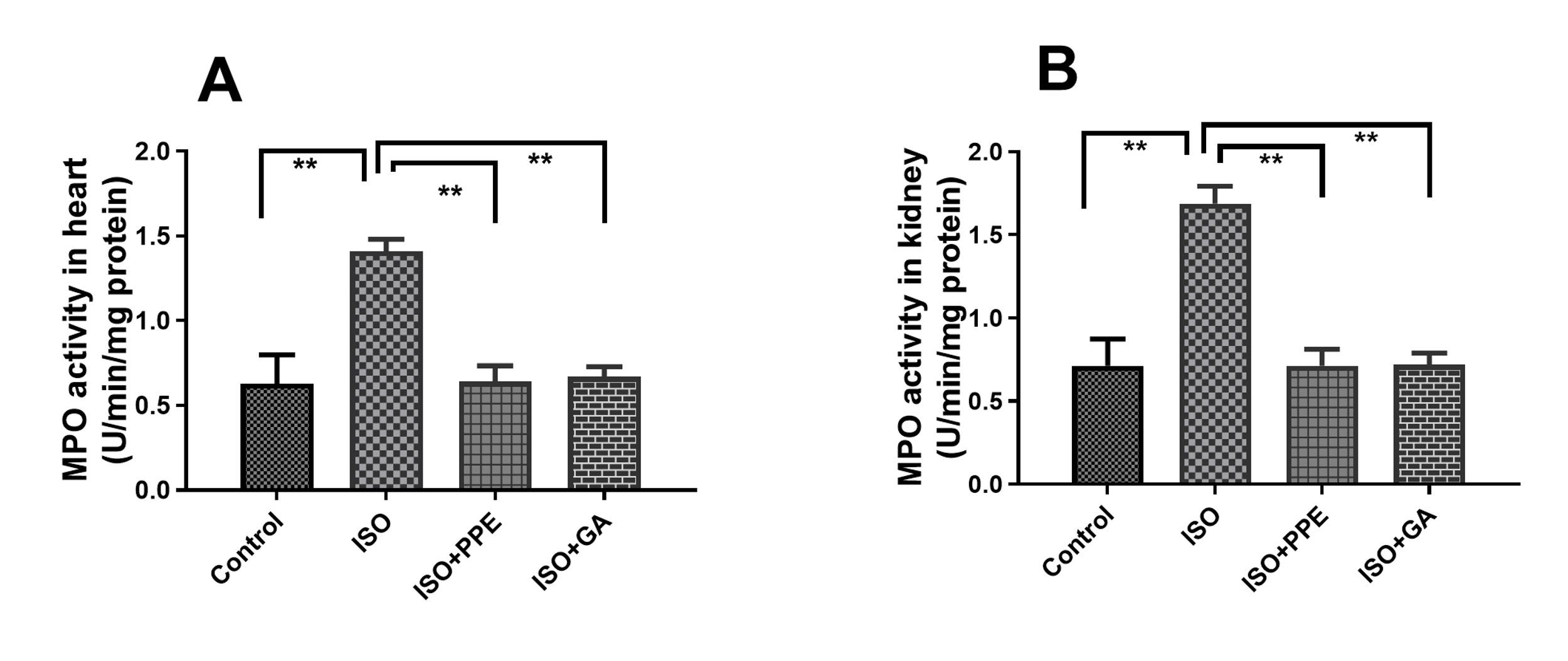
PPE treatment reduced MPO activities in heart and kidney in ISO-administered rats
The activity of MPO in the cardiac tissue was slightly increased due to isoproterenol administration in contrast to the control group (Figure 4A). Isoproterenol administration also increased the MPO activity in the kidney tissue (Figure 4B). Isoproterenol-administered rats that received the PPE demonstrated significant reduction of MPO activity in the heart tissue and the kidneys (Figure 4A and 4B). Treatment with gallic acid in isoproterenol-administered rats also exhibited a noteworthy reduction in the activity of MPO in the heart and kidney (Figure 4A and 4B).
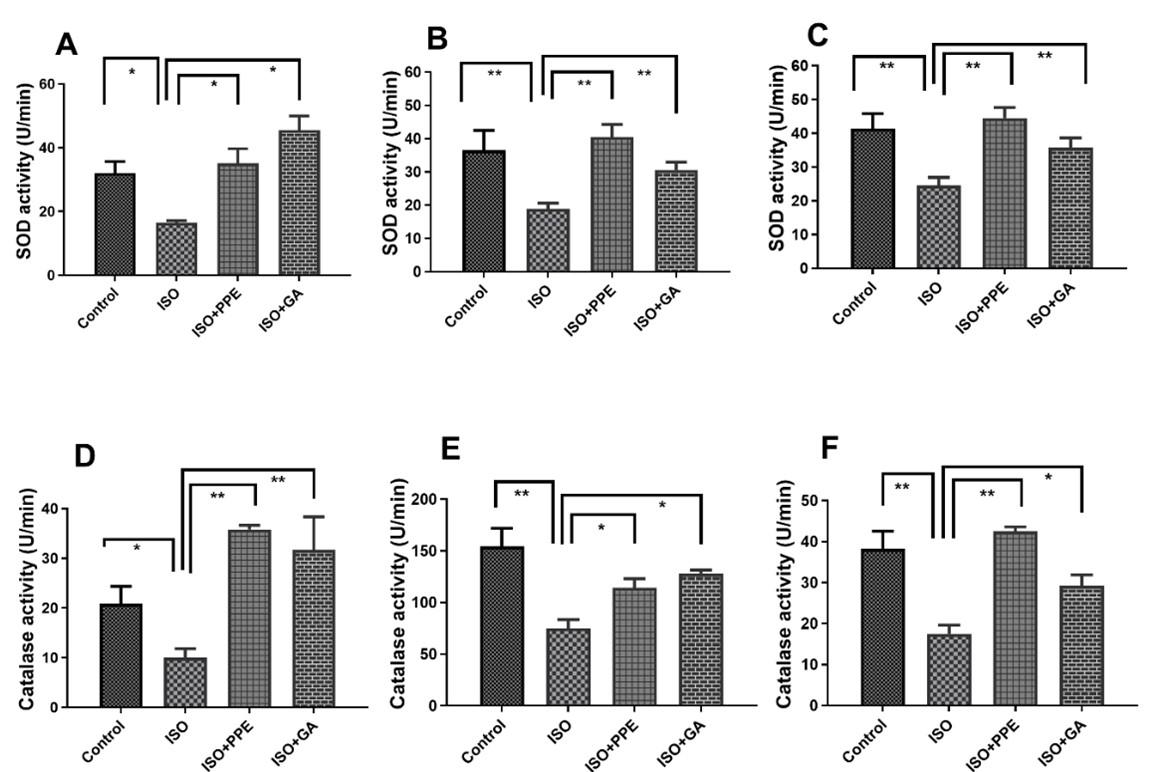
PPE treatment restored SOD and catalase activities in plasma, heart and kidney in ISO-administered rats
Rats administered with isoproterenol exhibited a significant (p<0.05) reduction in SOD activity in the plasma, heart, and kidney, as depicted in Figure 5A, 5B, and 5C. However, treatment with PPE or gallic acid was significantly restoring SOD activity in the plasma, heart, and kidneys.
The administration of isoproterenol resulted in a significant (p<0.05) reduction in cellular antioxidant capacity, as evidenced by decreased catalase activities in the plasma, hearts, and kidneys, when compared to control rats (Figure 5D, 5E, 5F). However, our selected extract, PPE led to a substantial (p<0.05) increase in catalase activities, as shown in Figure 5D, 5E, and 5F, when compared to those rats administered with isoproterenol alone. Similar kind of increasing catalase activities (p<0.05) was noticed in the administration of gallic acid with isoproterenol-administered rats respectively. This observation indicates the positive pharmacological effects of both PPE and gallic acid (Figure 5D, 5E, 5F).
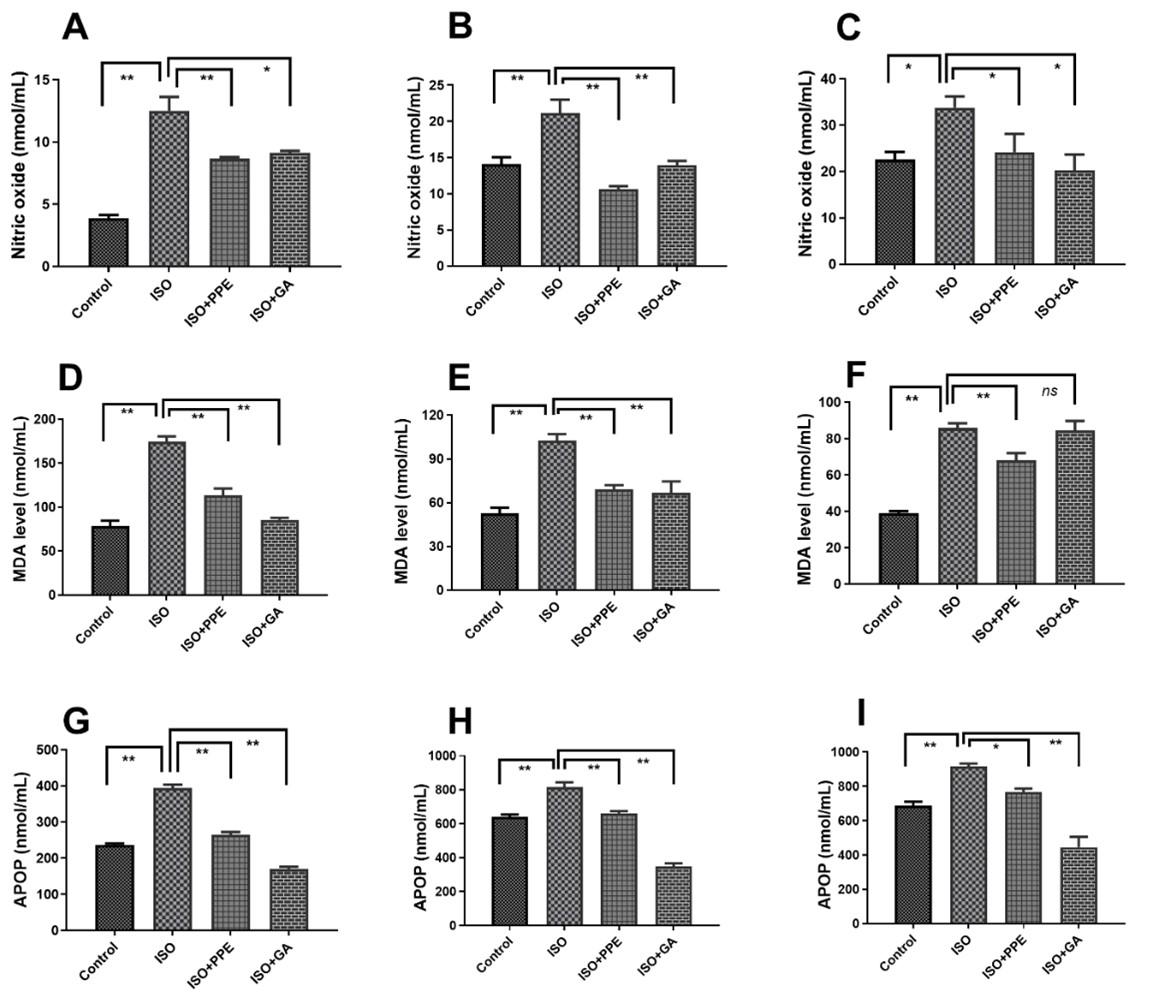
PPE treatment reduced oxidative stress parameters in plasma, heart and kidney in ISO-administered rats
Oxidative stress marker NO concentration in plasma, heart, and kidney was increased significantly (p<0.05) by the administration of isoproterenol when compared to the control rats (Figure 6A-C). In response to this, PPE treatment resulted in a significant (p<0.05) decrease in NO concentration in the heart, plasma, and kidney. On the other hand, when isoproterenol-administered rats were treated with gallic acid, significant (p<0.05) decreases in NO concentration were noticed as well in plasma, heart, and kidneys, comparable to the levels in the isoproterenol-administered rats (Figure 6A-C).
Furthermore, isoproterenol administration significantly (p<0.05) elevated the concentration of another biomarker, MDA in the respective samples as seen in Figure 6D, 6E, and 6F, compared to control rats. PPE treatment in isoproterenol-administered rats resulted in significant (p<0.05) decreases in MDA concentration. Similarly, gallic acid treatment also led to significant (p<0.05) decreases in MDA concentration in the respective homogenates compared to isoproterenol-administered rats (Figure 6D, 6E, 6F). Advanced protein oxidation products (APOPs), another dependable biomarker of oxidative stress was also used and depicted similar increasing portrait in isoproterenol-administered rats as illustrated in Figure 6G, 6H, and 6I. Similar decreasing graphs were led in the treatment with PPE and gallic acid respectively when compared to isoproterenol-administered rats (Figure 6G, 6H, 6I).
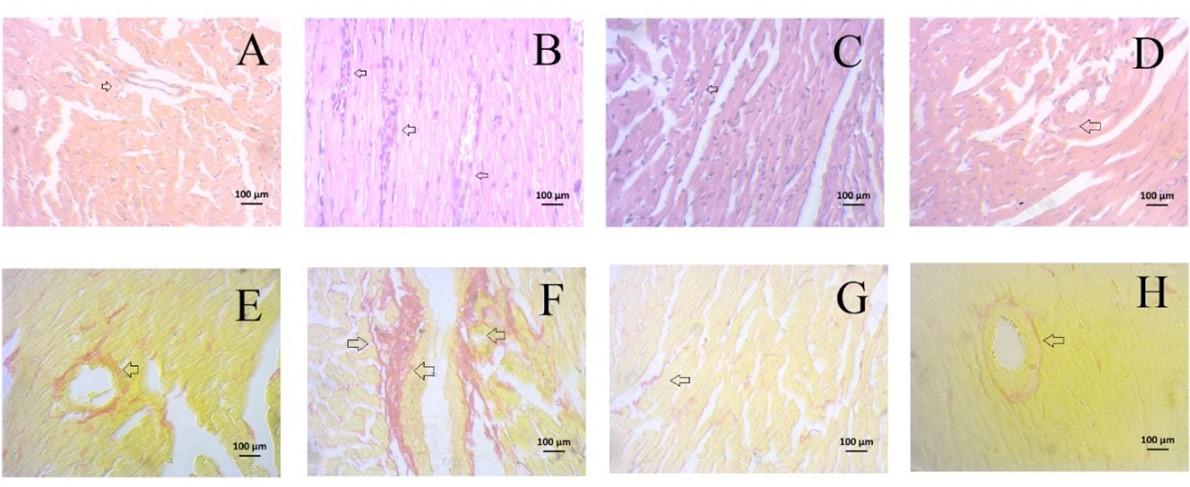
Histological assessments
Staining with hematoxylin and eosin revealed that control rats displayed a well-organized arrangement of cardiomyocytes in the left ventricle as shown in Figure 7A (upper panel). In contrast, isoproterenol-administered rats exhibited different arrangement due to necrosis, scar tissue deposits, and infiltration of inflammatory cells in the heart as shown in Figure 7B (upper panel). Treatment with PPE effectively prevented scar formation and reduced inflammatory cell infiltration in the hearts of isoproterenol-administered rats, as observed in Figure 7C (upper panel). Similar kind of prevention was observed in gallic acid treatment as shown in Figure 7D (upper panel).
Sirius red staining was performed to investigate collagen deposition in the heart. Control rats displayed no collagen deposition in the perivascular and peri-cardiomyocyte zones [Figure 7E (lower panel)]. On the other hand, isoproterenol-administered rats exhibited marked collagen deposition in these zones [Figure 7F (lower panel)]. However, PPE and gallic acid treatment effectively prevented collagen deposition in the perivascular and peri-cardiomyocyte zones in the hearts of isoproterenol-administered rats, as demonstrated in Figure 7G and 7H (lower panel).
DISCUSSION
Isoproterenol, a synthetic catecholamine at a dose range 50-100 mg/kg, can induce morphological and functional alterations in such a way that may ultimately lead to myocardial necrosis [5, 11]. Due to the oxidative degradation of isoproterenol, it also causes the initiation and propagation of free radical generating reactions which eventually culminate in cardiotoxicity. In this investigation, administration of the PPE orally reduced oxidative stresses and inflammation in the heart of ISO administered rats.
Excessive production, in comparison to inadequate removal, of pro-oxidants like superoxide, peroxide, and MDA, contributes to oxidative stress, which ends up in the injury of heart muscle leading to myocardial infarction [21]. Autoxidation of ISO results in increased synthesis of free radicals which causes the initiation and maintains the propagation of lipid peroxidation [22]. For example, free radical-induced peroxidation of phospholipids that are the main component of cell membrane and membrane-bound organelles, causes sustained rise in intracellular calcium level and adenosine triphosphate (ATP) depletion; both of these are contributors of irreparable cell damage [23]. In our study, oral administration of pomegranate fruit peel extract decreased the level of lipid peroxidation both in plasma and heart, as was reflected in the amount of MDA, in isoproterenol treated rats. This might be attributed to the presence of gallic acid and rutin hydrate which was confirmed by HPLC-DAD analysis of the pomegranate extract. Gallic acid and rutin are strong scavengers of free radicals [24, 25]. This was in agreement with previous studies where researchers showed that both rutin [26, 27] and gallic acid [28] prevent isoproterenol induced myocardial infarction associated lipid peroxidation in rats.
Enzymes such as ALT and AST activities are usually elevated in the plasma due to tissue injury [29]. In isoproterenol treated rats, the increased activities of ALT and AST were observed. Oral administration of PPE resulted in decreased activities of AST and ALT in plasma. Gallic acid administration also normalized the activities of AST and ALT in plasma of ISO-administered rats which are notably comparable to the oral administration of PPE extract. Additionally, the pathogenesis of acute myocardial infarction (MI) was confirmed by the increased activity of CK-MB in the plasma of ISO-administered rats compared to control rats. CK-MB is generally expressed in the heart muscle, which makes it a reliable biomarker for the diagnosis of MI, since impairment in the myocardial tissue would result in further elevation of CK-MB activity [30]. Thus, our observations are consistent with previous studies which showed that CK-MB activity was increased in rats treated with ISO [30, 31]. In compliance with this, PPE treatment was able to reduce ISO-induced elevated activity of CK-MB significantly which is comparable to the effect of gallic acid treatment showed decreased CK-MB activity in plasma. Furthermore, MPO is an inflammatory enzyme released in oxidative stress and excessive circulatory MPO level increases the risk of cardiovascular dysfunction [12, 16]. This marker transforms hydrogen peroxide into hypochlorous acid that further induces tissue damage. In this study, treatment with PPE reduces the MPO level in ISO-administered rats and therefore revealed cardioprotective effect.
Myocardial infarction triggers an upregulation in the gene expression of inducible nitric oxide synthase (iNOS) which led to an elevated biosynthesis of NO [32, 33]. In this current investigation, we observed that treatment with ISO resulted in an increase in nitric oxide levels. β-adrenergic stimulation also promotes the upregulation of iNOS gene expression, consequently leading to a significant increase in the production of nitric oxide [34]. Increased levels of NO in association with reactive oxygen species generate peroxynitrite (•ONOO-) radical, which is a powerful pro-oxidant [35]. Such conversion suggests that the biosynthesis of peroxynitrite might be suppressed by curbing the production of upstream reactive oxygen and nitrogen. As oral treatment of PPE significantly lowered the level of NO and MDA in isoproterenol treated rats, hence logically, it can be hypothesized that it may also interrupt the generation of superoxide and peroxynitrite. Previous studies supported these experimental results showed that inducible nitric oxide synthase (iNOS) in the colonic mucosal area was diminished by pomegranate extract [36]. On the other hand, gallic acid treatment in ISO administered rats significantly lowered the NO in plasma and heart, which is comparable to the PPE-administered rats.
Nowadays, it is very well established that free radical and pro-oxidant mediated damage to cells, tissue, and organs can be prevented and even cured by antioxidants. Antioxidant enzymes such as SOD, catalase, and glutathione peroxidase are endogenous molecular machinery acting as safeguard against oxidative and nitrosative stress [37]. In general, lipid peroxidation and activities of these enzymes are inversely related to the cellular level [38]. Moreover, SOD and catalase in cardiac muscle are damaged and enfeebled by free radical production due to isoproterenol induced myocardial injury. Thus, isoproterenol induced MI qualitatively and quantitatively attenuated the defense mechanism against oxidative stress and free radicals. In this study, significantly lower activities of CAT and SOD were found in the heart and kidney tissues of isoproterenol-administered rats in comparison to control rats. Gallic acid treatment restored the SOD and CAT activity in isoproterenol-administered rats that is further supported by the previous study [28, 39]. On the other hand, treatment with PPE compensated for the catalase and SOD activities in isoproterenol-treated rats. Our observations were also in concert with previous studies where researchers demonstrated that pomegranate seed and peel extracts caused the reduction of pro-oxidant level and restoration of antioxidant defense by maximizing the activities of SOD and catalase in experimental animals [40, 41].
Our study also revealed that PPE is a rich source of polyphenolic phytochemicals such as gallic acid and rutin hydrate. Gallic acid is one of the most remarkable phenolic compounds, plays positive roles in inflammation, immunomodulation, cardiovascular diseases, and metabolic disorders [42, 43]. For example, evidence was found for gallic acid showed that this treatment prevented myocardial damage in STZ-induced type-1 diabetic animal [44]. It also protects the myocardium against isoproterenol-induced oxidative stress, which was confirmed by the quantification of enzymatic and non-enzymatic antioxidants levels, histopathological findings, and evaluation of biochemical parameters in rats [45]. Our investigation also revealed that PPE prevented inflammatory cells infiltration in the necrotic zone of left ventricles of isoproterenol-administered rats. Moreover, PPE also decreased collagen deposition and fibrosis in the left ventricle of isoproterenol-administered rats. Oxidative stress and inflammation are the causative factors that may stimulate the extracellular matrix deposition in the heart [46]. Thus, the reduction of oxidative parameters and restored antioxidants in the heart of isoproterenol-administered rats due to the PPE administration could be responsible for reducing cardiac fibrosis. Previous studies also suggest that pomegranate flower extract reduced the cardiac fibrosis in Zucker diabetic fatty rats by modulating the NF-кB signaling pathway [47].
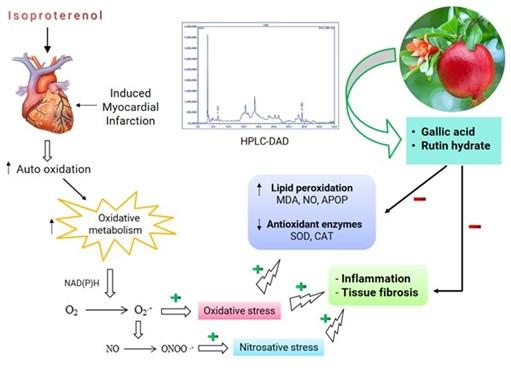
Natural secondary metabolites play significant roles in preventing diseases and maintaining human and animal health. The pharmaceutical, food, and nutraceutical industries extensively use natural products, including fruit and plant products, to formulate different herbal medications, dietary supplements, and nutrients for numerous illnesses [48, 49]. The pomegranate fruits are nutritious, having diverse medicinal values. Correspondingly, the peels of pomegranate are the by-products of the food industry which may be also added value in pharmaceutical industry and help in waste management system. This investigation provides evidence that pomegranate peel extract could be potential sources of crucial phenolic antioxidants. A proposed mechanism of action for pomegranate peel extract polyphenols are depicted in Figure 8.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the logistic support and laboratory facilities provided by the Department of Pharmaceutical Sciences, North South University, Bangladesh and Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh. This research work was partially supported by the R and D research grant from the Ministry of Science and Technology of Bangladesh, granted to Dr. Nusrat Subhan (Grant No. R & D 2021-2022/43).
AUTHOR CONTRIBUTIONS
FK, KN, MMR, SH, and HH conducted the investigation. FK, KN, MMR, NS, MAA, and MAH contributed to analysing and interpreting data and drafting the manuscript. MNI, NS, MAA, and MAH contributed to the conceptualization, visualization and editing of the draft. NS, MAA, and MAH coordinated the research, revised the manuscript, and approved the final version for publication. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
- [2]Whelton P. The challenge of hypertension and atherosclerotic disease in economically developing countries. High Blood Press. 1995;4:36-45.
- [3]Zaragoza C, Gomez-Guerrero C, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011.
- [4]Davel AP, Brum PC, et al. Isoproterenol induces vascular oxidative stress and endothelial dysfunction via a giα-coupled β 2-adrenoceptor signaling pathway. PloS One. 2014;9:e91877.
- [5]Akter N, Chowdhury FI, et al. Polyphenolics in ramontchi protect cardiac tissues via suppressing isoprenaline-induced oxidative stress and inflammatory responses in long-evans rats. J Funct Foods. 2020;75:104250.
- [6]Martinson B, Hans MP, et al. Readers' choice: Hot papers downloaded in 2006. Elsevier Science; 2007.
- [7]Rahimi HR, Arastoo M, et al. A comprehensive review of Punica granatum (pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran J Pharm Res. 2012;11:385.
- [8]Mutahar S S, Mutlag M A-O, et al. Antioxidant activity of pomegranate (Punica granatum l.) fruit peels. Food and Nutrition Sciences. 2012;2012.
- [9]Garachh D, Patel A, et al. Phytochemical and pharmacological profile of Punica granatum: An overview. Int Res J Pharm. 2012;3:65-8.
- [10]Middha SK, Usha T, et al. A review on antihyperglycemic and antihepatoprotective activity of eco-friendly Punica granatum peel waste. Evid based Complement Altern Med. 2013;2013.
- [11]Rahman MM, Ferdous KU, et al. Polyphenolic compounds of amla prevent oxidative stress and fibrosis in the kidney and heart of 2k1c rats. Food Sci Nutr. 2020;8:3578-89.
- [12]Mamun F, Rahman MM, et al. Polyphenolic compounds of litchi leaf augment kidney and heart functions in 2k1c rats. J Funct Foods. 2020;64:103662.
- [13]Niehaus Jr W, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126-30.
- [14]Khan S, Rahman MM, et al. Trichosanthes dioica roxb. Prevents hepatic inflammation and fibrosis in CCl4-induced ovariectomized rats. Clin Nutr Exp. 2020;33:1-17.
- [15]Tracey WR, Tse J, et al. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: Pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther. 1995;272:1011-5.
- [16]Rahman MM, Shahab NB, et al. Polyphenol-rich leaf of Aphanamixis polystachya averts liver inflammation, fibrogenesis and oxidative stress in ovariectomized long-evans rats. Biomed Pharmacother. 2021;138:111530.
- [17]Witko-Sarsat V, Friedlander M, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304-13.
- [18]Nayan SI, Chowdhury FI, et al. Leaf powder supplementation of senna alexandrina ameliorates oxidative stress, inflammation, and hepatic steatosis in high-fat diet-fed obese rats. PLOS One. 2021;16:e0250261.
- [19]Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-5.
- [20]Chance B, Maehly A. The assay of catalases and peroxidases. Methods Biochem Anal. 1954:1:357-424.
- [21]Singal PK, Beamish RE, et al. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Myocardial Injury. 1983:391-401.
- [22]Liaudet L, Calderari B, et al. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail Rev. 2014;19:815-24.
- [23]Mladěnka P, Hrdina R, et al. Cardiac biomarkers in a model of acute catecholamine cardiotoxicity. Human Exp Toxicol. 2009;28:631-40.
- [24]Badhani B, Sharma N, et al. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Advances. 2015;5:27540-57.
- [25]Enogieru AB, Haylett W, et al. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid Med Cell Longev. 2018;2018.
- [26]Karthick M, Prince PSM. Preventive effect of rutin, a bioflavonoid, on lipid peroxides and antioxidants in isoproterenol‐induced myocardial infarction in rats. J Pharm Pharmacol. 2006;58:701-7.
- [27]Ali M, Mudagal M, et al. Cardioprotective effect of tetrahydrocurcumin and rutin on lipid peroxides and antioxidants in experimentally induced myocardial infarction in rats. Die Pharmazie. 2009;64:132-6.
- [28]Priscilla DH, Prince PSM. Cardioprotective effect of gallic acid on cardiac troponin-t, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in wistar rats. Chem Biol Interact. 2009;179:118-24.
- [29]Farvin KS, Anandan R, et al. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol Res. 2004;50:231-6.
- [30]Rosalki SB, Roberts R, et al. Cardiac biomarkers for detection of myocardial infarction: Perspectives from past to present. Clin Chem. 2004;50:2205-13.
- [31]Ahmed KM, Rana A, et al. Effect of calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomed. 2004;11:327-30.
- [32]Wilmes V, Scheiper S, et al. Increased inducible nitric oxide synthase (iNOS) expression in human myocardial infarction. Int J Legal Med. 2020;134:575-81.
- [33]Selim S, Akter N, et al. Flacourtia indica fruit extract modulated antioxidant gene expression, prevented oxidative stress and ameliorated kidney dysfunction in isoprenaline administered rats. Biochem Biophys Rep. 2021;26:101012.
- [34]Li D, Qu Y, et al. Inhibition of inos protects the aging heart against β-adrenergic receptor stimulation-induced cardiac dysfunction and myocardial ischemic injury. J Surg Res. 2006;131:64-72.
- [35]Beckman JS, Beckman TW, et al. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci. 1990;87:1620-4.
- [36]Larrosa M, González-Sarrías A, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717-25.
- [37]Wu J, Hecker JG, et al. Antioxidant enzyme gene transfer for ischemic diseases. Adv Drug Deliv Rev. 2009;61:351-63.
- [38]Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: Roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001;89:187-206.
- [39]Yu Z, Song F, et al. Comparative pharmacokinetics of gallic acid after oral administration of gallic acid monohydrate in normal and isoproterenol-induced myocardial infarcted rats. Front Pharmacol. 2018;9:328-.
- [40]Chidambara Murthy KN, Jayaprakasha GK, et al. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. Journal of Agricultural and Food Chemistry. 2002;50:4791-5.
- [41]Mohan M, Patankar P, et al. Cardioprotective potential of Punica granatum extract in isoproterenol-induced myocardial infarction in wistar rats. J Pharmacol Pharmacother. 2010;1:32-7.
- [42]Ryu Y, Jin L, et al. Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and smad3 binding activity. Sci Rep. 2016;6:1-14.
- [43]Harikrishnan H, Jantan I, et al. Modulation of cell signaling pathways by phyllanthus amarus and its major constituents: Potential role in the prevention and treatment of inflammation and cancer. Inflammopharmacol. 2020;28:1-18.
- [44]Patel SS, Goyal RK. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacog Res. 2011;3:239.
- [45]Priscilla DH, Prince PSM. Cardioprotective effect of gallic acid on cardiac troponin-t, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in wistar rats. Chem Biol Interact. 2009;179:118-24.
- [46]Suthahar N, Meijers WC, et al. From inflammation to fibrosis—molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14:235-50.
- [47]Huang TH, Yang Q, et al. Pomegranate flower extract diminishes cardiac fibrosis in zucker diabetic fatty rats: Modulation of cardiac endothelin-1 and nuclear factor-κB pathways. J Cardiovasc Pharmacol. 2005;46:856-62.
- [48]Islam N, Khan MF, et al. Neuropharmacological insights of african oil palm leaf through experimental assessment in rodent behavioral model and computer-aided mechanism. Food Biosci. 2021;40:100881.
- [49]Haque MA, Jantan I, et al. Tinospora species: An overview of their modulating effects on the immune system. J Ethnopharmacol. 2017;207:67-85.