Effect of Gynura procumbens leaf extract with biological nanoparticles on streptozotocin-induced hyperglycemia in a rat model
Abstract
Herbal medicines, a popular choice for treatments, are effective against diabetes when used with nanoparticles. Thus, this study investigates the effects of Gynura procumbens ethanolic extract with chitosan nanoparticles on streptozotocin-induced hyperglycemia in a rat model. Twenty-eight days old 16 male rats were randomly divided into 4 groups such as T0 = control (normal), T1 = streptozotocin-induced diabetes, T2 = streptozotocin-induced diabetes treated with ethanolic extract of Gynura Procumbens (150 mg/kg body weight), and T3= streptozotocin induced diabetes treated with ethanolic extract of Gynura Procumbens (150 mg/kg) and chitosan nanoparticles (25 mg/kg). Blood glucose levels were significantly higher in the diabetic group T1 (26.27±1.76) compared to treatment T2 (18.32±.715) and T3 (15.45±1.33) groups. The body weight of T1 (115.75±0.08c) significantly decreased compared to control T0 (216.25±4.27) on the 21st day. Interestingly, there was significant (p<0.01) weight gain in T2 and T3 than diabetic group T1. Also, insulin level peaked in T3 (0.41 µU/ml) statistically significant at (p<0.01), while it decreased in diabetic group T1 (0.41 µU/ml) compared to control T0 (0.34 µU/ml). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were normal in control T0 (181.50 U/L and 139.00 U/L), while diabetic group T1 showed an increased level (463.75 U/L and 322.00 U/L). Interestingly, treatment of the plant extracts and nanoparticles (T3) effectively reduced ALT and ASL levels within the 21 days of treatment. In conclusion, Gynura procumbens ethanolic extract and chitosan nanoparticles can be used as an effective anti-diabetic treatment.
INTRODUCTION
Metabolic diseases are of increasing public health concern, which include diabetes, cardiovascular diseases, arteriosclerosis, obesity, and a variety of cancers. Diabetes mellites, a disease recognized by abnormalities of blood glucose levels, caused by damage and eventual loss of β-cells in the endocrine pancreas, results in insulin deficiency and is often accompanied by symptoms of polyuria, polydipsia, polyphagia, and weight loss [1]. The chronic hyperglycemia of diabetes is highly correlated with long-term organ damage, dysfunction, and failure of various organs, including eyes, kidneys, nerves, heart, and blood vessels [2]. In 2017, approximately 462 million individuals were affected by type 2 diabetes, corresponding to 6.28% of the world's population (4.4% of those aged 15-49 years, 15% of those aged 50-69, and 22% of those aged 70+), or a prevalence rate of 6059 cases per 100,000 and 80 lacs people in Bangladesh suffer from diabetes [3]. Bangladesh has an estimated 7.1 million cases of diabetes, with almost equal numbers of cases going undiagnosed, given that 75% of the country's population lives in rural areas with little access to healthcare. According to data released by The International Diabetes Federation, by 2025, this number should have doubled. The cost of medications, hospital stays, and laboratory testing prevents nearly 45% of rural residents from receiving diabetes treatment [4].
The main risk factors for type 2 diabetes include being obese, obesity, lack of physical activity, old age, family history, altered level of high-density lipoprotein (HDL) and triglycerides, history of gestational diabetes, polycystic ovary syndrome (PCOS), or being in certain races or ethnicities [5-7]. Management of type 2 diabetes includes healthy eating, regular exercise, weight loss, diabetes medication or insulin therapy, blood sugar monitoring. These steps will help keep blood sugar level closer to normal, which can delay or prevent complications [8].
Some oral drugs are used to control diabetes such as metformin, sulfonylureas, glinides, thiazolidinediones, DPP-4 inhibitors, SGLT2 inhibitors. Long-term use of those oral drugs have major risk/side effects of abdominal pain, congestive heart failure, bladder cancer (pioglitazone), risk of bone fractures, joint pain, and risk for urinary tract infections etc. [9]. Insulin is another therapy to keep blood sugar levels within a target range. There are some disadvantages of insulin injections that raise the risk of hypoglycemia. Moreover, some people may be uncomfortable about injecting, which could affect employment if drive for a living [10]. Long-term taking of insulin may cause allergic reactions, localized itching, and rash to life-threatening anaphylaxis [10].
Traditional plants are a popular choice for treatment of diabetes, although its usage is not studied conscientiously in Bangladesh. Thus, these herbal formulations have a noteworthy research potential, especially with escalating cases of diabetes mellitus in Bangladesh. Thus, the comparative use of herbal medicinal plants such as Gynura procumbens as the treatment for diabetes is easy to use. Gynura procumbens (commonly known as longevity spinach is one of those precious medicinal herbs of Asteraceae that are still included in an unutilized herb despite the variety of useful pharmacological properties it possesses [11-13]. This plant is popular in Southeast Asian countries, as it is considered as treatment of diabetes mellitus, along with other important diseases such as rheumatism, kidney diseases, diabetes mellitus, cancer, and hypertension [14].
Nanoparticles are promising vehicles for drug delivery; efficient take-up of nanoparticles by attachment to the surface or integrating into the matrix, compared to larger macromolecules, make them the ideal transport and delivery system for drugs [15]. Nano systems with different compositions and biological properties have been extensively investigated for drug and gene delivery applications. Chitosan, a carbohydrate-based biopolymer, has gained scientific attention for treating diabetes mellitus, being a suitable carrier for drugs and active ingredient delivery. Chitosan has also been shown to recover impaired insulin secretion, glucose metabolism and altered lipid metabolism associated with diabetes mellitus [16, 17].
Recently received particular attention of Gynura procumbens is used as an anti-diabetic medicinal plant probably because of its empirical evidence and efficiency in the management of diabetes. Biological nano particles such as polysaccharide chitosan have been used as drug delivery systems. Systemic drug treatment for diabetes mellitus carries many risks with adverse effects such as gastrointestinal reactions, skin allergies, hypo glycaemia and other side effects. Due to high expenditure, and many side effects of synthetic drug of diabetes, the patients have tendency to move on the herbal products. Very limited data using Gynura procumbens with nanoparticles on diabetes is available. Thus, the current study investigated the effect of ethanolic extract of Gynura procumbens leaf with nanoparticles in streptozotocin-induced hyperglycemia in rat model by analyzing body weight, blood glucose, insulin, alanine transaminase (ALT), aspartate aminotransferase (AST) and histopathological changes of liver and kidney in hyperglycemic rats.
MATERIALS AND METHODS
Reagents and equipment
The following equipment have been used such as Whatman filter paper, test tube, volumetric flask, beakers, funnels, measuring cylinder, glass rod, semi-automatic pipettes, 1-3 ml syringes, heater, pH meter (Acustrip, South Carolina, USA), grinding machine, evaporator machine, refrigerator, digital electronic balance (Mettler-Toledo, LLC, Columbus, USA), oral gavage, strip, normal saline, hand gloves, and glucometer. The following reagents have been used including ethanol, 5% glucose solution, 5% acetic acid, and citrate buffer. Chitosan and streptozotocin were collected from Sigma Aldrich Chemical Ltd., USA.
Collection and preparation of plant materials
Previously described methods were used to prepare ethanolic extract of 1 kg of Gynura procumbens leaves, freshly collected from Botanical Garden of Hajee Mohammad Danesh Science and Technology University [18]. As per this method, extract of these leaves was made after washing, blending, and mixing with 1.5 liters of 95% ethanol. The extract was further filtered and centrifuged at 3000 rpm for 20 minutes (Mikro 20 Zentaifugen, Hettich Instruments, Beverly, California, USA). The supernatant obtained was concentrated by evaporation in a rotary evaporator (BUCHI Rotavapor R-200, BUCHI Labortechnik AG, Switzerland) at 40°C and freeze dried (Sharp Corporation, Sakai, Osaka, Japan). A yellowish dark green powder of Gynura procumbens crude ethanolic extract was obtained, which was stored at -20°C until further use.
Experimental animals
Sixteen male rats aged 28 days were collected from Centre for Diarrheal Disease Research, Bangladesh which are pathogen free. The animals were allowed to acclimatize in the laboratory environment for a week before the commencement of experiment. The rats were housed in a wire cage measuring 43×27×17 cm at temperature (27±5)0C and 12/12 light /dark cycle under natural control and sawdust substrate was changed weekly. The rats were fed a standard commercial pellet diet from ICDDR,B and water ad-libitum throughout the experimental period of 21 days.
This research project has been approved by the animal ethics committee of the Department of Physiology and Pharmacology at Hajee Mohammad Danesh Science and Technology University in Bangladesh at meeting resolution number 15, which was held on August 17, 2022, because the experimental design of the study was not objectionable or subversive to animal ethics.
The number of 16 rats were assigned into four groups named T0, T1, T2, and T3 (4 rats/group). Only T1, T2, T3 were induced by streptozotocin subcutaneously at the dose rate of 60 mg/kg body weight of rats except T0 (normal control). Whereas T0 =control (normal), T1 =streptozotocin-induced diabetes), T2 =streptozotocin-induced diabetes treated with ethanolic extract of Gynura Procumbens (150 mg/kg body weight), and T3= streptozotocin induced diabetes treated with ethanolic extract of Gynura Procumbens (150 mg/kg) and chitosan nanoparticles (25 mg/kg). Gynura Procumbens ethanolic extract and chitosan nanoparticles were administered orally for 21 days.
Induction of diabetes in rats
Followed by an overnight fast, induction of diabetes in rats was done using intraperitoneal injection of 60 mg/kg of streptozotocin (Sigma Aldrich Chemical Co., USA) reconstituted in 0.1 mol/L cold citrate buffer (pH 4.5). After 72 h of streptozotocin administration, blood glucose level was measured in blood collected from tail vein puncture using blood glucose meter (Sugar Check) manufactured by M.R. Trading International Ltd., Dhaka, Bangladesh [19].
Gynura procumbence extract with chitosan
Chitosan nanoparticles are safe, and biodegradable and can release drugs at controlled rates to the target site. Chitosan nanoparticles can be synthesized by various methods such as ionic gelation method, emulsion polymerization method, etc. Chitosan is a strong base owing to the presence of primary amino groups and becomes a polyelectrolyte. As a result, chitosan dissolves in 1M solution of glacial acetic acid. Chitosan nanoparticles could be used at 25 mg/kg body [20].
Administration of Gynura procumbens extract with chitosan nanoparticle
Prepared Gynura procumbens extract and Gynura with chitosan nanoparticle extract were fed orally to different treatment groups to the experimental rats with the help of an oral gavage. The use of oral gavage ensured the administration of requisite quantity, which was ascertained based on body weight of each individual rats.
Sample collection and preparation
Blood collection from all groups was done using anesthesia with chloroform, followed by sacrificing them. Abdominal and thoracic cavities were surgically opened for direct blood collection from the heart using a sterile syringe and needle. 1 ml of blood was stored in a tube containing 3.8% sodium citrate as an anticoagulant for hematological studies. Plasma was separated by centrifugation at 4500 rpm for 10 minutes and stored at -20 ̊ C. Liver and kidney samples were collected from individual rats and stored in 10% formalin solution for histopathological examinations.
Determination of blood glucose level
Blood samples were collected from the tail vein at day 0 (pre-treatment), then streptozotocin-induced confirmed diabetes after that 1st, 7th, 14th, and 21st day for estimation of blood glucose level. Estimation of blood glucose level was performed by the Sugar check blood glucose monitoring system (Strip method) from M.R. Trading International Ltd., Dhaka, Bangladesh.
Measurement of insulin level
After the blood was centrifuged for 10 minutes at 3000 rpm by the centrifuge machine. Then the serum samples were immediately transferred to the laboratory for the estimations of insulin levels. Estimations were carried out by Architect l-1000SR/Vitros ECi System (J and J) Random Access Multibatch Immunoassay Analyzer, Abbott Core Laboratory, Abbott Park, Illinois, U.S.A. Then the Reports were delivered for further analysis.
Determination of alanine transaminase (ALT) and aspartate aminotransferase (AST)
ALT is one of the enzymes that help the liver convert food into energy. The AST test is also a blood test of a liver profile. The ALT and AST tests were carried out in the laboratory with the help of Hemolyzer® 5 NG (open mode), Analyticon Biotechnologies GmbH, Lichtenfels, Germany. The value was expressed in U/L.
Determination of body weight
The body weight of all groups was recorded before treatment on the 1st day and the effect of the treatment at the end of the experiment on the 7th,14th, and 21st day with the help of electric balance.
Histopathological analysis
Histopathology was performed for the livers and kidneys in normal and hyperglycemic rats in the following ways. Firstly, the tissue sections of the liver and kidney were prepared from the preserved samples. Then kept under running tape water drop by drop overnight. Washed at (50%, 70%, 80%, 95%) alcohol for 1 h, respectively. Three times washing in 100% alcohol within 1 h. Again, two times chloroform washing within 1.5 h was then kept in a paraffin bath (56℃) for 3 h. Then paraffin block was prepared and then tissue sectioning was done by using a Microtome machine and cut at 6 µm. Staining was performed by using xylene, and alcohol (100%, 95%, 80%, 70%) for 2 minutes, respectively. After that slides were kept under distilled water and Hematoxylin dye for 10 minutes individually. Next, the slides were kept under Eosin dye with a few drops of lithium carbonate for 30 seconds. DPX solution was applied and made the slides permanent by putting cover slips over there and observing under the microscope at 10x objective.
Statistical analysis
The results of various biochemical and immunological parameters were expressed as mean ±SEM. Data were analyzed by using SPSS version 22 and Microsoft Excel. Statistically significant differences between group means were determined by analysis of variance (ANOVA). P value ≤ 0.05 was considered statistically significant.
RESULTS
Effect of Gynura procumbens plant extracts and chitosan nanoparticles on blood glucose level
Table 1 shows the blood glucose levels among the treated groups. Blood glucose levels were significantly higher in the diabetic group T1 (26.27±1.76) in comparison to treatment groups T2 (18.32±.715) and T3 (15.45±1,33). Among different combination of treatments, lowest blood glucose was observed in group T3.
Table 1. Effect of Gynura procumbens plant extracts and Chitosan Nanoparticles on blood glucose level in streptozotocin-induced diabetic rats.
Effect of Gynura procumbens plant extracts and chitosan nanoparticles on biochemical parameters
Insulin was normal in control group T0 with a rate of insulin (0.34 µU/ml) while the diabetic group T1 showed a decreased level of insulin (0.17 µU/ml). In addition, the insulin level was at its peak in the T3 group within the 21st day of treatment and the rate was 0.41 µU/ml which was statistically significant.
Table 2 represents the ALT and AST levels of normal and treated rat groups which were significantly different at a 5% level of significance. ALT and AST were normal in control group T0 with the rate of ALT (181.50 U/L) and AST (139.00 U/L) while the diabetic group T1 showed an increased level of ALT 463.75 U/L and AST 322.00 U/L.
In addition, a lower level of ALT and AST was found in the T3 group after the 21st day of treatment and the levels of ALT (237.00 U/L) and AST (165.50 U/L) were statistically significant.
Table 2. Effect of Gynura procumbens plant extracts and Chitosan Nanoparticles on biochemical parameters in streptozotocin-induced diabetics rats.
Effect of Gynura procumbens plant extracts on live body weight
Table 3 showed that after induction of diabetes, body weight in different groups by supplementation of different treatments was statistically significant(p≤0.01). Here, the weight of diabetic group T1 (115.75±0.08) significantly decreased from the control T0 (216.25±4.27), treated group T2 (193.00±3.21), and T3 (204.75±2.62) at the last 21st day. After the treatment, T2 and T3 showed a slight weight gain compared to the diabetic group.
Table 3. Effect of Gynura procumbens plant extracts on live body weight in streptozotocin-induced hyperglycemic rats.
Effect of Gynura procumbens plant extracts on histopathology in liver and kidney
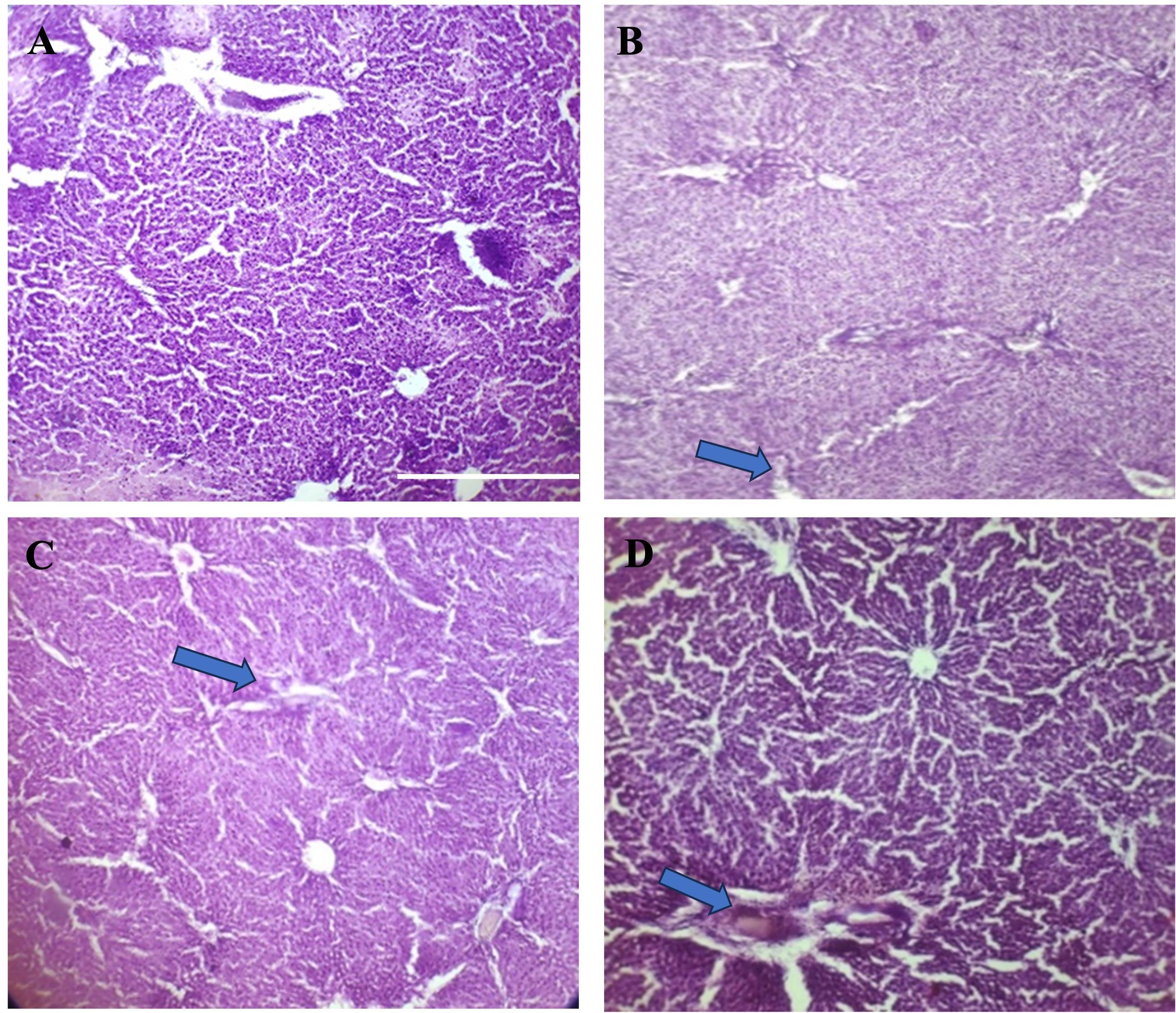
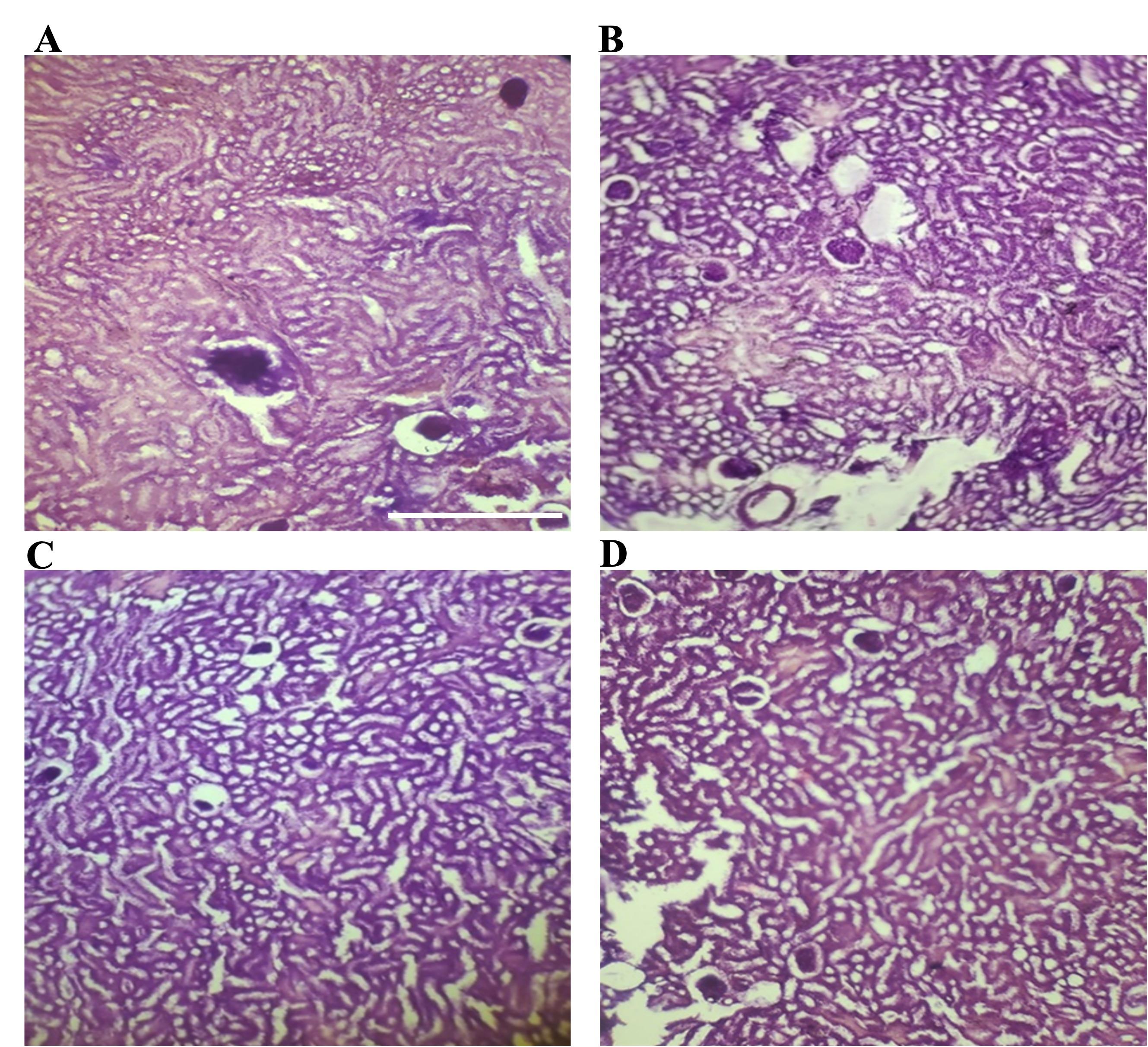
Increasement of the interlobular space in rat liver was observed by microscopic examination (Figure 1B-D). Sections of kidney showed no hemorrhagic lesions, no inflammatory infiltration and no other distinct changes as observed by microscopic examinations (Figure 2A-D).
DISCUSSION
The experiment was conducted to determine the effect of Gynura procumbens ethanolic extract with chitosan nanoparticles on streptozotocin-induced hyperglycemia in rats. Blood glucose level, body weight, lipid profile, and insulin levels were measured. Microscopic changes were also compared with the normal and treated groups. The present study revealed that the blood glucose levels were normal in the control group T0 on the 21st day. The blood glucose levels were significantly increased in the diabetic group (T1) as compared with T2 and T3 groups. A similar findings showed the increasement of blood glucose level during diabetes [21, 22].
In a previous study, it was determined that after 14 days of treatment, Gynura procumbens lowered high blood glucose levels because of its hypoglycemic qualities and capacity to absorb glucose in vitro in RIN-5F cells [23] and additionally, another study showed that Gynura procumbens extract directly affected peripheral glucose absorption and utilization [24].
This study also demonstrated that level of insulin in the diabetic group T1 was lower than that of the control group T0. Additionally, insulin level of T3 group peaked after 21 days of treatment. These results are consistent with a study that examined the impact of Gynura procumbens therapy on insulin levels and found that Gynura procumbens extract stimulated insulin-secreting cells [25].
Insulin activity at the cellular level is imitated by aqueous extract of Gynura procumbens, where chitosan nanoparticles are used for controlled release of insulin [26], a different study explored the hypoglycemic activity of differently regioselective chitosan sulfates in alloxan-induced diabetic rats [15].
The levels of ALT and AST were normal in control group T0 while the diabetic group T1 showed an increased level of ALT and AST. Also, lower level of ALT and AST on T3 group with 21st days of treatment was observed which is very identical to previous research [27-29]. Gynura procumbens has the potential ability to improve the heart and liver function of diabetic animals by lowering the ALT, AST, and ALP levels [27, 30, 31].
At the end of the 21st day, the body weight of the diabetic group T1 was considerably lower than that of the control group T0. In comparison to the diabetic group, T2 and T3 after the treatment showed a modest weight gain, which is very comparable to previous studies [19]. The body weight increased in the treatment group may be due to long-term glucocorticoid therapy [32, 33].
It is stated that in a hyperglycemic state, the body cells seldom access glucose, and that lipids and tissue proteins are broken down for energy supply, causing body weight loss and muscle wastage [19, 34-36]. Also, a decreased body weight is correlated with metabolic defects that are brought on by toxicity [32, 37].
The liver and kidney sections were examined under a microscope to look for any hemorrhagic lesions, inflammatory infiltration, or other obvious alterations. In case of kidney, we found no significant changes in kidney and this findings were consistent with earlier studies [34]. Further, microscopic examination revealed that the liver of the streptozotocin-induced group of rats had a modest increase in the interlobular gap. This result was also consistent with earlier studies [38, 39].
CONCLUSIONS
It is concluded that ethanolic extract of Gynura procumbens with chitosan nanoparticles had a significant effect on blood glucose, plasma insulin, body weight, ALT, and AST levels. This study also indicated that Gynura procumbens with chitosan nanoparticles has possessed a significant antihyperglycemic effect in streptozotocin-induced diabetic rats. For further study, it is suggested that nanoparticles could be used with other natural or synthetic products to see their efficacy on drug delivery systems against various diseases.
ACKNOWLEDGMENTS
This research received no external funding. The authors are honored to express their deepest sense of gratitude and sincere appreciation to Dr. Khadija Al-Ferdous, Assistant Professor, Department of Anatomy and Histology, Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh for her helpful advice and cooperation in preparing histopathological slides.
AUTHOR CONTRIBUTIONS
Conceptualization and design of the research, MMMP and RI; methodology, RI; experimental investigation, SAB, SS, and RI; sample resources, RI; writing—original draft preparation, SAB, MMMP, and MMH; writing—review and editing, SAB, SS, MBR, VK, and MMH; supervision, RI, SS, and MMMP project administration, RI. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Cooke DW, Plotnick L. Type 1 diabetes mellitus in pediatrics. Pediatrics in review. 2008;29:374-85.
- [2]Bloomgarden ZT, Einhorn D. Hemoglobin a1c in diabetes diagnosis: Time for caution. Endocrine Practice. 2010;16:5-6.
- [3]Khan MAB, Hashim MJ, et al. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. Journal of epidemiology and global health. 2020;10:107.
- [4]Mohiuddin A. Diabetes fact: Bangladesh perspective. Community and Public Health Nursing. 2019:39.
- [5]Burch E, Williams LT, et al. Short-term improvements in diet quality in people newly diagnosed with type 2 diabetes are associated with smoking status, physical activity and body mass index: The 3d case series study. Nutrition & diabetes. 2020;10:25.
- [6]Kooti W, Farokhipour M, et al. The role of medicinal plants in the treatment of diabetes: A systematic review. Electronic physician. 2016;8:1832.
- [7]Singh P, Jain P, et al. Phytotherapeutic review on diabetes. Spatula DD. 2016;6:1-9.
- [8]Apovian CM, Okemah J, et al. Body weight considerations in the management of type 2 diabetes. Advances in therapy. 2019;36:44-58.
- [9]Miller R. Implementation of the center for disease control (cdc) prediabetes risk test in the medical weight loss setting. 2021.
- [10]Haastrup MB, Henriksen JE, et al. Insulin allergy can be successfully managed by a systematic approach. Clinical and Translational Allergy. 2018;8:1-6.
- [11]Shahadat MN, Islam R, et al. Antidiabetic evaluations of some traditional plants in alloxan induced diabetic mice model. IOSR J Agric Vet Sci. 2019;12:59-68.
- [12]Rahman A, Asad M. Chemical and biological investigations of the leaves of gynura procumbens. International Journal of Biosciences. 2013;3:36-43.
- [13]Toumpanakis A, Turnbull T, et al. Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: A systematic review. BMJ Open Diabetes Research and Care. 2018;6:e000534.
- [14]Shathi SA, Aziz FB, et al. Journal of bangladesh agricultural university. J Bangladesh Agril Univ. 2022;20:141-9.
- [15]Xing R, He X, et al. Antidiabetic activity of differently regioselective chitosan sulfates in alloxan-induced diabetic rats. Marine Drugs. 2015;13:3072-90.
- [16]Sarkar S, Das D, et al. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydrate polymers. 2020;247:116594.
- [17]Wang Z, Gleichmann H. Glut2 in pancreatic islets: Crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47:50-6.
- [18]Zhang X, Tan B. Effects of an ethanolic extract of gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore medical journal. 2000;41:9-13.
- [19]Algariri K, Meng KY, et al. Hypoglycemic and anti–hyperglycemic study of gynura procumbens leaf extracts. Asian Pacific Journal of Tropical Biomedicine. 2013;3:358-66.
- [20]Wardani G, Eraiko K, et al. The role of antioxidant activity of chitosan-pinus merkusii extract nanoparticle in against lead acetate-induced toxicity in rat pancreas. Veterinary medicine international. 2019;2019.
- [21]Gansau JA, Chin L, et al. Hypoglycemic effects of gynura procumbens fractions on streptozotocin-induced diabetic rats involved phosphorylation of gsk3β (ser-9) in liver. Sains Malays. 2012;41:969-75.
- [22]Lee H-W, Hakim P, et al. Antidiabetic effect of gynura procumbens leaves extracts involve modulation of hepatic carbohydrate metabolism in streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research. 2012;6:796-812.
- [23]Hassan Z, Yam MF, et al. Antidiabetic properties and mechanism of action of gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules. 2010;15:9008-23.
- [24]Kamaruzaman KA, Noor M. Gynura procumbens leaf improves blood glucose level, restores fertility and libido of diabetic-induced male rats. Sains Malaysiana. 2017;46:1471-7.
- [25]Rasadah M, Hamid M, et al. Study on anti-diabetic properties of gynura procumbens merr. Poster presented at Natural Products Seminar, Kota Kinabalu2002. p. 23-4.
- [26]Li J, Wu H, et al. Alginate calcium microbeads containing chitosan nanoparticles for controlled insulin release. Applied Biochemistry and Biotechnology. 2021;193:463-78.
- [27]Ziaul Amin M, Afrin M, et al. Evaluation of medicinal effects of gynuraprocumbens leave extracts on oxidative, glycemic, lipidomics, and enzymatic profiles in alloxan-induced diabetic mice. J Diabetes Metab J Diabetes Metab. 2021;12:876.
- [28]Singh D, Lawrence K, et al. In-vivo hyperglycemic, antioxidant, histopathological changes, and simultaneous measurement of kaempferol verified by high-performance thin layer chromatography of setaria italica in streptozotocin-induced diabetic rats. Saudi Journal of Biological Sciences. 2022;29:3772-90.
- [29]Tithi TI, Tahsin MR, et al. An in vivo and in silico evaluation of the hepatoprotective potential of gynura procumbens: A promising agent for combating hepatotoxicity. Plos one. 2023;18:e0291125.
- [30]Samadi‐Noshahr Z, Hadjzadeh MAR, et al. The hepatoprotective effects of fennel seeds extract and trans‐anethole in streptozotocin‐induced liver injury in rats. Food science & nutrition. 2021;9:1121-31.
- [31]Choi S-I, Park MH, et al. Gynura procumbens extract alleviates postprandial hyperglycemia in diabetic mice. Preventive Nutrition and Food Science. 2016;21:181.
- [32]Grüβner R, Nakhleh R, et al. Streptozotocin-induced diabetes mellitus in pigs. Hormone and metabolic research. 1993;25:199-203.
- [33]Borsha RP, Islam R, et al. Study of steroid induced hyperglycemia against traditional hypoglycemic plant extracts in rat model. Asian Journal of Pharmacy and Pharmacology. 2020;6:254-60.
- [34]Evan AP, Mong SA, et al. The effect of streptozotocin and streptozotocin-induced diabetes on the kidney. Kidney and Blood Pressure Research. 1984;7:78-89.
- [35]Jacobs RL, House JD, et al. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967-70.
- [36]Tahsin MR, Tithi TI, et al. In vivo and in silico assessment of diabetes ameliorating potentiality and safety profile of gynura procumbens leaves. Evidence-Based Complementary and Alternative Medicine. 2022;2022.
- [37]Zhiyong W, Gleichmann H. Glut2 in pancreatic islets. Diabetes. 1998;47:50-6.
- [38]Laguens SC, Hernández RE, et al. Streptozotocin-induced liver damage in mice. Hormone and Metabolic Research. 1995;23:455-9.
- [39]Evan SAM, Gattone VH, et al. The effect of streptozotocin and streptozotocin-induced diabetes on the kidney. Kidney and Blood Pressure Research. 1984;7:78-89.