Evaluation of the effectiveness of low-level laser therapy on proliferation of fibroblasts isolated from chronic wounds in human in vitro
Abstract
Chronic wounds pose significant challenges in healthcare due to impaired healing mechanisms. Fibroblast cells play a crucial role in wound healing by orchestrating proliferation and migration. This study aimed to assess fibroblast cells derived from chronic wounds and explore the impact of low-level laser therapy (LLLT) on their growth and migration. Dermal samples from chronic pressure ulcers and diabetic ulcers were obtained from 20 patients at three sites. Fibroblasts from wound base, margins, and adjacent healing skin were isolated and characterized. Proliferation and migration capabilities of these cells were evaluated. LLLT was applied at various energy levels (2.5, 3, 3.5, 4, and 5 J/cm2) to assess its effect on cell count. Fibroblasts from chronic wounds exhibited slower proliferation and migration rates compared to normal dermal fibroblasts. Notably, LLLT intervention at different energy levels led to a significant increase in cell count, with the most pronounced effect observed at 3 J/cm2. LLLT at an energy level of 3 J/cm2 demonstrated a notable enhancement in fibroblast migration. These findings underscore the potential of LLLT as a therapeutic approach for chronic wounds, offering insights into its efficacy in augmenting fibroblast functions crucial for wound healing.
Keywords
INTRODUCTION
Chronic wounds present a significant healthcare challenge globally, particularly affecting aging populations and individuals with chronic ailments. The prevalence of chronic wounds, associated with aging and chronic diseases, has considerable societal and economic implications. In the United States alone, around 5.7 million individuals encountered chronic wounds in 2017, leading to an estimated annual healthcare expenditure of $20 billion for their management. The profound impact of these wounds extends beyond the physical realm, substantially diminishing patients' quality of life, inducing pain, psychological distress, and often necessitating extended hospitalization to avert severe complications [1].
Despite the availability of diverse wound treatment methods encompassing traditional and contemporary approaches [2], the exigency persists for novel therapeutic avenues to complement existing strategies. Low-level laser therapy (LLLT), also known as photo biomodulation (PBM), stands as a promising medical modality harnessing low-intensity laser energy to stimulate cellular functions. The biphasic dose-response relationship characterizing LLLT underscores the importance of precise parameters such as wavelength, fluence, irradiance, and illumination duration for optimal therapeutic outcomes [3, 4]. It's crucial to differentiate LLLT from photodynamic therapy, as they diverge in mechanisms; LLLT modulates cellular functions via low-level light energy, whereas photodynamic therapy activates exogenous photosensitizers to generate reactive oxygen species [5]. LLLT has exhibited encouraging outcomes in stimulating stem cell activity and enhancing tissue healing, demonstrating efficacy in managing various dermatological conditions [6].
Fibroblast cells are pivotal in the intricate process of wound healing. Recent investigations into LED and laser technologies aimed at augmenting fibroblast activity and collagen synthesis endeavor to establish cost-effective approaches for wound repair without reliance on costly stationary light systems. Given the critical role of fibroblasts in wound healing, numerous studies have explored LLLT's impact on fibroblast behavior, including growth, migration, and collagen production [7-9]. Experimental evidence supports the notion that near-infrared LED light treatment enhances mitochondrial oxidative metabolism and expedites cell and tissue repair [10-14].
The promise of LLLT in enhancing chronic wound management presents an opportunity for healthcare systems globally, including Vietnam, to potentially improve patient outcomes. This study seeks to provide further evidence regarding the cellular-level efficacy of LLLT, specifically investigating its impact on wound development and healing processes in fibroblast cells in vitro.
MATERIALS AND METHODS
The study sample consisted of tissue samples obtained from 10 patients diagnosed with pressure ulcers and 10 patients diagnosed with diabetic foot ulcers who received inpatient treatment at the Vietnam National Institute of Burns. The inclusion criteria were patients aged 18 years and older with stage IV ulcers. Additionally, healthy fibroblast cells derived from the foreskin of children were used as a control group.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Vietnam National Institute of Burns (approval code 1237/QD-HCQY).
Biopsy for fibroblast cell collection
For optimal biopsy sample suitability, it is crucial to effectively cleanse the biopsy site. Patients are recommended to undergo a thorough full-body shower 24 hours prior to the biopsy procedure. The biopsy site must be carefully cleaned and prepared following the specific instructions provided in the medical guidelines [15, 16]. The skin surrounding the biopsy site is carefully washed using Microshield medical soap (Schulke, India) and sterile water. Subsequently, the actual biopsy site is treated with Povidone solution (Pharmedic, Vietnam) and thoroughly rinsed with sterilized 0.9% saline solution. Finally, the area is gently dried by blotting, ensuring proper care is taken during the process [17].
Biopsy procedures were conducted at three distinct locations within each wound: the wound base (Site 1), the wound margin (Site 2), and the surrounding healthy skin (Site 3). Each biopsy specimen, measuring 0.5 × 0.5 cm, encompassed the complete skin thickness, comprising the epidermis, dermis, and subcutaneous tissue [18]. Immediately following collection, the tissue samples were immersed in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and collagenase. To preserve their integrity, the samples were stored at a temperature of 4°C until further isolation and subsequent culture of fibroblast cells could be performed [10].
Isolation and cultivation of fibroblast cells
The established protocol developed by Freshney [19] was employed for the isolation and cultivation of fibroblast cells. Initially, the skin samples underwent cleansing with Phosphate Buffered Saline (PBS) solution (Invitrogen, USA) and were subsequently sectioned into 2 mm-sized specimens [10].
The tissue specimens were placed in a 25 cm2 Corning cell culture flasks (Corning, USA) containing 5 ml of DMEM culture medium with 10% (FBS) and collagenase. The flask was then incubated under controlled conditions at 37°C with 5% CO2 [20]. Following a 2-day incubation period, an additional 1 ml of culture medium was added to facilitate cell growth. During the culture transition phase, typically lasting 3-5 days, the medium volume was adjusted to reach a total volume of 5 ml. Regular medium changes were conducted every 3-4 days, and the cell population was diligently monitored until achieving at least 50% confluence on the flask surface [17].
For preservation, fibroblast cells derived from the third passage culture were selected and stored using DMEM storage medium supplemented with 10% dimethyl sulfoxide (DMSO) under cryogenic storage conditions utilizing liquid CO2 [17].
Counting living cultured fibroblast cells
The cell quantification for appropriate seeding and assessment of cell proliferation was conducted utilizing a Neubauer counting chamber following the protocol outlined by Bich Phuong et al. [17]. Cells were stained with a 0.4% trypan blue dye solution and enumerated under a microscope within a unit area of 1 mm2. The cell density was determined using the formula:
![]()
Where, C denotes the cell density (cells/mL) and n signifies the number of cells counted in the Neubauer counting chamber.
Determining the viability of cultured fibroblast cells
The viability of fibroblast cells at various culture stages was evaluated according to the protocol outlined by Bich Phuong et al. [17]. A 0.4% Trypan Blue staining solution (Gibco, USA) was utilized for assessing viability. This stain selectively permeates the membranes of impaired (nonviable) cells, resulting in their visualization as blue entities that can be differentiated under microscopic examination [21].
To perform the cell viability assessment, 1 mL of the cell suspension was transferred to a centrifuge tube, and 50 μL of a 0.4% Trypan Blue stain was added. The mixture was incubated for 5 minutes. Subsequently, the solution was transferred to a hemocytometer and examined under an inverted microscope for the quantification of both the total cell count and the number of cells stained blue, indicating non-viable cells [17]. The cell viability percentage (%) is calculated using the formula:
![]()
Assessing the effect of LLLT on fibroblast proliferation
The proliferation of fibroblasts, specifically wound base fibroblasts, was evaluated using a modified model based on the research conducted by Pansani et al. in 2014 [22]. The cells from the third generation (G3) were cultured in a 6-well plate (Corning, USA) at a density of 5x104 cells per well in 2 mL of DMEM supplemented with 10% FBS and 1% AB 1X (Gibco, USA). The cell culture was maintained at 37°C with 5% CO2. The number of viable cells, cultured for 24 hours, was determined using the previously described method [17].
To assess the impact of LLLT on fibroblast cell proliferation, the cells were seeded into a 6-well plate and divided into two groups: a control group without laser irradiation and an LLLT group. The LLLT group was further divided into subgroups with varying energy levels (2.5, 3, 3.5, 4, and 5 J/cm2) of 808nm laser and corresponding irradiation times (30-60 seconds). Laser irradiation was administered daily for three consecutive days.
The B-Cure Laser Pro device (Good Energies®, Israel) was utilized for LLLT in the study. The laser employed in the research belonged to the GaAlAs category, which refers to Gallium Aluminum Arsenide and represents a solid-state laser diode. The maximum power output of the laser was 250 mW, ensuring the intensity of the laser irradiation. The wavelength of the laser fell within the infrared range and measured 808 nm, enabling effective penetration into the target tissues. The pulse frequency was set at 13 kHz, indicating the rate at which laser pulses were emitted per second. Furthermore, the laser pulse duration was 26 μs, signifying the duration of each individual laser pulse. To maintain a consistent distance between the laser probe and the sample surface, a distance of 1 cm was maintained throughout the experiments. These specific laser parameters were deliberately selected and implemented to deliver the desired effects of LLLT in the study.
Following the final irradiation, the number of viable cells in both the control group and the LLLT group was determined using the trypan blue staining method described earlier. The cell count was performed at the wound sites and compared with the control group, which did not undergo laser irradiation.
Statistical analysis
The statistical analysis of the research findings was performed using SPSS 22 statistical software. The cell count was presented as the mean value (±) standard deviation. The non-parametric Mann-Whitney test was employed for data analysis, with a significance level set at p < 0.05 to determine statistical significance.
RESULTS
Effectiveness of culturing primary fibroblast cells
Primary fibroblast cells were successfully isolated from the wound base (Site 1), wound margin (Site 2), and adjacent healthy skin (Site 3). No bacterial or fungal contamination was detected at any site, confirming aseptic culture conditions and lack of microbial growth. The number of viable fibroblast cells obtained was consistent across all three sites, with 20 viable samples per site. This indicates successful culture and maintenance of viability of the isolated primary fibroblasts. Additionally, no non-proliferating cells were found at any site, suggesting the fibroblasts retained proliferative potential essential for wound healing and tissue regeneration. In summary, the data demonstrates the effective isolation of viable, proliferating primary fibroblast cells from the wound base, margin, and surrounding healthy skin under sterile conditions. This provides a robust foundation for further investigating fibroblast behavior and function in wound healing across these sites.
Comparative analysis of fibroblast cell generations
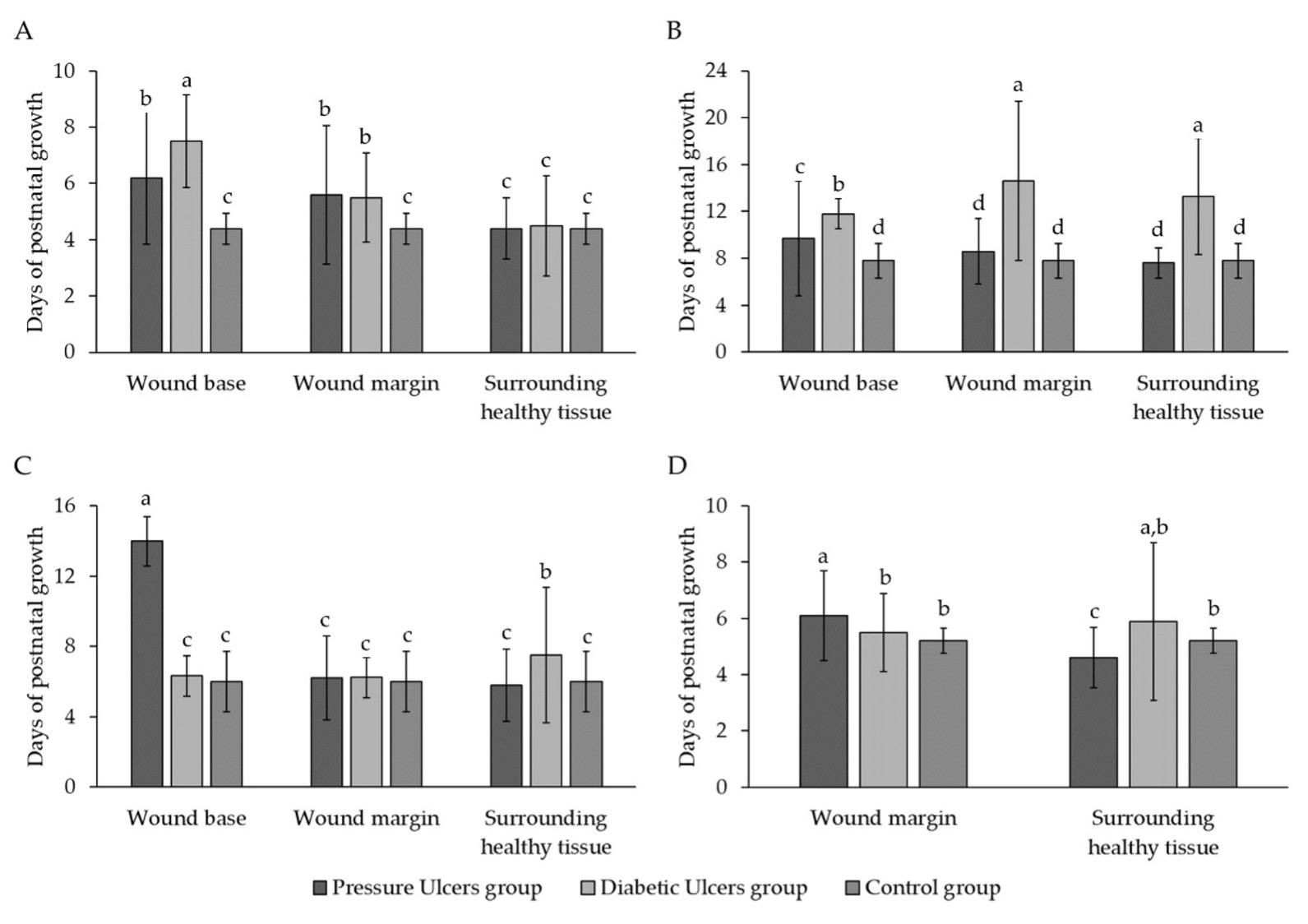
Figure 1A shows fibroblast growth during the initiation phase of cell culture across three groups - pressure ulcers, diabetic ulcers, and control. Growth was measured at Site 1, Site 2, and Site 3 over time in days. In the pressure ulcer group, mean growth at Sites 1, 2, and 3 was 6.2±2.35, 5.6±2.46, and 4.4±1.08 respectively. No significant differences were found between Sites 1 and 2 or between Sites 2 and 3, but Sites 1 and 3 were significantly different (p < 0.05).
In the diabetic ulcer group, mean growth was 7.5±1.65, 5.5±1.58, and 4.5±1.78 at Sites 1, 2, and 3 respectively. Site 1 significantly differed from Sites 2 and 3, but no difference was seen between Sites 2 and 3. No significant differences were found between the pressure ulcer and control groups. The diabetic ulcer group differed significantly from control only at Site 1 (p < 0.05). Overall, figure 1A provides an overview of variation in early fibroblast growth across wound sites between pressure and diabetic ulcers and control samples. The results highlight differences in initial fibroblast behavior and characteristics during culture initiation relevant to wound healing pathology.
Figure 1B shows the average growth time of 1st generation fibroblasts (G1) across wound sites for pressure ulcers, diabetic ulcers, and control groups. In the pressure ulcer group, G1 growth times were 9.7±4.9, 8.6±2.79, and 7.6±1.27 days at Sites 1, 2, and 3 respectively, with no significant differences between sites. Growth times significantly differed between the diabetic ulcer and control groups at Sites 1 and 2, but not Site 3.
Figure 1C presents the average growth time of 2nd generation fibroblasts (G2). In the pressure ulcer group, G2 growth time was 14±1.41 days at Site 1 versus 6.2±2.39 and 5.8±2.04 days at Sites 2 and 3 - significantly different from Site 1 but not each other. In the diabetic ulcer group, G2 growth times were 6.33±1.15, 6.25±1.16, and 7.5±3.86 days at Sites 1, 2, and 3 respectively, with no significant differences. Growth time only significantly differed between the pressure ulcer and control groups at Site 1.
Figure 1D shows growth times for 3rd generation fibroblasts (G3). In the pressure ulcer group, no growth was observed at Site 1. G3 times were 6.1±1.59 and 4.6±1.07 days at Sites 2 and 3 respectively - significantly different. In the diabetic ulcer group, no G3 growth occurred at Site 1. Times were 5.5±1.38 and 5.89±2.8 days at Sites 2 and 3 respectively - not significantly different.
Overall, the data indicates differences in fibroblast growth patterns and times across wound locations and generations between chronic wound groups compared to normal healing. This suggests impaired proliferation and migration capabilities in chronic wound fibroblasts, particularly at the non-healing wound base site.
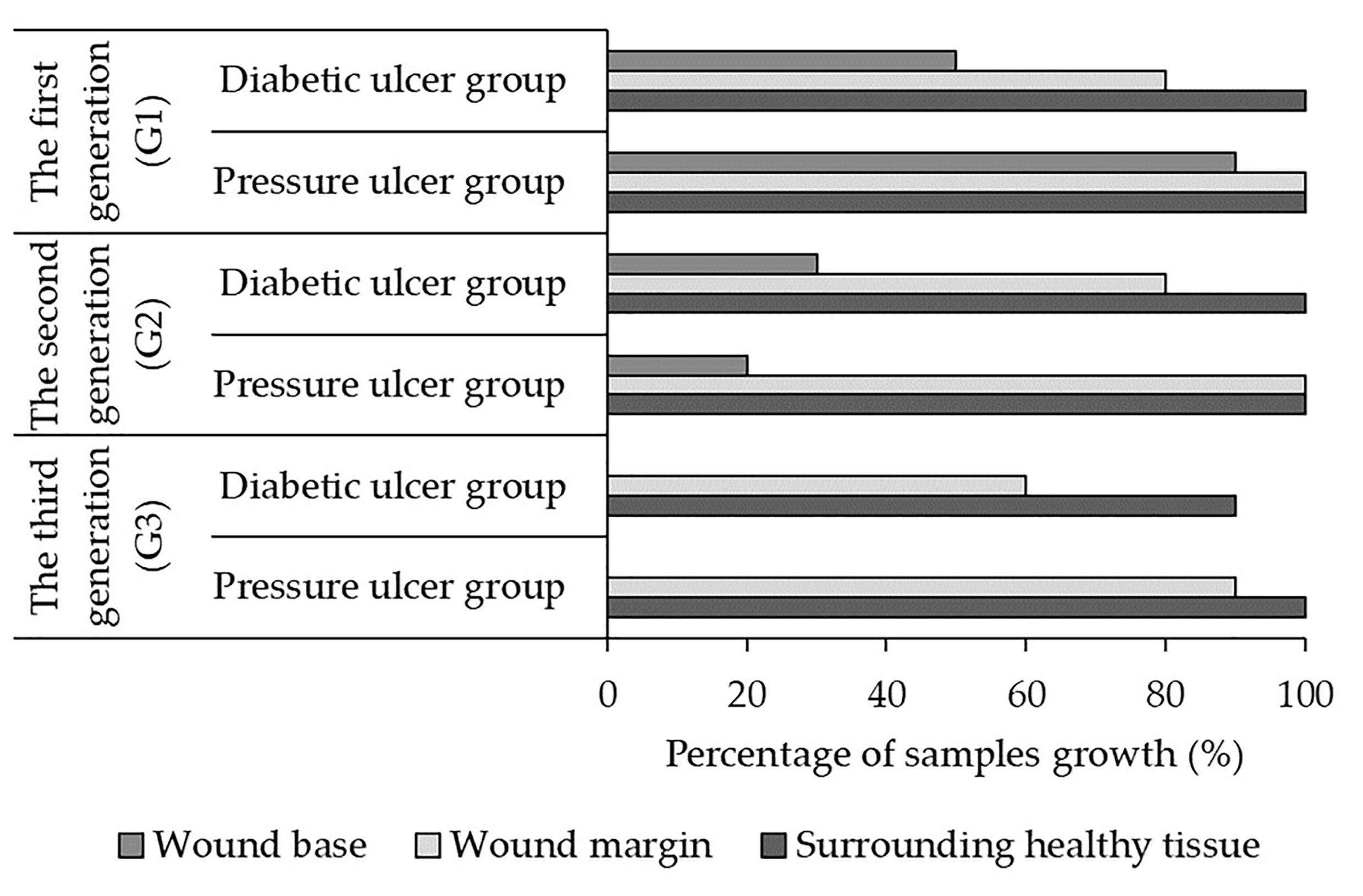
Fibroblasts cultured over generations for different patients
Figure 2 shows the percentage of fibroblast samples successfully cultured across three generations (G1, G2, G3) at the wound base (Site 1), margin (Site 2), and adjacent skin (Site 3) for pressure and diabetic ulcer groups. In generation 1, a higher percentage of samples were cultured at all sites for both groups compared to subsequent generations. The pressure ulcer group had 90-100% success at all sites, while the diabetic ulcer group had 50-100% success (p = 0.05 for comparison between G1 and G2). In generation 2, the percentage of cultured samples decreased, especially at Site 1 - just 20% and 30% for pressure and diabetic ulcers respectively (p < 0.05 for decrease at Site 1). Success remained higher at Sites 2 and 3. By generation 3, no fibroblast growth occurred at Site 1 for either group. The pressure ulcer group had 90-100% success at Sites 2 and 3, while the diabetic ulcer group had 60-90% success (p = 0.05).
Overall, the data shows declining fibroblast culture success across generations specifically at the wound base site (Site 1), suggesting reduced proliferation or availability of viable cells over time at this location. The other sites generally maintained higher proliferation over multiple generations. Limitations in proliferation were particularly evident at the non-healing wound base site (Site 1), with reductions in culture success by the third generation. Further research into factors impacting multi-generational fibroblast growth and viability is warranted, especially investigating the differences between wound locations.
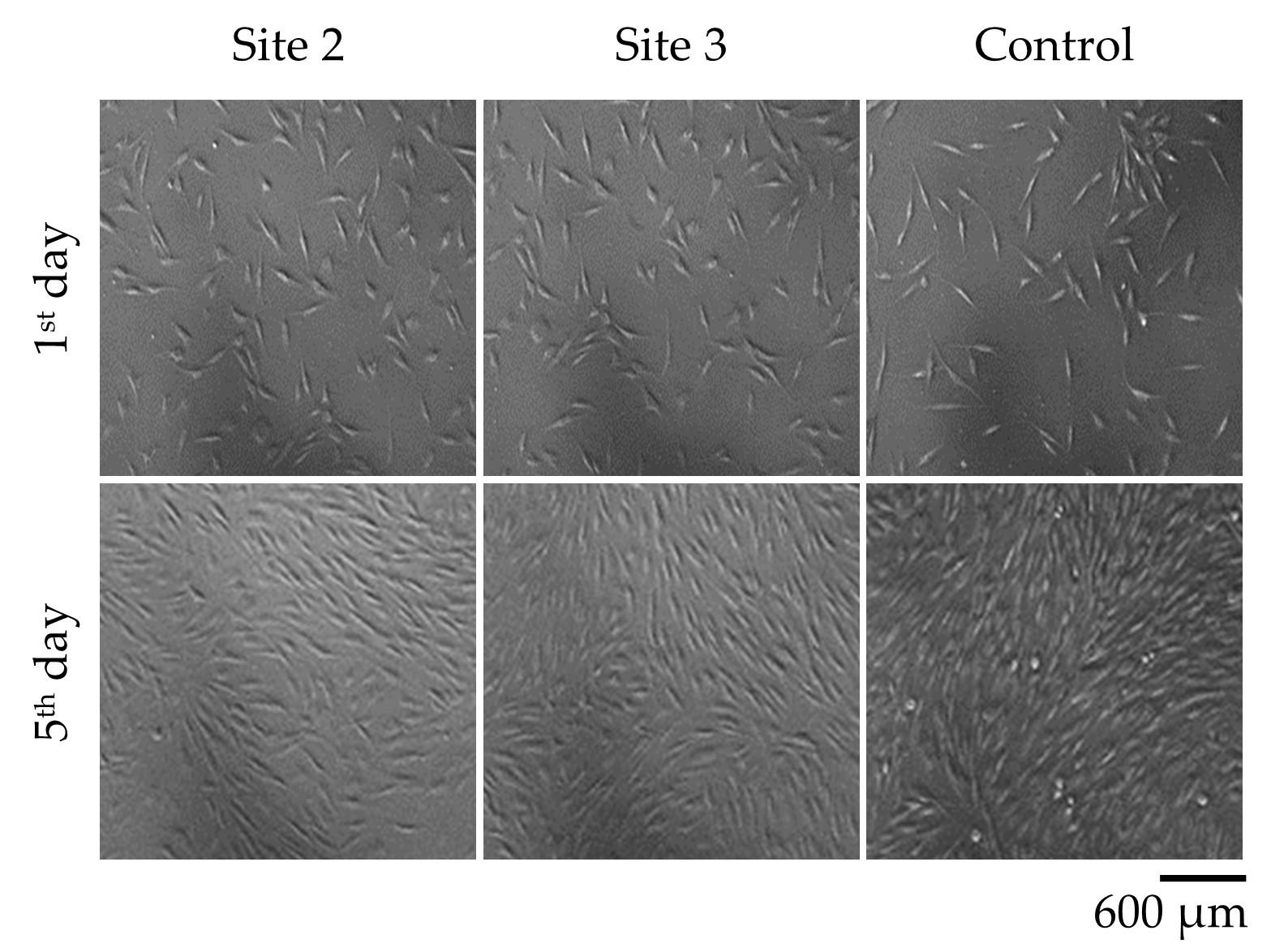
Visualizing fibroblast cells in the third generation
Figure 3 demonstrates the morphology and growth characteristics of fibroblast cells in the third generation of cell culture. The fibroblast cells exhibited a diamond-shaped morphology and adhered to the surface of the culture dish, forming a monolayer. The images presented in Figure 3 provide visual evidence of the fibroblast cell proliferation and adherence patterns at different time points. It is worth noting that these fibroblast cells were derived from the third generation of fibroblasts cultured from samples taken from Pressure Ulcer Wounds.
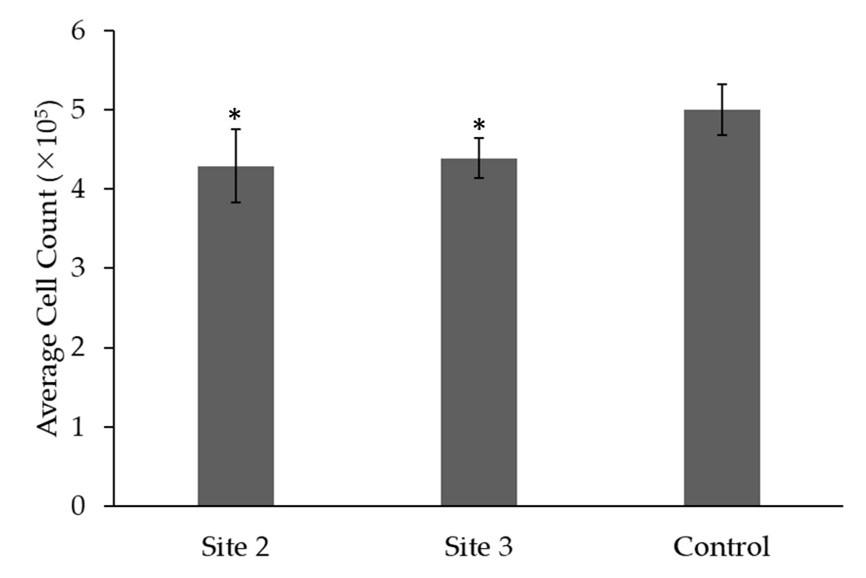
Figure 4 shows the average cell count (x105) of cell proliferation in samples from Site 2, Site 3, and the control group derived from pressure ulcer wounds after 5 days of cultivation in the 3rd generation. The p-value indicates statistical significance when compared to the control group (p = 0.05). Figure 4 displays the mean cell count for each site along with the corresponding standard deviation. The average cell count for Site 2 is (4.29 ± 0.46)x105. Similarly, Site 3 has an average cell count of (4.39 ± 0.25)x105, showing slightly higher cell count compared to Site 2. In comparison, the control group has an average cell count of (5.0 ± 0.32)x105, indicating a slightly higher cell count compared to both Site 2 and Site 3 (p > 0.05).
Overall, the results demonstrate successful culture of 3rd generation fibroblasts from pressure ulcers, though proliferative capacity was reduced at wound sites compared to normal fibroblasts. The microscopy images provide visual confirmation of typical fibroblast growth patterns and morphology. Further quantification underscores the decreased cell expansion at wound locations.
Impact of LLLT on fibroblast cell growth
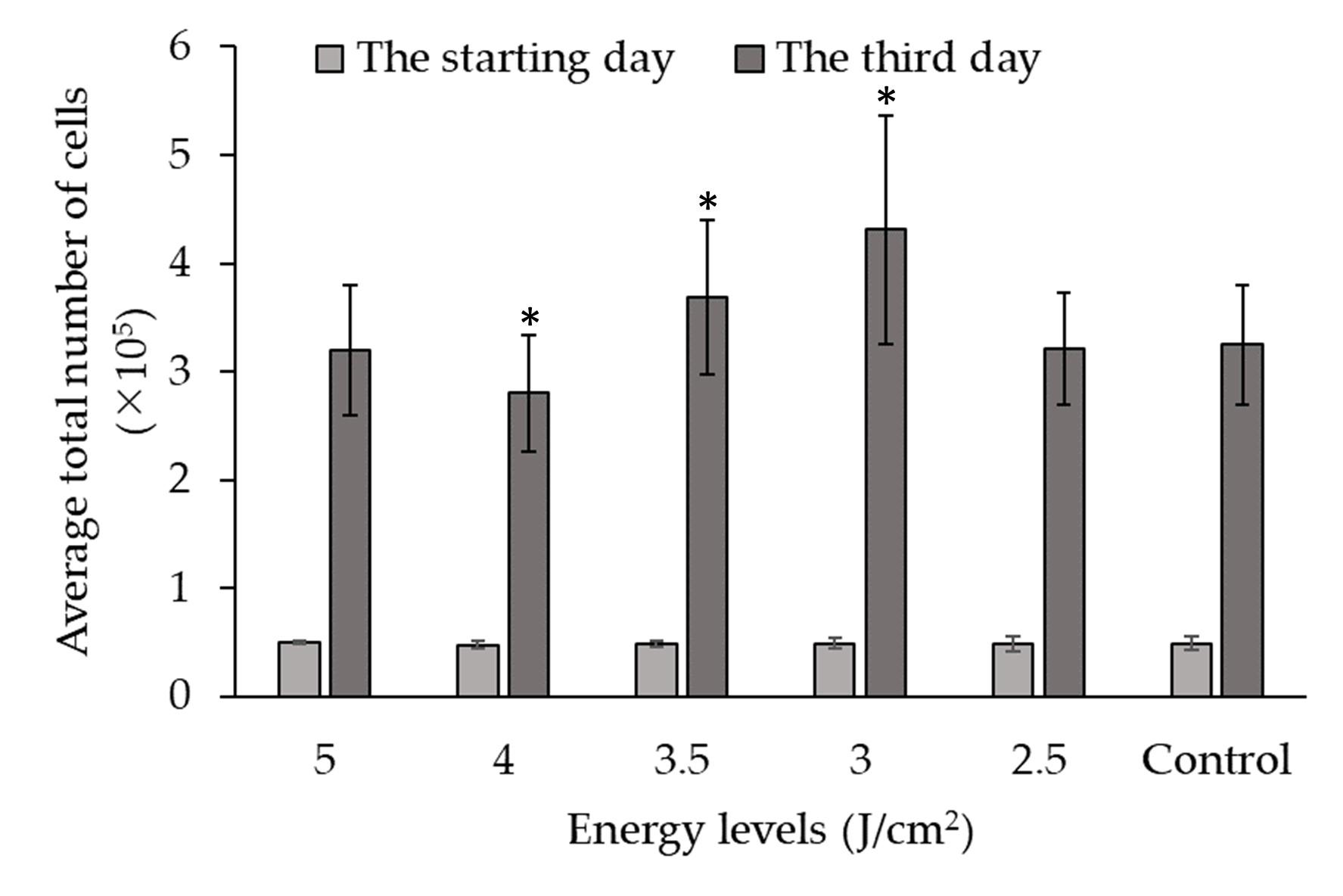
Figure 5 presents the data on the impact of LLLT on the growth of fibroblast cells from at different energy levels. The experiment involved culturing fibroblast cells and subjecting them to varying levels of energy during LLLT. The growth of fibroblast cells was measured and compared between the different energy levels and a control group (in units of total cells x105).
On the starting day, the average cell counts were relatively similar across all energy levels and the control group, ranging from 0.47±0.04 to 0.50±0.01 x105 total cells. However, on the third day of culture, significant differences in cell growth were observed among the energy levels. The average cell counts varied across the different energy levels, with values ranging from 2.80±0.54 to 4.31±1.06 x105 total cells. Statistical analysis using p-values was conducted to compare each energy level with the control group. The p-values indicate the significance of the differences observed.
The analysis revealed that at energy levels of 3 and 3.5 J/cm2, there was a significant increase in cell growth compared to the control group (p < 0.05). At the energy level of 2.5 and 5 J/cm2, although there was an increase in cell growth compared to the control group, the difference was not statistically significant (p < 0.05). This decreased cell count observed with 4 J/cm2 irradiation may potentially be attributed to technical errors introduced during the cell culture procedure for this specific energy level experiment.
The results indicate that specific energy levels, such as 3.5, and 3 J/cm2, can significantly enhance cell growth, while other energy levels may not have a significant effect. It can be concluded that the use of the 3 J/cm2 energy level in LLLT has the most beneficial effect on fibroblast cell growth.
Overall, the results demonstrate LLLT can enhance fibroblast growth in an energy-dependent manner. A level of 3 J/cm2 provided optimal and significant stimulation of cell proliferation compared to control. This indicates LLLT at 3 J/cm2 could be leveraged to positively influence fibroblast cell expansion for tissue engineering and regenerative medicine applications requiring effective cell growth.
DISCUSSION
Based on current research, investigations into therapeutic approaches for chronic wounds primarily focus on healthy fibroblast cells due to evidence suggesting reduced proliferation and premature aging in fibroblast cells derived from chronic wounds [23]. Successful isolation of fibroblast cells from chronic wounds serves as a fundamental basis for experimental studies exploring potential cell-based therapies for managing chronic wounds. Previous research publications have documented the successful isolation of fibroblast cells from venous ulcers, diabetic ulcers, and pressure ulcers [18, 24, 25]. There are two commonly utilized techniques for tissue sample separation in isolation procedures: purely mechanical techniques and enzyme-based techniques. Purely mechanical techniques are limited in their suitability to certain tissue types and often yield a low quantity of cells. On the other hand, enzyme-based techniques are easier to implement and can result in a higher cell yield, albeit at a higher cost [19]. In this particular study, 60 biopsy samples were collected from pressure ulcers and diabetic ulcers in 20 patients. Prior to sampling, all tissues were prepared following an established protocol to ensure they were free from infection. The trypan blue method is a straightforward approach used to evaluate cytotoxicity. Nevertheless, this method is not without its limitations. These limitations include potential errors in calculating results for individual samples, challenges in distinguishing between dead cells and stained debris, inconsistencies among operators, and the time and labor-intensive nature of processing multiple samples [26].
In this study, a total of 60 fibroblast cell samples were examined. Among these samples, only at Site 1 (wound base), the third-generation cell regeneration was not sustained. Fibroblast cells obtained from the wound margin (Site 2) and surrounding healthy skin (Site 3) exhibited no noticeable morphological changes, appearing spindle-shaped and forming a single layer when adhered to the culture dish. Cells from Sites 2 and 3 demonstrated robust proliferative capacity, allowing their isolation up to the third, fourth, and fifth generations. Notably, Brem et al. observed that fibroblast cells isolated from four tissue samples of patients with venous ulcers retained their characteristic phenotype. Fibroblast cells from the surrounding healthy skin displayed the most favorable response, while those from the wound base exhibited an average response, and the cells from the wound margin showed comparatively less development [18].
Cellular aging is characterized by a decline in growth capacity and morphological alterations. Comparing the proliferation process between Site 2 (wound margin) and Site 3 (surrounding healthy skin) with healthy fibroblast cells revealed no significant differences. However, fibroblast cells derived from chronic wounds exhibited a noticeable deceleration in proliferation compared to healthy fibroblast cells (p < 0.05). The cell counts in samples from Sites 2 and 3 was significantly lower than that of the control group (p < 0.05), indicating a slower proliferation rate of fibroblast cells from chronic wounds compared to healthy fibroblast cells. These findings are consistent with seminal studies conducted by Miriam Loots et al., which demonstrated a reduced proliferative capacity of fibroblast cells isolated from type II diabetic ulcers [24]. Wall et al. also observed decreased proliferation, impaired wound closure ability, and premature aging in fibroblast cells from chronic wounds compared to normal fibroblast cells. Additionally, they highlighted the accumulation of senescent fibroblast cells, accounting for over 15%, in both chronic wounds and non-wounded skin [25]. Vande Berg and Robson found that fibroblast cells in decubitus ulcers and venous ulcers exhibited reduced proliferation and displayed signs of cellular senescence. Fibroblast cells from decubitus ulcers demonstrated a slower rate of replication compared to normal fibroblast cells as well [27].
Studying fibroblast cells derived from chronic wounds provides a foundation for investigating experimental interventions, such as LLLT, for wound treatment. Numerous studies have demonstrated the effectiveness of LLLT in promoting wound healing, with outcomes dependent on the dosage and wavelength employed [28-30]. Laser irradiation at 808 nm was applied to fibroblast cells at various energy levels in this study, aiming to determine the dosage with optimal stimulation of biological effects on cell proliferation. The results revealed a significant increase in cell count following LLLT treatment with dosages of 3 and 3.5 J/cm2, with 3 J/cm2 yielding the most favorable outcome (p < 0.05). The findings of this study indicate that 3 and 3.5 J/cm2 represent stimulatory doses, whereas 4 J/cm2 exhibits suppressive effects compared to the control group (p < 0.05). These findings align with previous research by Basso et al. [31-33], which explored the impact of LLLT on the proliferation and migration of gingival fibroblast cells in humans and identified the optimal energy dosage as 3 J/cm². A comprehensive review of cell culture studies conducted by AlGhamdi et al. [34] further supported these findings, demonstrating that LLLT at energy levels ranging from 0.5 to 4.0 J/cm2 enhanced the proliferation rate of various cell lines. Kreisler et al. [35] also observed an increase in fibroblast cell proliferation in vitro following direct and continuous LLLT irradiation. Similarly, Ma et al. [36] demonstrated that irradiation of healthy fibroblast cells with an 830nm laser wavelength promoted both cell proliferation and collagen synthesis. These findings are consistent with previous investigations highlighting the beneficial effects of LLLT on cells through the upregulation of growth factor expression [32, 37]. The stimulation of cell proliferation by low-level laser treatment may occur through several key cellular mechanisms. These include increased generation of reactive oxygen species [38, 39], enhanced production of ATP [40, 41], alterations in the activity of membrane-bound mitochondrial enzymes [41], changes in expression of growth factors regulating cell division [39, 40, 42], and shifts in levels of proteins involved in critical signaling pathways [42-44]. By influencing these critical processes governing energy availability, proliferation signals, and pathway communication, low-level laser interaction can modulate cell behavior to drive expanded cell division. While previous studies have implicated these mechanisms, further research is still needed to fully elucidate the specific pathways mediating the effects of low-level lasers on photo biomodulation and cell growth. Elucidating these photobiological mechanisms will strengthen our understanding and implementation of laser-based approaches for stimulating cellular proliferation.
CONCLUSION
The study underscores the potential of LLLT at an energy level of 3 J/cm2 in enhancing fibroblast migration, a crucial aspect of wound healing in chronic wounds. The findings distinctly address the research objective, affirming the substantial impact of LLLT on pivotal fibroblast functions. The significant increase in cell count post-LLLT intervention highlights its promising role as a therapeutic approach for chronic wounds. This outcome, derived from our investigation into fibroblast behavior in chronic wound environments, indicates a clear path for the clinical application of LLLT. In essence, the research emphasizes the potential of LLLT as an adjunctive treatment modality for chronic wounds. The observed enhancement in fibroblast migration opens avenues for further exploration, potentially improving the management and healing outcomes for individuals affected by chronic wounds.
ACKNOWLEDGMENT
We acknowledge the cooperation and support of outpatients, and collaborators at the Vietnam National Institute of Burns for the time and effort they devoted to the study. We also thank you for the support from Ho Chi Minh city Hospital of Rehabilitation and Occupational Disease (Ho Chi Minh City, Vietnam) and Can Tho University of Medicine and Pharmacy (Can Tho City, Vietnam).
AUTHOR CONTRIBUTIONS
MHP and BPNT designed outlines and drafted the manuscript. MHP and BPNT performed the experiments and analyzed the data. VHD and THN wrote the initial draft of the manuscript. NNPT, HHN, and LTS reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Alam W, Hasson J, et al. Clinical approach to chronic wound management in older adults. Journal of the American Geriatrics Society. 2021;69:2327-34.
- [2]Addis R, Cruciani S, et al. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. International Journal of Medical Sciences. 2020;17:1030-42.
- [3]Chung H, Dai T, et al. The nuts and bolts of low-level laser (light) therapy. Annals of Biomedical Engineering. 2012;40:516-33.
- [4]Phan MH, Pham TNN, et al. Features of mesenchymal stem cells derived from umbilical cord lining membranes and their potential use in burn injury therapy. J of Southwest Jiaotong University. 2023;58:146-52.
- [5]Freitas LFd, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of Selected Topics in Quantum Electronics. 2016;22:348-64.
- [6]Santos CMd, Rocha RBd, et al. A systematic review and meta-analysis of the effects of low-level laser therapy in the treatment of diabetic foot ulcers. The International Journal of Lower Extremity Wounds. 2020;20:198-207.
- [7]Oliveira DAAP, De Oliveira RF, et al. Assessment of cytoskeleton and endoplasmic reticulum of fibroblast cells subjected to low-level laser therapy and low-intensity pulsed ultrasound. Photomedicine and Laser Surgery. 2009;27:461-6.
- [8]Hsieh YL, Cheng YJ, et al. The fluence effects of low-level laser therapy on inflammation, fibroblast-like synoviocytes, and synovial apoptosis in rats with adjuvant-induced arthritis. Photomedicine and Laser Surgery. 2014;32:669-77.
- [9]Amorim FCM, Arisawa EÂL, et al. Preclinical study of experimental burns treated with photobiomodulation and human amniotic membrane, both isolated and associated. Rev Lat Am Enfermagem. 2023;31:e3726.
- [10]Yang TS, Nguyen LTH, et al. Biophotonic effects of low-level laser therapy at different wavelengths for potential wound healing. Photonics2022.
- [11]Erdle BJ, Brouxhon S, et al. Effects of continuous-wave (670-nm) red light on wound healing. Dermatologic Surgery. 2008;34:320-5.
- [12]Corazza AV, Jorge J, et al. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomedicine and Laser Surgery. 2007;25:102-6.
- [13]Dall Agnol MA, Nicolau RA, et al. Comparative analysis of coherent light action (laser) versus non-coherent light (light-emitting diode) for tissue repair in diabetic rats. Lasers in Medical Science. 2009;24:909-16.
- [14]Trelles MA, Allones I. Red light‐emitting diode (led) therapy accelerates wound healing post‐blepharoplasty and periocular laser ablative resurfacing. Journal of Cosmetic and Laser Therapy. 2006;8:39-42.
- [15]Greenwood JD, Merry SP, et al. Skin biopsy techniques. Primary Care: Clinics in Office Practice. 2022;49:1-22.
- [16]Manchanda M, Torres M, et al. Metabolic reprogramming and reliance in human skin wound healing. Journal of Investigative Dermatology. 2023.
- [17]Nguyen TBP, Dinh VH, et al. In vitro assessment of the effect of low level laser therapy on the proliferation and migration of fibroblasts derived from patients with chronic wounds. Vietnam Medical Journal. 2023;525:230-5.
- [18]Brem H, Golinko MS, et al. Primary cultured fibroblasts derived from patients with chronic wounds: A methodology to produce human cell lines and test putative growth factor therapy such as gmcsf. Journal of Translational Medicine. 2008;6:75.
- [19]Freshney RI. Culture of animal cells: A manual of basic technique and specialized applications: John Wiley & Sons; 2015.
- [20]Roberts WG, Berns MW. In vitro photosensitization i. Cellular uptake and subcellular localization of mono-l-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin ii. Lasers in Surgery and Medicine. 1989;9:90-101.
- [21]Kaltenbach JP, Kaltenbach MH, et al. Nigrosin as a dye for differentiating live and dead ascites cells. Experimental Cell Research. 1958;15:112-7.
- [22]Pansani TN, Basso FG, et al. Effects of low-level laser therapy on the proliferation and apoptosis of gingival fibroblasts treated with zoledronic acid. International Journal of Oral and Maxillofacial Surgery. 2014;43:1030-4.
- [23]desJardins-Park HE, Foster DS, et al. Fibroblasts and wound healing: An update. Regenerative Medicine. 2018;13:491-5.
- [24]Loots MAM, Lamme EN, et al. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Archives of Dermatological Research. 1999;291:93-9.
- [25]Wall IB, Moseley R, et al. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. Journal of Investigative Dermatology. 2008;128:2526-40.
- [26]Gupta R, Rajpoot K, et al. Chapter 7 - methods and models for in vitro toxicity. In: Tekade RK, editor. Pharmacokinetics and toxicokinetic considerations: Academic Press; 2022. p. 145-74.
- [27]Vande Berg JS, Rose MA, et al. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-β1. Wound Repair and Regeneration. 2005;13:76-83.
- [28]Gupta A, Keshri GK, et al. Superpulsed (ga-as, 904 nm) low-level laser therapy (lllt) attenuates inflammatory response and enhances healing of burn wounds. Journal of Biophotonics. 2015;8:489-501.
- [29]Wanitphakdeedecha R, Iamphonrat T, et al. Local and systemic effects of low-level light therapy with light-emitting diodes to improve erythema after fractional ablative skin resurfacing: A controlled study. Lasers in Medical Science. 2019;34:343-51.
- [30]Nilforoushzadeh MA, Kazemi khoo N, et al. An open-label study of low-level laser therapy followed by autologous fibroblast transplantation for healing grade 3 burn wounds in diabetic patients. Journal of Lasers in Medical Sciences. 2019;10:S7-S12.
- [31]Basso FG, Soares DG, et al. Low-level laser therapy in 3d cell culture model using gingival fibroblasts. Lasers in Medical Science. 2016;31:973-8.
- [32]Peplow PV, Chung TY, et al. Laser photobiomodulation of proliferation of cells in culture: A review of human and animal studies. Photomedicine and Laser Surgery. 2010;28:S-3-S-40.
- [33]Basso FG, Pansani TN, et al. In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. International Journal of Dentistry. 2012;2012:719452.
- [34]AlGhamdi KM, Kumar A, et al. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers in Medical Science. 2012;27:237-49.
- [35]Kreisler M, Christoffers AB, et al. Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers in Surgery and Medicine. 2002;30:365-9.
- [36]Ma H, Yang JP, et al. Effect of low-level laser therapy on proliferation and collagen synthesis of human fibroblasts in vitro. J Wound Manag Res. 2018;14:1-6.
- [37]Woodruff LD, Bounkeo JM, et al. The efficacy of laser therapy in wound repair: A meta-analysis of the literature. Photomedicine and Laser Surgery. 2004;22:241-7.
- [38]George S, Hamblin MR, et al. Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. Journal of Photochemistry and Photobiology B: Biology. 2018;188:60-8.
- [39]Bai J, Li L, et al. Low level laser therapy promotes bone regeneration by coupling angiogenesis and osteogenesis. Stem Cell Research & Therapy. 2021;12:432.
- [40]Zhang Q, Zhou C, et al. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene x-1 deficiency. Journal of Cerebral Blood Flow & Metabolism. 2014;34:1391-401.
- [41]Lima PLV, Pereira CV, et al. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. Journal of Photochemistry and Photobiology B: Biology. 2019;194:71-5.
- [42]Kim JE, Woo YJ, et al. Wnt/β-catenin and erk pathway activation: A possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers in Surgery and Medicine. 2017;49:940-7.
- [43]Li Q, Chen Y, et al. Laser irradiation promotes the proliferation of mouse pre-osteoblast cell line mc3t3-e1 through hedgehog signaling pathway. Lasers in Medical Science. 2017;32:1489-96.
- [44]Shingyochi Y, Kanazawa S, et al. A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of akt, erk, and jnk. PLOS ONE. 2017;12:e0168937.