Detection of multi-antibiotic resistant Vibrio parahaemolyticus isolated from fresh produce in Dhaka, Bangladesh
Abstract
The demand for fresh produce is rising daily as it's an excellent way to maintain a healthy lifestyle. Thus, the current study aimed to employ culture-based and molecular techniques for isolating and identifying the pathogenic Vibrio parahaemolyticus from fresh produce. A total of 112 fresh produces of 14 different types were purchased from venders. The colonial appearance on thiosulfate citrate bile salt sucrose agar and CHROMagar culture plates revealed the prevalence of Vibrio spp. and V. parahaemolyticus in all examined samples, which were 45/112 (40.2%) and 40/112 (35.7%), with the highest load of up to 2.0x105cfu/g and 6.4x103cfu/g, respectively. Sixty isolates in all were chosen for further molecular characterization. Multiplex PCR results exhibited that all tested isolates were positive for tlh gene and six for tdh gene but none of isolate for trh gene. According to obtained data on antimicrobial susceptibility, the major portion of tested strains was found to be resistant to ampicillin (90%), tetracycline (80%), and streptomycin (70%). The range of the multiple antibiotic index was 0-0.33. This study finding revealed that the existence of potential pathogenic V. parahaemolyticus in tested fresh produce samples constitutes a risk to public health and consumer safety, thus urging continued surveillance.
INTRODUCTION
Several government authorities advise eating fresh produce for a healthy diet because of its medicinal and nutritional benefits [1] and low energy content [2]. Fresh produce consumption has significantly increased in recent years in numerous nations, including Bangladesh, the United States, the United Kingdom, Canada, Thailand, and India. As a result, during the past several years, the production of fresh produce on a global scale has increased to 30%rising from 30 to 60 million tons [3]. According to Faustat, the total consumption of vegetables in Bangladesh reached 4049 kt in 2013 and it is 0.226% more than the previous year [4]. Despite the substantial health advantages of fresh produce, outbreaks of food-borne illnesses associated with contaminating fresh produce have grown over the past few decades [5]. Fresh produce may come into contact with bacterial contaminants during the handling of pre-and post-harvest processing [6]. According to several publications [7], Vibrio spp. outbreaks of human infections have risen globally during the past ten years. Gastroenteritis caused by V. parahaemolyticus is extensively documented due to its increasing outbreaks [8]. V. parahaemolyticus can lead to further complications like septicemia when consumed raw or undercooked food or comes into contact with the aquatic environment [8, 9]. V. parahaemolyticus is a prevalent food-borne pathogen in Asia [10], and it has been estimated that in Japan, V. parahaemolyticus is responsible for 20 to 30% of food poisoning illnesses. Because people frequently eat raw or undercooked seafood in Southeast Asian countries, virulent V. parahaemolyticus strains are to blame for about 50% of outbreaks [11]. Acute gastroenteritis is thought to be caused by the presence of hemolysin genes, such as tdh, which codes for the thermostable direct hemolysin, and/or trh, which codes for the TDH-related hemolysin, TRH [12]. Vegetable samples tested positive for virulent V. parahaemolyticus, according to Tunung et al. [13]. Therefore, it is of the utmost significance to gather data on V. parahaemolyticus connected with raw vegetable samples in order to understand the risk of the disease caused by the intake of raw vegetables [13]. Over time V. parahaemolyticus has increasingly been found in foods like seafood, meat and meat products, cereal products, egg products, fruits and vegetables, boxed food, and others [12-16]. Global foodborne illness outbreaks are linked to the intake of raw fruits, vegetables, and juices [13, 14]. Foodborne outbreaks responsible for V. parahaemolyticus have been documented in Asia, Europe, the US, South America, and Africa [12, 16, 17]. There have been a few cases of V. parahaemolyticus in Bangladesh as well [17, 18]. Based on published research in 2020, we know that vegetable samples obtained from several local marketplaces in Dhaka city were the source of V. parahaemolyticus, which was isolated based on selected culture medium and biochemical tests [19]. However, as fresh produce production and consumption have increased, worries have been raised regarding the rising incidence of V. parahaemolyticus in Bangladesh.
Hundreds of boats and launches every day arrive at and depart from Sadarghat situated in the southern part of Dhaka facilitating communication mostly with the southern districts and many of those boats and launches transport fresh produce from village to city. Many businesses emerge based on this busy port such as markets for fresh produce, wholesale shops, street food shops, restaurants, etc. River water has also been used for washing different goods including fresh produce. Given the rise in fresh produce consumption, there is a chance that the infectious agents in the fresh produce sold in Sadarghat's market places might transferred easily to customers, increasing the risk of outbreaks of food-borne illness. Though the microbiological quality of some fresh produce generally consumed raw in some parts of the world has been reported, but so far as we know information is limited on contamination levels, prevalence, molecular identification and multiple antibiotics resistance profiles of V. parahaemolyticus from fresh produce sold at different markets in Sadarghat. Therefore, this study aimed to inquire the presence of V. parahaemolyticus on fresh produce sold in Sadarghat, Dhaka, Bangladesh.
MATERIALS AND METHODS
Sampling
A total of 112 fresh produce of 14 different types were purchased from vendors who brought fresh produce by big boats from different areas of the southern part of Bangladesh in Sadarghat, Dhaka, Bangladesh. Fresh produce samples were collected in sterile plastic zipper bags separately and transported carefully to the laboratory in cooler boxes surrounded by ice packs to keep the temperature between 4 and 10°C. Within two hours of collection, the samples were processed for bacteriological investigation.
Enumeration and phenotypic characterization of V. parahaemolyticus
Each fresh produce sample was chopped by a sterile knife in a sterile tray. Twenty-five gm of each chopped sample was aseptically weighed and placed into an autoclaved homogenizing glass beaker (Pyrex, Germany) containing 100 ml of sterile phosphate buffered solution (PBS; Merck, Darmstadt, Germany) after then agitated on a rotator (Digisystem, New Taipei, Taiwan) for 2 min. The serial dilution plate technique [20] was used for the enumeration of total vibrio count (TVibC). According to this technique, one ml of pre-enrichment sample was transferred to nine ml of sterile normal saline for ten-fold (1:10) dilution and further diluted up to 105 dilutions based on requirement and spread onto thiosulfate citrate bile salt sucrose agar (TCBS; Himedia, Mumbai, India) using a sterile glass spreader. The inoculated media plates were then incubated at 35 ± 2 °C for18-24 h. Following incubation, the overall colony count is calculated, and their concentrations in the original fresh produce sample were estimated in cfu/gm. In order to complete the isolation process, one ml of the homogenate sample was enriched in 9 ml of the alkaline peptone water and incubated at 35 °C for 18 to 24 hours. After incubation, suspected colonies on TCBS agar (Himedia, Mumbai, India) as Vibrio species were subsequently screened and subcultured once again on plates of TCBS agar (Himedia, Mumbai, India) and CHROMagar (CHROMagar, Paris, France). Colonies of green to blue-greenin TCBS agar (Himedia, Mumbai, India) and mauve to pink in CHROMagar (CHROMagar, Paris, France) plates were selected as V. parahaemolyticus. Subsequently, the selected bacterial strains were then inoculated onto Gelatin Agar (GA; Himedia, Mumbai, India) plates to check the activity of the enzyme gelatinase for further confirmation. Tryptic soy agar (TSA; Himedia, Mumbai, India) plate was used to maintain and Luria Bertani (LB) broth (Himedia, Mumbai, India) containing 30% glycerol (Nepa, Tangerang, Indonesia) to preserve the pure culture of bacterial isolates. A number of biochemical assays were performed in accordance with the earlier defined approach to identify isolated bacterial strains [21].
Molecular characterization of V. parahaemolyticus
Sixty colonies from TCBS agar (Himedia, Mumbai, India) and CHROMagar (CHROMagar, Paris, France) plates were selected for further molecular identification. After being inoculated into a 5 ml LB broth (Himedia, Mumbai, India) containing 3% NaCl (Merck, Darmstadt, Germany), isolated colonies of V. parahaemolyticus from GA plates (Himedia, Mumbai, India) were kept for an overnight incubation at 37°C. After incubation, the bacterial culture was centrifuged to harvest the bacterial cells. In accordance with the manufacturer's instructions, total genomic DNA was extracted from centrifuged bacterial cells using the RED Extract-N-Amp Tissue PCR Kit from Sigma, St. Louis, MO, USA. The extracted genomic DNA from bacterial cells was tested to detect the presence of species-specific tlh gene and virulence tdh and trh genes for V. parahaemolyticus by multiplex PCR method using specific primers (tlh-F: aaagcggattatgcagaagcactg and R: gctactttctagcattttctctgc; tdh-F: gtaaaggtctctgacttttggac and R: tggaatagaaccttcatcttcacc; and trh-F: ttggcttcgatattttcagtatct and R: cataacaaacatatgcccatttccg) [17]. V. parahaemolyticus strain (ATCC BAA-238) was used as positive control. All PCR-amplified products were separated by electrophoresis in a 1.5% agarose gel and visualized under UV light with a GelDoc Go imaging system (Bio-Rad, California, United States).
Antimicrobial susceptibility test and multiple antibiotic resistances index
Twelve antibiotic discs (Oxoid, Thermo Scientific, New York, USA) including ampicillin (10 μg), cefotaxime (30 μg), cefuroxime (30 µg), imipenem (10 μg), gentamicin (30 μg), amikacin (30 μg), streptomycin (10 µg), tetracycline (30 μg), ciprofloxacin (5 µg), nalidixic acid (30 μg), trimethoprim-sulfamethoxazole (25 µg), and cholramphenicol (30 µg) were used to conduct an antibiogram test of V. parahaemolyticus isolates by using the Kirby-Bauer disc diffusion technique [22], Ten bacterial isolates were inoculated onto Mueller-Hinton Agar (MHA) (Merck, Darmstadt, Germany) and then left to dry for several minutes. Overnight incubation at 37°C was performed with the antibiotic discs (Oxoid, Thermo Scientific, New York, USA) on the MHA (Merck, Darmstadt, Germany) plates. As per the recommendations from the Clinical and Laboratory Standards Institute [CLSI] [23] zones of inhibition were measured in millimeters and classified as susceptible, moderate, or resistant. Following the steps described by Ayandele et al. [24], the multiple antibiotic resistance index (MARI) of each isolate positive for the tlh gene to various antibiotics was performed. In the MARI computation, the number of antibiotics to which a organism is resistant was divided by the overall number of antibiotics to which the organism was exposed.
RESULTS
Prevalence and phenotypic confirmation of V. parahaemolyticus in fresh produce
In the present study, a total of 112 samples were analyzed for isolation of V. parahaemolyticus from locally available fresh produce. The prevalence (40.2 %.) of vibrios in the total examined samples was 45/112, according to the colonial appearance of yellow, green, and/or blue-green colonies on TCBS agar. The vibrios load of different fresh produce samples ranged from 1.0x101 to 2.0x105 cfu/g with the lowest in capsicum and highest in carrot except no count found in lemon. The prevalence of V. parahaemolyticus showing green and/or blue-green colonies on TCBS agar and mauve to pink in CHROMagar plates (Figure 1) was 40/112 (35.7%).
In the current study, the load of V. parahaemolyticus ranged between 2.0x101 and 6.4x103cfu/g (Table 1). Compared to fresh produce with smooth and rough surfaces, leafy types were more infected with V. parahaemolyticus (Table 1). As mentioned in Table 1, sixty (60) isolates were chosen for further molecular analysis.
Multiple colonies were chosen for some positive samples because of their color ranges, which on TCBS agar ranged from green to blue-green and on CHROMagar from mauve to pink with comparatively large colony sizes. The multiplex PCR assay for tlh, tdh and trh genes detection was performed for molecular confirmation of the presence of V. parahaemolyticus following identification by TCBS agar, CHROMagar culture plates, and biochemical test (data not shown).
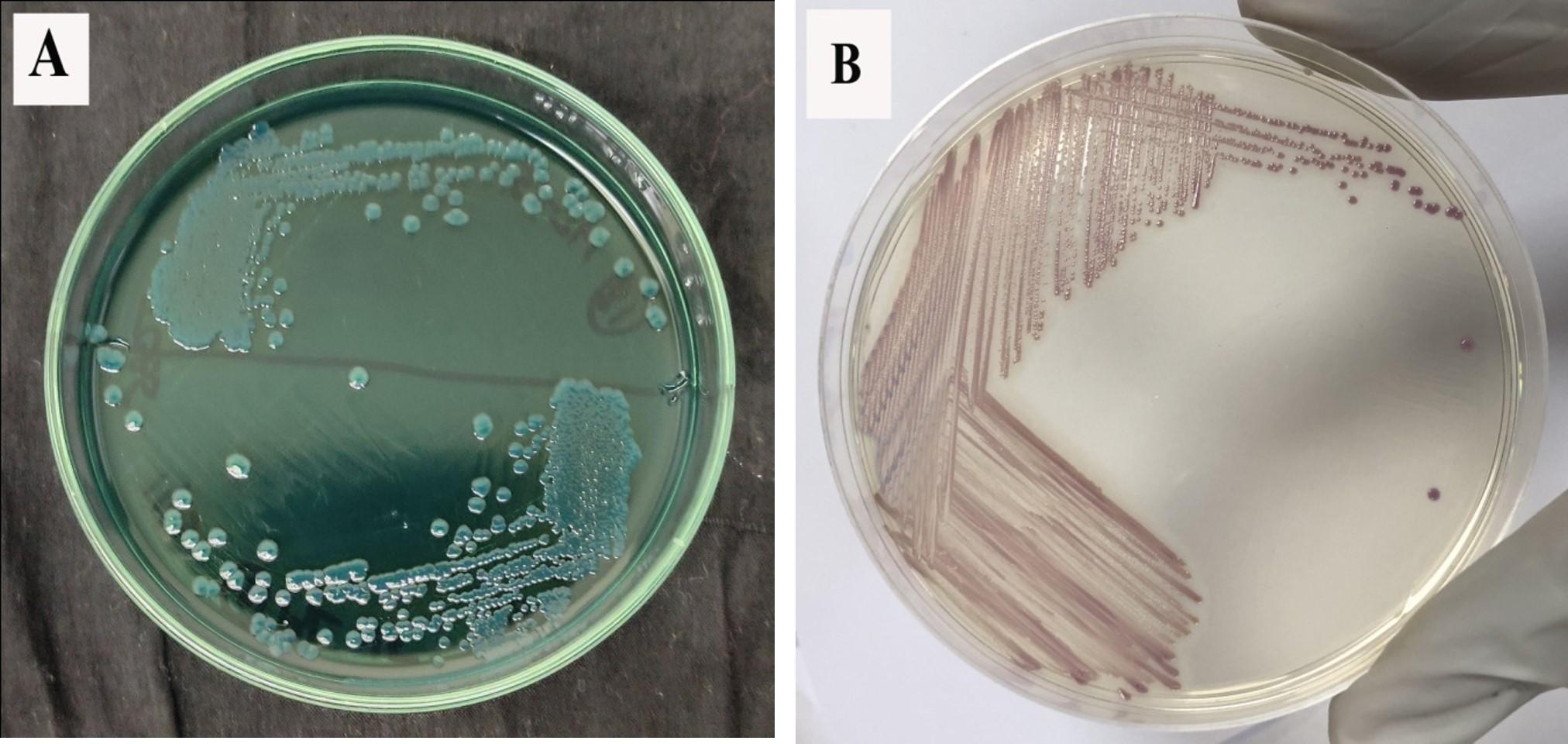
Table 1. Prevalence of vibrios from fresh produce based upon cultural and PCR.
Molecular confirmation of V. parahaemolyticus in fresh produce
The tlh gene fragment (~450 bp) that is specific for V. parahaemolyticus was amplified from all isolates. Detection for trh and tdh gene fragments in isolates revealed negative results for trh gene whereas 6 isolates showed positive results for tdh gene (Figure2).
Among the tdh-positive isolates, 2 (50.0%), 1 (33.4%), 1 (33.4%), 1 (25.0%) and 1 (25.0%) were from lettuce, coriander leaves, carrot, thankunipata and raddish samples, respectively (Table 2). From the samples of tomato, cucumber, green chili, naga chili, spring onion, long coriander leaves, and mint leaves, not a single tdh-positive isolate was discovered.
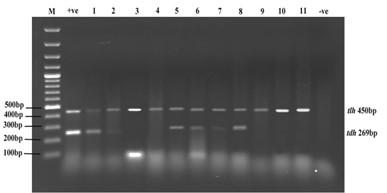
Table 2. Virulence-associated tdh gene frequency in V. parahaemolyticus.
Antibiotic susceptibility profile of V. parahaemolyticus in fresh produce
Antibiotic susceptibility test was performed on six isolates positive for both tlh and tdh genes and four isolates positive for only tlh gene using twelve antibiotics selected from eight different groups. Ampicillin (90%) and tetracycline (80%) were shown to be highly resistant to V. parahaemolyticus isolates. Varying levels of antimicrobial resistance were identified against streptomycin (70%), amikacin (60%), and gentamicin (50%). Marked lower antimicrobial resistance was observed against cefuroxime (10%). However, higher frequencies of antimicrobial sensitivity were determined against chloramphenicol (90%) followed by imipenem (80%), ciprofloxacin (80%), nalidixic acid (80%), cefotaxime (70%), cefuroxime (70%), trimethoprim-sulfamethoxazole (70%), and gentamicin (50%) (Table 3 and 4).
Table 4 shows the MARI results obtained in this study. Only one (10%) out of ten isolates gave MARI of < 0.20 and that was isolated from the mint leaves sample (ML2 0.16). Other nine (90%) isolates gave higher MARI (resistant to 3 or more classes of antibiotics).
Table 3. Interpretation of antimicrobial resistance profile of V. parahaemolyticus isolates.
Table 4. Multiple antibiotic resistance indexof V. parahaemolyticus isolates.
DISCUSSION
Fresh produce, whether eaten raw or partially cooked, is important for human nutrition due to its high nutritional value and health benefits. Since fresh produce is typically exposed to microbial contamination throughout the entire supply chain, from the farm's production stage to the market and even to the consumer, it can also be a major source of food-borne infections [3]. Poor handling practices and an unhygienic surrounding environment might lead to indirect contamination. Contamination may also happen after harvesting due to contaminated wash water and cross-contamination from a foodhandler with an infection [25]. When fresh fruits and vegetables are washed, rinsed, and sprinkled with contaminated water, they might become infected as well [26]. A study conducted by Nipa et al. [27] also reported fresh produce were highly contaminated and the contamination rate was 100% from Bangladesh. Other studies by Mathur et al. [28] and Mritunjay & Kumar [25] reported that bacterial contamination rate of fresh produce was 85.4‒100% from India. The results from all these studies verified that although containing high nutritional compounds and potential health benefits for human, they are also a good source for growth of microorganisms. Since V. parahaemolyticus in humans is frequently the causative microorganism of food-borne gastroenteritis, the occurrence of disease-causing strains of this bacterium in fresh produce is quite concerning for public health. Previous studies in Bangladesh have exhibited that fresh fruits and vegetables contains Vibrio spp. [19] but our present study revealed firstly the existence and identification of V. parahaemolyticus in fresh produce employing culture-based approach and molecular PCR methods. The level of vibrio prevalence in our studied fresh produce samples (40.2%) seems to be higher than that presented by Kabir et al. [29], Biswas et al. [30], Aurin et al. [19], and Igbinosa et al. [12] in which 14.3%, 20%, 23% and 24.6% respectively of the observed samples were found to be positive to vibrios but close to findings of Okafo et al. (33.3%) [31]. The count vibrio was higher in carrot than tomato and capsicum. Because the surface of carrot was wrinkled, tomato, and capsicum were smooth surfaces. A study conducted by Alemu et al. [6] also observed that smooth surface fresh produce holds less contaminant bacteria. In samples of fresh produce, the detection of V. parahaemolyticus reported by Li et al. [32], Tunung et al. [14], Xie et al. [15], Aurin et al. [19], Igbinosa et al. [12], Tunung et al. [33], and Beshiru et al. [34] was 6.12%, 12%, 7.2%, 6%, 11%, 13.7% and 11.1%, respectively and those results were lower than the current study result (35.7%). While no V. parahaemolyticus was found to be positive in fresh produce samples in Korea [35], the frequency of occurrence in vegetables obtained from a wet market in Selangor, Malaysia was 32.05% [13], which was similar with the current investigation. In this investigation, the leafy fresh produce samples were the most often contaminated. Compared to vegetables with smooth surfaces and narrow exposed outer surfaces, leafy fresh produce is justified in having a larger surface area and a courser surface that makes it easier for contaminants to adhere [6, 33]. Eating raw or slightly undercooked vegetables for preserving flavor and natural taste can, unfortunately, make pathogen attachment and survival easier because of their complex surface and porosity, which leads to food-borne infection and disease outbreaks [36]. Fresh produce samples containing V. parahaemolyticus pose concerns for the public's health for people who eat raw or undercooked fresh produce. There is still ambiguity about the entirety of the pathogenicity of V. parahaemolyticus [17]. Since certain illnesses are caused by isolates missing tdh, trh, or T3SS2, the risk is significant regardless of whether pathogenic islands are present in genomes of circulating V. parahaemolyticus [9]. Out of 60 V. parahaemolyticus isolates studied, only 6 percent were tdh positive, whereas none were trh positive. These findings are not unexpected because the majority of clinical isolates of V. parahaemolyticus carrying tdh and/or trh genes, while strains containing tdh+ or trh+ gene found in seafood and environment their prevalence is estimated to be lower, making up 1–10% of isolates [9, 37]. The findings of our investigation correlate with other research that documented food samples contaminated by V. parahaemolyticus carrying the tdh gene but not the trh gene [33, 37]. V. parahaemolyticus isolates harboring tdh virulent gene also detected from vegetable samples [12, 14, 34]. V. parahaemolyticus isolated from fresh produce available in Dhaka of Bangladesh is positive for tdh gene being the first published report.
Since penicillin's discovery, the use of antibiotics has greatly improved both human and animal health. Expanding studies have exhibited that the rise in V. parahaemolyticus cases, which may be resistant to antibiotics, poses a serious risk to global human growth in the fields of public health and economics [8, 34]. Antibiotic resistance in vibrios is probably mostly caused by the overuse and inappropriate use of antibiotics for bacterial infections prophylactics and the spread of disease-causing microorganisms. Antimicrobial resistance in Vibrio strains is acquired by horizontal gene transfer and mobile genetic elements as a result of prolonged exposure to these antibiotics through the environment [38]. Tested antibiotics of the present study are suggested by CDC as treatments for illnesses linked to Vibrio species [39]. The majority of the tested isolates in current study showed resistance to ampicillin, tetracycline, streptomycin, amikacin, and gentamicin. These findings are consistent with other studies published in various kinds of sample sources [12, 17, 34, 38] and since these antibiotics are found to be utilized for treating infections in humans, this finding raises concerns for public health. The intrinsic resistance to ampicillin exhibited by Vibrio spp. elsewhere is equivalent to the resistance to ampicillin seen in 90% of tested isolates of V. parahaemolyticus in this investigation [34, 40, 41]. The rise of resistant bacteria in recent years has decreased the effectiveness of penicillin group, despite being one of the most beneficial antibiotic classes for primary care [38]. In V. parahaemolyticus, resistance to ampicillin and streptomycin is quite prevalent [15, 17, 42]. A significant portion of the studied isolates that were recovered from fresh produce showed antibiotic resistance, which is consistent with this trend. Furthermore, in accordance with other studies, a subset of tested isolates from our investigation showed lower resistance to cefuroxime (10%), a third-generation cephalosporin [17]. Similar to this, V. parahaemolyticus isolated from the United States was found to exhibit lower cefotaxime resistance by Shaw et al. [39]. Cefotaxime resistance was shown to be higher in Letchumanan et al. [9] and Igbinosa et al. [12] than in the present study. Cefotaxime resistance was shown to be higher in Letchumanan et al. [9] and Igbinosa et al. [12] than in the present study. High susceptibilities to antibiotics such as chloramphenicol (90%), imipenem (80%), ciprofloxacin (80%), nalidixic acid (80%), cefotaxime (70%), cefuroxime (70%), trimethoprim-sulfamethoxazole (70%), and gentamicin (50%) were discovered among the examined isolates of the present investigation. These findings are in agreement with earlier studies [17, 37, 40, 42, 43] that found most V. parahaemolyticus to be susceptible to chloramphenicol and ciprofloxacin. Physicians may prescribe these drugs to treat illnesses linked to V. parahaemolyticus as they remain effective against these bacteria [17, 40]. Similar to the study findings of Ahmed et al. [38], every isolate of V. parahaemolyticus that was investigated in this investigation showed multidrug resistance (MDR). Additionally, the MDR ratio is closely similar to earlier reports [17, 18, 34].
Multiple antimicrobial resistance to at least 2 antibiotics was shown by all the tested isolates, with a MARI range of 0.16 to 0.33 and this MARI value which is lower and contrasts the findings from previous studies [34] and is similar to earlier reports [17, 18, 37]. In our investigation, 90% of the isolates had a MARI of more than 2, which is higher than in previous investigations [12, 34, 37]. Different sample sources, geographic distribution, and test methodologies could all be contributing factors to the variation in the MARI [12, 13, 34]. The MARI score of 0.2 or higher, which 90% of the isolates in this investigation displayed, is noteworthy and indicates that samples originated from a high-risk contamination source where plenty of antibiotics were utilized [44]. Due to a widespread utilization of antibiotics in modern medicine, aquaculture, agriculture, dairy production, and poultry farming, antimicrobial residues are becoming more and more prevalent in the environment [17]. Fresh produce's ability to harbor Antibiotic resistance (AR) V. parahaemolyticus can pose a serious threat to public health in managing and controlling the illness. Antibiotic application, prophylaxis, and treatment regimens are all directly impacted by MDR in V. parahaemolyticus [34]. Nevertheless, keeping an eye out it's important to monitor antimicrobial resistance for identifying the efficacy of new generations of antibiotics as well as ensuring food safety [38, 41].
CONCLUSION
This is the first thorough investigation that details the prevalence, pathogenicity, and antimicrobial activity of V. parahaemolyticus isolating from fresh produce in Dhaka, Bangladesh. According to the current study's findings, fresh produce contaminated by pathogenic strains of V. parahaemolyticus might put consumers in danger, particularly if they eat raw or lightly cooked vegetables. This investigation exhibited the potential level of V. parahaemolyticus contamination in fresh produce, considering the handling practice usually carried out by workers, vendors, and environment in which they display and transport the fresh produce and the possibility of food-related outbreaks of disease; proper and vigorous washing of fresh produce with safe running water before consuming required to reduce the number of microorganisms. Both, food handlers and consumers must adopt good hygiene practices to reduce the risks of foodborne illness through fresh produce as vehicles. The hygienic regulations and recommendations should be properly followed to by those handling various sorts of food. The local vendors are a significant part of the urban food supply chain with the minimal risk of food-borne disease, following control measures should be undertaken to minimize the microbial density of fresh produce. Additionally, the government must step in to protect consumers and ensure that the criterion of safety for these items can be maintained considering the current local circumstances.
Consequently, the results of this study might be employed to guide other investigations evaluating the microbiological quality of fresh produce both sold and grown locally, helping to develop risk management regulations for Dhaka city as well as the whole country of Bangladesh.
ACKNOWLEDGEMENT
To the members of the research team who helped this study be completed successfully, their knowledge, devotion, and dedication were crucial in achieving our study goals.
AUTHOR CONTRIBUTIONS
SA and ZF designed outlines and drafted the manuscript. FT and MAS collected study samples and SA, FT, MNH, MTA and MAS operated the experiments and analyzed the results data. All authors of present study reviewed and approved the final article.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Berger CN, Sodha SV, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environmental microbiology. 2010;12(9):2385-2397.
- [2]Charlton K, Kowal P, et al. Fruit and vegetable intake and body mass index in a large sample of middle-aged Australian men and women. Nutrients. 2014;6(6):2305-2319.
- [3]Balali GI, Yar DD, et al. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today’s world. International journal of microbiology. 2020.
- [4]FAO W. Country nutrition paper Bangladesh. In Joint FAO/WHO International Conference on Nutrition. 2014;21:47.
- [5]Ailes EC, Leon JS, et al. Microbial concentrations on fresh produce are affected by postharvest processing, importation, and season. Journal of Food Protection. 2008;71(12):2389-2397.
- [6]Alemu G, Mama M, Siraj M. Bacterial contamination of vegetables sold in Arba Minch town, Southern Ethiopia. BMC research notes. 2018;11:1-5.
- [7]Crim SM, Iwamoto M, et al. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US sites, 2006–2013. Morbidity and Mortality Weekly Report. 2014;63(15):328.
- [8]Lopatek M, Wieczorek K, Osek J. Antimicrobial resistance, virulence factors, and genetic profiles of Vibrio parahaemolyticus from seafood. Applied and environmental microbiology. 2018;84(16):e00537-18.
- [9]Letchumanan V, Chan KG, Lee LH. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Frontiers in microbiology. 2014;5:705.
- [10]Lee JK, Jung DW, et al. Occurrence of Vibrio parahaemolyticus in oysters from Korean retail outlets. Food Control. 2008;19(10):990-994.
- [11]Nelapati S, Nelapati K, Chinnam BK. Vibrio parahaemolyticus-An emerging foodborne pathogen-A Review. Vet World. 2012;5(1):48-62.
- [12]Igbinosa EO, Beshiru A, et al. Prevalence and characterization of food-borne Vibrio parahaemolyticus from African salad in southern Nigeria. Frontiers in Microbiology. 2021;12:632266.
- [13]Tunung R, Margaret SP, et al. Prevalence and quantification of Vibrio parahaemolyticus in raw salad vegetables at retail level. J. Microbiol. Biotechnol. 2010;20(2):391-396.
- [14]Tunung R, Ghazali FM, et al. Rapid detection and enumeration of pathogenic Vibrio parahaemolyticus in raw vegetables from retail outlets. International Food Research Journal. 2011;18(1).
- [15]Xie T, Xu X, et al. Prevalence, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus from ready-to-eat foods in China. Frontiers in microbiology. 2016;7:549.
- [16]Chen L, Wang J, et al. Epidemiological characteristics of Vibrio parahaemolyticus outbreaks, Zhejiang, China, 2010–2022. Front. Microbiol. 2023;14:1171350.
- [17]Ali S, Hossain M, et al. Diversity of Vibrio parahaemolyticus in marine fishes of Bangladesh. Journal of applied microbiology. 2021;131(5):2539–2551.
- [18]Siddique AB, Moniruzzaman M, et al. Characterization of Pathogenic Vibrio parahaemolyticus Isolated from Fish Aquaculture of the Southwest Coastal Area of Bangladesh. Frontiers in microbiology. 2021; 12:635539.
- [19]Aurin SA, Chowdhury SP, et al. Characterization of multi-drug resistant Gram-negative bacteria present in fresh leafy & salad vegetables in Dhaka, Bangladesh. European Journal of Engineering and Technology Research. 2020;5(11):1322-1327.
- [20]Greenberg AE, Connors JJ, et al. Standard methods for examination of water and wastewater (20th ed.). APHA, Washington DC. 1998.
- [21]Cheesbrough M. Medical laboratory manual for tropical countries in microbiology.1st ed. English Language Book Society, London. 1985;40-57.
- [22]Bauer AW, Kirby WMM, et al. Antibiotic susceptibility testing by a standardized single disk method. Amer J Clin Pathol. 1966;45:493-496.
- [23]Clinical and Laboratory Standards Institute [CLSI]. Performance Standards for Antimicrobial Susceptibility Testing. in CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. 2017
- [24]Ayandele AA, Oladipo EK, et al. Prevalence of Multi-Antibiotic Resistant Escherichia coli and Klebsiella species obtained from a Tertiary Medical Institution in Oyo State, Nigeria. Qatar medical journal. 2020;2020(1):9.
- [25]Mritunjay SK, Kumar V. A study on prevalence of microbial contamination on the surface of raw salad vegetables. 3 Biotech. 2017;7:1-9.
- [26]Olyaei A, Hajivandi L. Parasitological contamination of markets and farms in vegetables consumed in southern Iran. Global Veterinaria. 2013;10(3):327-331.
- [27]Nipa MN, Mazumdar RM, et al. Prevalence of multi drug resistant bacteria on raw salad vegetables sold in major markets of Chittagong city, Bangladesh. Middle-East Journal of Scientific Research. 2011;10(1):70-77.
- [28]Mathur A, Joshi A, Harwani D. Microbial contamination of raw fruits and vegetables. Internet Journal of Food Safety. 2014;16:26-28.
- [29]Kabir A, Das AK, Kabir MS. Incidence of antibiotic resistant pathogenic bacteria in vegetable items sold by local and super shops in Dhaka city. Stamford Journal of Microbiology. 2014;4(1):13-18.
- [30]Biswas B, Azad MAK, et al. Isolation and Identification of Pathogenic Bacteria from Fresh Fruits and Vegetables in Chittagong, Bangladesh. Journal of Microbiology Research.2020;10(2):55-58.
- [31]Okafo CN, Umoh VJ, Galadima M. Occurrence of pathogens on vegetables harvested from soils irrigated with contaminated streams. Science of the total environment. 2003;311(1-3):49-56.
- [32]Li XC. Active surveillance of foodborne pathogens in foods in Wenzhou in the past 5 years. Chinese Journal of Health Inspection. 2007;17(10):1843-1845
- [33]Tunung R, Jeyaletchumi P, et al. Detection and quantification of Vibrio parahaemolyticus in vegetables and environmental samples at farm level. Food Research. 2022;6(5):310-318.
- [34]Beshiru A, Igbinosa EO. Surveillance of Vibrio parahaemolyticus pathogens recovered from ready-to-eat foods. Scientific Reports. 2023;13(1):4186.
- [35]Chung MS, Kim CM, HA SD. Detection and enumeration of microorganisms in ready to eat foods, ready to cook foods and fresh cut produce in Korea. Journal of Food Safety. 2010;30(2):480-489.
- [36]Mir SA, Shah MA, et al. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control. 2018;85:235-244.
- [37]Narayanan SV, Joseph TC, et al. Prevalence, virulence characterization, AMR pattern and genetic relatedness of Vibrio parahaemolyticus isolates from retail seafood of Kerala, India. Frontiers in Microbiology. 2020;11:592.
- [38]Ahmed HA, El Bayomi RM, et al. Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and V. cholerae isolated from crustaceans and humans. International journal of food microbiology. 2018;274:31-37.
- [39]Shaw KS, Rosenberg Goldstein RE, et al. Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland Coastal Bays. PloS one. 2014;9(2):e89616.
- [40]Sudha S, Mridula C, et al. Prevalence and antibiotic resistance of pathogenic Vibrios in shellfishes from Cochin market. 2014.
- [41]Yu Q, Niu M, et al. Prevalence and ant imicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shellfish in Shanghai. Food control. 2016;60:263-268.
- [42]Xie T, Wu Q, et al. Comparison of Vibrio parahaemolyticus isolates from aquatic products and clinical by antibiotic susceptibility, virulence, and molecular characterisation. Food Control. 2017;71:315-321.
- [43]Huang A, Wang Y, et al. Antibiotic Resistance and Epidemiology of Vibrio parahaemolyticus from Clinical Samples in Nantong, China, 2018-2021. Infection and drug resistance. 2023;16:7413-7425.
- [44]Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to Identify high-risk sources of fecal contamination of foods. Applied and environmental microbiology. 1983;46(1):165-170.