Hypoglycemic effects of propolis extract on polycystic ovarian syndrome in Wistar rats
Abstract
Polycystic ovarian syndrome (PCOS) is an endocrinopathy that causes various metabolic syndromes, including insulin resistance in 20-40% of patients. The treatment of the condition is often carried out using metformin, but it has been reported to have different gastrointestinal side effects. Consequently, several studies have recommended the use of propolis as an alternative, which has proven effective in improving insulin resistance in diabetic rats and causes fewer side effects. Thus, the current study aimed to compare the effects of propolis and metformin in treating insulin resistance based on homeostasis model assessment of insulin resistance (HOMA-IR) value. This study used 30 Wistar rats in randomized control trial design. The animals were divided into 5 groups: control, PCOS (PCO), PCOS with metformin (PCO+M), PCOS with propolis extract (PCO+P), and PCOS with metformin and propolis extract (PCO+MP). Subsequently, blood sampling, ovarian tissue sampling, and vaginal smearing were performed after 28 days of treatment. PCO+P and PCO+M groups experienced a significant decrease in testosterone concentration and preantral follicle count compared to PCOS group, but the anovulation cycle was similar. Fasting blood glucose concentration in PCO+MP group showed a significant decrease compared to others. However, fasting insulin concentration and HOMA-IR value in treatment groups did not show any reduction compared to PCOS group. Based on the results, propolis extract and metformin could not improve insulin resistance based on HOMA-IR value. However, propolis extract had better hypoglycaemic effects compared to metformin. In conclusion, the combination of both treatments had the better potential to lower fasting glucose concentration.
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is a prevalent endocrine abnormality affecting 8-13% of women of reproductive age. In addition, the abnormality is typically defined and diagnosed using the Rotterdam Criteria Consensus (2003). The diagnosis of PCOS requires the presence of 2 out of 3 essential features, including persistent oligo-ovulation or anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries. Several studies have shown that it often leads to metabolic syndromes in 20-40% of patients, such as glucose intolerance, insulin resistance, type 2 diabetes mellitus, and cardiovascular disease in the long term [1,2]. Recent reports also provided information on its potential pathogenesis, such as PCOS correlation with dysbiosis of the gut microbiota, obesity, and the high concentration of autoantibodies associated with chronic low-grade inflammation. A previous report revealed that chronic low-grade inflammation could cause insulin resistance, a condition characterized by the inadequate effects of insulin on fat, muscle, and liver, leading to hyperinsulinemia and increased blood glucose concentration [3,4]. Hyperinsulinemia, in turn, leads to abnormal ovaries function and increased androgen production, which inhibit the ovulation cycle and antral follicle growth, thereby causing abnormal morphology of the ovaries [2,4].
To overcome these challenges, several studies have proposed the use of metformin in PCOS treatment to improve insulin sensitivity. The medicine has proven to be effective in reducing hyperinsulinemia, basal and stimulated luteinizing hormone (LH) levels, and free testosterone concentration in overweight women with PCOS. In addition, approximately 40% of anovulatory women have been shown to ovulate and become pregnant due to weight loss caused by its use [5]. Metformin can also affect total antioxidant capacity (TAC) and total oxidant status (TOS), thereby exerting a scavenging ability that inhibits low-grade inflammation and insulin resistance [6]. Various studies have reported its combination with several other medicines or herbal treatments to obtain better therapeutic results, but it had gastrointestinal side effects, such as nausea, vomiting, stomachache, and diarrhea, depending on the dosage. The unpleasant side effects typically cause reluctance among patients toward long-term usage [7].
In line with these findings, there is a pressing need to develop new treatment options against insulin resistance, with high effectiveness and compliance among patients. A potential candidate is propolis, which is widely used as a supplement for many health problems in Indonesia. This material has been reported to possess several bioactivities, including antioxidant, anti-inflammatory, antibacterial, antifungal, and antiviral effects. The antioxidant and anti-inflammatory components of propolis are beneficial to PCOS patients, who typically have low-grade inflammation that can cause insulin resistance. Cai et al. reported that ethanol extract of propolis supplementation significantly reduced fasting insulin level and homeostasis model assessment of insulin resistance (HOMA-IR) value in high-fat diet-induced insulin resistance in rats [8]. The extract also reduced fasting blood glucose concentration and increased insulin sensitivity index in diabetic-induced rats [9]. Despite the availability of literature on propolis effects on diabetic animal models, there are limited reports regarding the impact of propolis on insulin resistance in PCOS. Previous reports showed the presence of large similarities between rodents and humans at the metabolic level, making rodents suitable models for investigation. The animals also have similar food intake patterns, which is often challenging for humans [10]. Therefore, this study aims to compare the effects of propolis and metformin on fasting blood glucose (FBG) concentration, fasting insulin (FINS) concentration, and HOMA-IR value in PCOS.
MATERIALS AND METHODS
Animal study
A total of 30 healthy female Wistar rats aged 8 weeks with a mean weight of 100 g were obtained from Airlangga University Animal Laboratory, Indonesia. Rats were housed under regulated lighting (12-hour light/dark cycle), and adaptation was carried out for one week. In addition, standard meals were given ad libitum with a composition of 10.3% fat, 65.5% carbohydrate, and 24.2% protein. The animals were fed twice a day in the morning and evening, with the provision of drinking water (aquades) ad libitum and housing based on treatment group. All the experiments were conducted in line with the ethics and guidelines of animal care. The study procedures were approved by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Diponegoro, Indonesia (Number: 138/EC/H/FK-UNDIP/XII/2021).
This was a laboratory experimental study with a post-test-only control group design. Rats were randomly divided into 5 groups, including control, PCOS (PCO), PCOS with metformin supplementation (PCO+M), PCOS with propolis extract supplementation (PCO+P), and PCOS with metformin and propolis extract supplementation (PCO+MP). PCOS was induced by a daily testosterone propionate (TP) intramuscular injection with a dosage of 10 mg/kg body weight (mg/kg BW) for 28 days, starting at estrous phase [11]. Propolis extract dosage in PCO+P group was 240 mg/kg BW [9], metformin dosage in PCO+M was 90 mg/kg BW (dose modified from Wessels et al. [12]), and PCO+MP group was administered propolis extract (120 mg/kg BW) and metformin (45 mg/kg BW). The propolis extract supplementation used was HDI Bee Propolis (CC Pollen Co., Phoenix, AZ, USA), which contained 3.85 g of flavonoids in every 100 g of bee propolis and 2.93 g of phenol in 50 g of bee propolis (spectrophotometry UV-vis). Metformin HCl 500 mg, produced by Hexpharm Jaya, Jakarta, Indonesia, was used in this study. Propolis extract and metformin supplementation were given orally starting on day 29 for 4 weeks.
Collection and analysis of samples
Blood sampling for serum total testosterone (TT) levels, fasting blood glucose, and fasting insulin was carried out on day 57 after the rats fasted for 12 hours under 60 mg/kg BW ketamine-xylazine anesthesia. Serum concentration of total testosterone was measured using Rat Testosterone Competitive ELISA kit (BT Lab, Zhejiang, China). In addition, HOMA-IR value was calculated by multiplying fasting glucose concentration (mg/dl) with fasting insulin concentration (mIU/L) and dividing by 405. FBG levels from blood vessels were examined using glucometers, while FINS levels were assessed with Rat Insulin ELISA kit (BT Lab, Zhejiang, China). ELISA was a labeled immunoassay that was considered the gold standard of immunoassays and sensitive to detect and quantify substances, including antibodies, antigens, proteins, glycoproteins, and hormones.
Rats were sacrificed by injecting 100 mg/kg BW ketamine-xylazine intramuscularly, and an incision on the abdomen was made to obtain ovaries tissues. The samples obtained were then fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, mounted on a glass side, and stained with hematoxylin and eosin (HE). Subsequently, analysis was carried out with a Nikon E100 light microscope (Nikon Instruments Inc., New York, USA) with Optilab Advance Plus 12 MP digital camera, followed by image editing with Image Raster software. The samples were examined to identify follicles by the shape and counted the number with 100-400 times magnification. Ovarium morphological changes were defined by preantral follicles count. The estrous cycle in rats averaged 4-5 days and had four stages, namely proestrus, estrus, metestrus, and diestrus. Vaginal smears were obtained on days 1 and 57 by inserting and flushing 0.2 ml saline into the vaginal orifice at approximately 5-10 mm depth. The drop of the samples was then placed on a slide, air dried, stained by Giemsa stain, and evaluated with a Nikon E100 light microscope (Nikon Instruments Inc., New York, USA) with 100 times magnification. The estrous cycle was determined by examining the relative neutrophils, nucleated epithelial cells, and relative cell density.
Statistical analysis
Statistical analysis was conducted using One-Way ANOVA test, followed by a post hoc test to determine the significance of the differences between each treatment. Kruskal-Wallis test and Mann-Whitney U test were used for data, that were not distributed normally. A significant difference was determined by a p-value of less than 0.05, and SPSS Version 26 was used to analyze the data obtained.
RESULTS
PCOS clinical manifestations in experimental animals
PCOS was defined by Rotterdam criteria, which required 2 out of 3 manifestations, including anovulation, hyperandrogenism, and polycystic ovary morphology. Serum TT concentrations in the study groups were significantly different, as shown in Table 1. The control group had significantly lower TT concentrations compared to PCO group (p=0.037) indicating that PCO model had hyperandrogenism. The estrous cycle obtained by vaginal smear on days 1 and 57 showed that in PCO group, all Wistar rats had an anovulation cycle (ANOV). Meanwhile, in control group, 2 rats had ANOV, indicating the absence of a significant difference in estrous cycle between both groups. Preantral follicles (PA) count was used to determine ovarian morphological abnormalities and the results showed the presence of a significant difference. Control group had a significantly lower PA count compared to PCO group (p=0.009), revealing that PCO group had polycystic ovaries. PCO group had 2 of the 3 features required to diagnose PCOS, including an increase in TT concentrations and PA count compared to control group.
PCO+P and PCO+M groups significantly decreased TT concentrations compared to PCO group. PCO+P group had a lower TT concentration compared to PCO+M group, but there was no significant difference. In addition, PCO+P group had the lowest TT concentration compared to other samples. The results showed that PCO+P and PCO+M groups had a lower PA count compared to PCO group, as shown in Figure 1. These results revealed that propolis and metformin monotherapy had the same effects on PA count in PCOS-induced rats, but the combination did not successfully reduce PA count. PCO+MP group had the lowest number of rats with ANOV but the anovulation status between the samples was not significantly different and found to be similar.
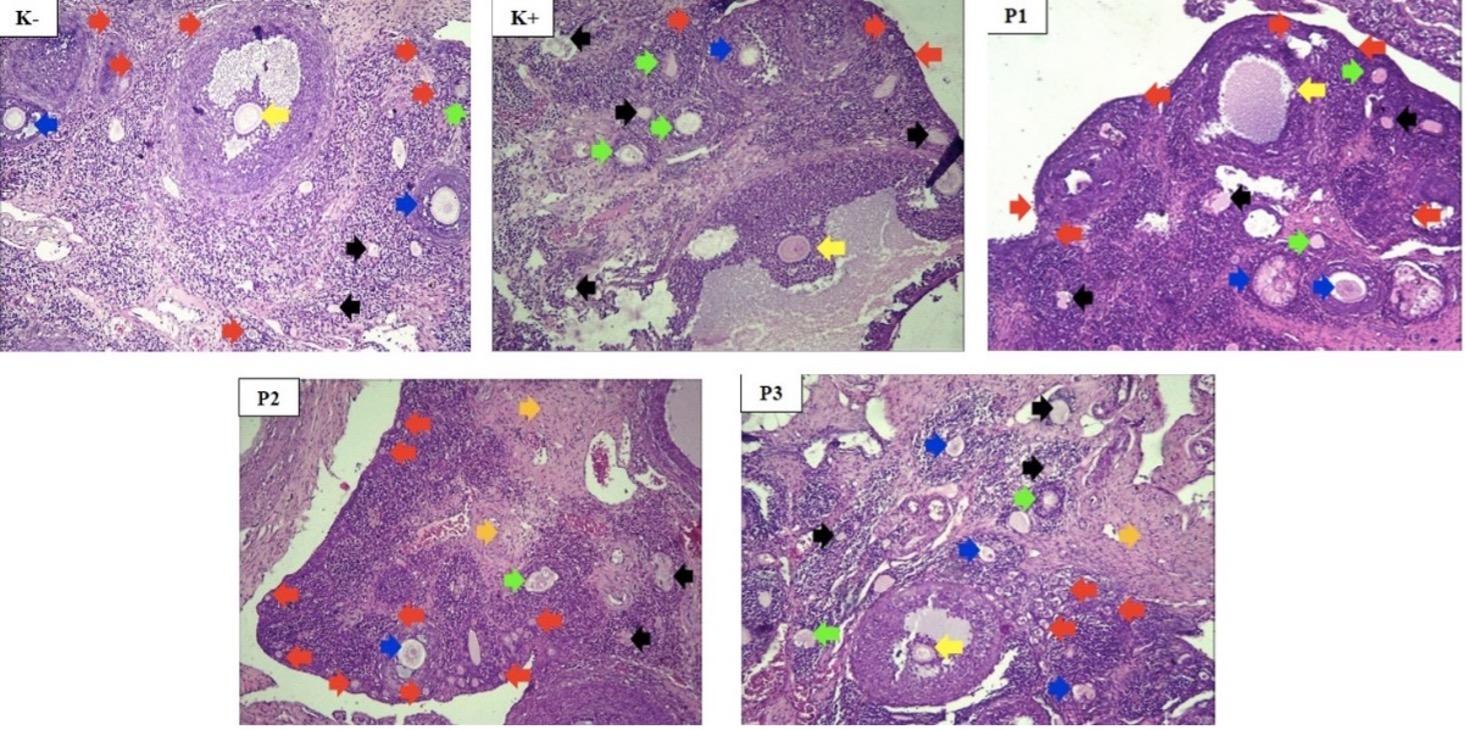
Table 1. PCOS clinical manifestations in study groups.
Effects of Propolis extracts on insulin resistance in PCOS rats
FBG in PCO+MP group was the lowest among treatment groups and was lower compared to the healthy control group, as shown in Table 2 and Figure 2. In addition, there was a significant difference in FBG concentration between the study groups. PCO+P group had lower FBG concentration compared to PCO+M group, suggesting that propolis extract had better hypoglycemic effects than metformin. The combination of propolis extract and metformin reduced FBG concentration compared to monotherapy.
FINS levels in control group were lower than PCO group, and PCO+P had the lowest fasting insulin concentration but was not statistically significant. Propolis extract alone had better potency for improving FINS concentration compared to other treatments. In addition, HOMA-IR value in control group was the lowest and was significantly different among all groups (p=0.002). PCO+P and PCO+MP groups had similar HOMA-IR value, which was lower than PCO+M group, but higher than PCO group.
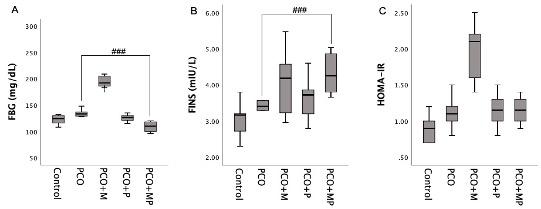
Table 2. Insulin resistance of study groups.
DISCUSSION
Propolis had been considered as a promising alternative treatment for metformin due to its beneficial effect in improving insulin resistance in diabetes-induced animals. In addition, it had the ability to repair glucose metabolism in diabetes-induced rats. Xue et al. reported that diabetes-induced rats treated with propolis 240 mg/kg BW had a hypoglycemic effect with improved insulin sensitivity [9]. These results were consistent with Rivera-Yanex et al., where diabetes-induced rats were given propolis 300 mg/kg BW for 15 days and experienced reduced fasting glucose levels from day 9 until the end of the study [13]. Propolis treatment and the combination with metformin in this study led to lower FBG concentration compared to PCOS group. This suggested that it had hypoglycemic effects. The active components of propolis, such as kaempferide, had the potential to prevent hyperglycemia by prompting glucose transporters-4 (GLUT-4) translocation in skeletal muscle [14]. Other flavonoid components, such as naringin, quercetin, or naringenin could increase hepatic glucokinase enzyme concentration and activity, leading to decreased hepatic glucose-6-phosphatase enzyme concentration [15]. Pahlavani et al. proposed 4 mechanisms through which propolis reduced glucose levels, namely by increasing glycolysis and glucose usage in liver cells. The second mechanism was associated with reducing the carbohydrate intake in gastrointestinal tracts and gut cells, while the third mechanism comprised activating insulin-sensitive GLUT-4 and glucose resorption by peripheral cells, including skeletal cells. The inhibition of glucose release from liver cells to blood circulation was the last mechanism [16].
Propolis had the ability to prevent the production of reactive oxygen species and inflammation apart from decreasing the incidence of hyperglycemia [17]. This condition was often associated with insulin resistance and dysfunction of islet β-cells. In addition, propolis played in enhancing insulin secretion from β -cells and modulating insulin receptor signals, which led to insulin sensitivity improvement. Insulin resistance or impaired sensitivity was marked by increasing fasting insulin level as a feedback mechanism [18,19]. Inconsistent results about propolis effects on insulin concentration were found in various studies. Beelosesky et al. revealed that testosterone-induced rats had normal or decreasing FBG levels and increasing FINS levels [11]. Li et al reported that oral administration of Chinese propolis for 10 weeks decreased FINS concentration in diabetes-induced rats [20]. Meanwhile, Xue et al. reported a different result, where treatment reduced FBG concentration in diabetes-induced rats but had no effect on insulin concentration [9].
Propolis extract had a hypoglycemic effect that played a role in repairing insulin resistance. Guan et al. reported that diabetes-induced rats that were given 600 mg/kg BW propolis extract experienced improved insulin resistance and sensitivity [21]. Zakerkish et al. had a similar result that it reduced HOMA-IR value in diabetes patients [22]. Propolis extract in this study had not yet succeeded in lowering HOMA-IR value. This result was similar to Samadi et al., where the extract in diabetes patients could decrease fasting glucose, HbA1c, triglyceride, and LDL cholesterol levels but no improvement in HDL levels and insulin resistance index [23].
Metformin had been used in PCOS treatment to improve insulin sensitivity and this medicine was included in international guidelines for insulin resistance in PCOS patients. The primary effect was a significant decrease in gluconeogenesis, thereby reducing glucose production by the liver. Metformin also increased the sensitivity of target tissues to insulin and reduced hyperinsulinemia in overweight women with polycystic ovaries [24]. In addition, it was recommended by current guidelines as treatment for overweight/obese women with PCOS to improve insulin resistance because insulin effect on metabolic improvement was most evident in patients with BMI of ≥ 25 kg/m2 [25]. Metformin in this study had no effect on insulin resistance because insulin usually functioned effectively in overweight or obese patients but there was no difference in rats body weight for all groups. Another possible reason was the role of metformin as an insulin sensitizer, which had a dose-dependent effect. There was an assumption that a higher dose of metformin than the dosage used in this study could show a different result. Li M et al. reported that metformin with a dosage of 500 mg/kg BW administered intragastrical for 12 weeks to diabetes-induced rats reduced fasting blood glucose concentration, thereby improving insulin resistance [26]. These results were in line with Za’abi et al., where fasting blood glucose concentration reduced after metformin treatment with 300 mg/kg BW given orally for 5 weeks in diabetes-induced rats [27]. The studies revealed that the different doses, administration routes, and duration could affect metformin action as an insulin sensitizer.
Propolis extract reduced serum total testosterone concentration but the combination with metformin did not have similar effects. Abbasi et al. reported that propolis extract treatment in PCOS patients for 3 months with a 100 mg/day dose could reduce TT concentration [28]. In addition, it reduced serum total testosterone concentration by reducing fasting glucose and insulin levels, which contributed to decreasing the condition of hyperinsulinemia in rats with PCOS. This was crucial as it affected the production of SHBG levels, which ultimately reduced serum total testosterone concentration. Metformin could also decrease TT concentration by inhibiting SHBG production in the liver. The results showed that metformin and propolis separately reduced TT concentration [29]. The number of PA counts decreased after propolis extract treatment and metformin caused a higher decrease but was not significantly different. Similar results were obtained by Sapmaz et al., where propolis treatment reduced the number of PA compared to PCOS rats without treatment [30]. Metformin also reduced androgen production and improved polycystic ovary morphology represented by PA through its role as an insulin sensitizer [31].
Although there are few publications investigating propolis extract as PCOS treatment, this is the first study comparing propolis with metformin. This study has several limitations, such as measuring estrous cycle only twice at the beginning and the end of treatment. Consequently, it was difficult to define anovulation from the data only. PCOS diagnosis was then defined by hyperandrogenism examined by high TT concentration and polycystic ovarian morphology represented by abundant PA. Treatment dose used was based on previous studies in diabetes-induced rats, and different dosages of propolis extract and metformin could have different results.
CONCLUSION
In conclusion, propolis extract and metformin monotherapy significantly decreased TT concentrations and PA count. Propolis extract dosage of 240 mg/kg BW had higher hypoglycemic effects compared to metformin monotherapy but could not improve FINS concentration and HOMA-IR value. In addition, the combination with metformin was beneficial for lowering FBG concentration but failed to treat insulin resistance in PCOS-induced rats. Metformin with a dosage of 90 mg/kg BW did not succeed in lowering FBG concentration, FINS concentration, and HOMA-IR value. Further studies with different dosages and longer treatment duration of propolis extract and metformin were needed to explore the full potency for insulin resistance treatment in PCOS patients.
ACKNOWLEDGMENT
None.
AUTHOR CONTRIBUTION
All authors equally contributed to the study. VDP: Conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing - original draft, writing - review & editing. ESL and NP: Formal analysis, investigation, methodology. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Teede HJ, Misso ML, et al. International PCOS Network. Erratum. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018; 33(9): 1602-18.
- [2]Chang JE. Yen and Jaffe’s Reproductive Endrocinology: Polycystic Ovary Syndrom and Hyperandrogenic States. Elsevier Saunders: Philadelphia, PA, USA, 2014.
- [3]Al-Karawi AS, Kadhim AS. Exploring the role of autoantibodies in Iraqi females with polycystic ovary syndrome. J Adv Biotechnol Exp Ther. 2024 Jan; 7(1): 147-156.
- [4]Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA) – a novel theory for the developement of polycyctic ovarian syndrome. Medical Hypotheses. 2012; 79: 104-12.
- [5]He L. Metformin and systemic metabolism. Trends Pharmacol Sci. 2021; 41(11): 868–81.
- [6]Nawar AS, Obaid ZH, Sheikh QI. Oxidant and antioxidant status of erythrocytes and plasma samples in polycystic ovary syndrome patients. J Adv Biotechnol Exp Ther. 2023 jan; 6(1): 17-24.
- [7]Pascale A, Marchesi N, et al. The role of gut microbiota in obesity, diabetes mellitus and effect of metformin: new insights into old diseases. Curr Opin Pharmacol. 2021; 49: 1–5.
- [8]Cai W, Xu J, et al. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Rest. Int. 2020; 130: 1-14
- [9]Xue M, Liu Y, et al. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed Pharmacother. 2019; 118: 109393.
- [10]Walters KA, Bertoldo MJ, et al. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab. 2018; 32(3): 271-81.
- [11]Shi D, Vine DF. Animal models of polycystic ovary syndrome : a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2020; 98(1): 185-193.
- [12]Wessels B, Ciapaite J, Van den Broek NMA, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS ONE. 2014: 9(6): 1-13.
- [13]Rivera-Yañez N, Rodriguez-Canales M, et al. Hypoglycaemic and antioxidant effects of propolis of Chihuahua in a model of experimental diabetes. Evid Based Complement Alternat Med. 2018: 1-10.
- [14]Al-Hariri M, Eldin TG, et al. Glycemic control and anti-osteopathic effect of propolis in diabetic rats. Diabetes Metab Syndr Obes. 2011; 4: 377-84.
- [15]Samadi N, Mozaffari-Khosravi H, et al. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. J Integr Med. 2017; 15(2): 124-134.
- [16]Pahlavani N, Malekahmadi M. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr Metab. 2020: 17: 65.
- [17]Nna VU, Bakar ABA, Mohamed M. Malaysian propolis, metformin and their combination, exert hepatoprotective effect in streptozotocin-induced diabetic rats. Life Sci. 2018; 211: 40-50.
- [18]Nia J, Chang Y, et al. Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-κB inflammatory pathways in diabetic mice and HepG2 cell models. J Agric Food Chem. 2017; 65(41): 9041-53.
- [19]Zullkiflee N, Taha H, et al. Propolis: Its role and efficacy in human health and diseases. Molecules. 2022; 27(18): 6120.
- [20]Li Y, Chen M, et al. Effect of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med. 2012: 1-8.
- [21]Guan R, Ma N, et al. Ethanol extract of propolis regulates type 2 diabetes in mice via metabolism and gut microbiota. J Ethnopharmacol. 2023; 310: 1-14.
- [22]Zakerkish M, Jenabi M, et al. The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep. 2019; 9: 7289.
- [23]Zullkiflee N, Taha H, Usman A. Propolis: Its role and efficacy in human health and diseases. Molecules. 2022; 27(18): 6120.
- [24]Prattichizzo F, Giuliani A, et al. Pleiotropic effects of metformin : Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018; 48: 87-98.
- [25]Oguz SH, Sendur SN, et al. Polycystic Ovary Syndrome: Targeting metabolism in the management of PCOS: Metformin and beyond. Elsevier Inc. 2022.
- [26]Li M, Hu X, et al. A possible mechanism of metformin in improving insulin resistance in diabetic rat models. Int J Endocrin. 2019; 2019: 3248527.
- [27]Za’abi MA, Ali BH, et al. The effect of metformin in diabetic and non-diabetic rats with experimentally-induced chronic kidney disease. Biomolecules 2021; 11: 814.
- [28]Ali A, Paramanya A, et al. The utilization of bee products as a holistic approach to managing polycystic ovarian syndrome-related infertility. Nutrients. 2023; 15(5): 1165.
- [29]Xue J, Li X, et al. Inulin and metformin ameliorate polycystic ovary syndrome via anti-inflammation and modulating gut microbiota in mice. Endocr. J. 2019; 66(10): 859-70.
- [30]Sapmaz T, Sevgin K, et al. Propolis protects ovarian follicular reserve and maintains the ovary against polycystic ovary syndrome (PCOS) by attenuating degeneration of zona pellucida and fibrous tissue. Biochem. Biophys. Rep. 2022; 636 (2): 97-103.
- [31]Zhao H, Xing C, et al. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta‑analysis. Reprod Health. 2021; 18: 171.