Phytase supplementation in Broilers: Influence on growth performance and physiological health
Abstract
Phosphorus is essential for broiler growth and bone development, but its bioavailability from plant-based feed is restricted by phytate binding. Phytase supplementation enhances nutrient utilization by breaking down phytate, improving phosphorus availability, growth performance, gut health, and reducing environmental phosphorus excretion. Thus, this study aimed to evaluate the impact of phytase enzyme supplementation on growth performance and hemato-biochemical markers as a potential alternative to antibiotic growth promoters in broilers. In this study, 300 Arbor Acres broiler chicks were randomly assigned to five treatment groups (T0–T4) with three replicates each. The control group (T0) received a basic diet (1–42 days), while experimental groups T1 supplemented with oxytetracycline and T2–T4 were supplemented with Phytase (Natuphos® E) from days 7–42. T1 received oxytetracycline (1000 mg/kg), while T2, T3, and T4 were supplemented with 500, 750, and 1000 FTU/kg of Phytase, respectively. Growth performance was assessed through body weight, feed intake, feed conversion ratio (FCR), and livability. Blood samples were analyzed for hematological parameters (CBC) and biochemical markers, including total protein, albumin, ALT, AST, cholesterol, triglycerides, LDL, and HDL. Results indicated significant improvements in body weight and FCR in phytase-supplemented groups, with T4 showing the best performance (body weight 2915.1 g, FCR 1.53). Phytase supplementation improved hematological and biochemical profiles in broilers, with 1000 FTU/kg showing the highest efficacy. These data suggest that phytase enhances growth, feed efficiency, and physiological parameters, making it a viable alternative to antibiotic growth promoters.
INTRODUCTION
The poultry industry has made significant progress in the livestock sector of Bangladesh over the past few decades [1]. The poultry industry is one of the major food industries in the world [2]. Bangladesh is now emerging as one of the fastest-growing poultry-producing nations, contributing significantly to the national economy with much-needed high-quality protein, and is a critical part of the ongoing drive towards poultry self-sufficiency [3]. This is a fast-growing industry covering 14% of total livestock production value [4]. Poultry is the largest contributor of animal protein in the country, accounting for about 22–27% of animal protein supply [5], followed by fish and cattle, which require a longer production period. Worldwide, poultry production is one of the most developed and rapidly growing sectors in the food industry [6]. The poultry sector (both commercial and backyard) contributes 1.53% of the total GDP of Bangladesh [7].
Feeding is important in efficient poultry production as poultry mainly relies on balanced nutrition for their production. Apart from this, farmers facing challenges from viruses, bacterial infections, and other infectious diseases are using various antibiotics in their animal feed, along with growth promoters, enzymes, and probiotics, to accelerate growth and increase profits. They act on the gastrointestinal tract and affect digestion, absorption, and improve gastrointestinal health [8].
Antimicrobial resistance poses a significant global health and development threat, primarily driven by the inappropriate and excessive use of antimicrobial agents in both humans and food animals [9, 10]. Antibiotics have been extensively used in poultry production to increase production, promote growth, and protect chickens against harmful microbes [11–13]. The addition of antibiotics in the broiler feed can result in a 5.8% increase in body weight gain [14]. The benefits include a good appetite, more efficient conversion of feed to animal growth, improved vitality, and even balance of intestinal microflora [15]. While antibiotics are essential, their misuse in animal farming has been related to the emergence of antimicrobial-resistant bacteria, which represents a human health risk [16–18], contamination of animal products and pollution of the environment [19, 20]. Consequently, the use of antibiotics as growth promoters was banned in the European Union in 2005 [21] and in China in 2020 [22]. For health reasons, consumers are moving towards conventional broiler meat, resulting in a global trend in poultry production toward the slaughter of antibiotic-free animals [11]. The ban on antimicrobials in animal feed, coupled with growing consumer demand, has driven researchers to explore alternatives to antibiotics [23]. This is a need that highlights the importance of sustainable feeding strategies, along with the engagement of possible antibiotic substitutes, to increase the production of antibiotic-free broiler meat [24]. Hence, this study was conducted to explore potential antibiotic substitutes focusing on enzymes used in broiler production.
Poultry has already started to reach some substitutes as feed additives. Since it is incorporated with plant and animal components, the largest part of the substances in feed are anti-nutritional and don't get ingested, which makes poultry perform poorly and diminishes the capacity of chickens to utilize feed. The adverse effect can be counteracted by the inclusion of physiologically active feed supplements. The supplementation with phytase to the poultry diet has been studied, and various colors of examination suggested how exactly this will help the utilization and absorption of nutrients [25]. Plants contain 60 to 70% of total phosphorus in the form of phytate phosphorus (myo-inositol) that is unavailable to poultry because of the very low level of endogenous phytase in the gastrointestinal tract [26]. Thus, inorganic phosphorus should be added either to the feed of poultry, or commercial phytase (the sources are mainly of microbial origin) should be added as phytase breaks down the phytate to inositol and inorganic phosphorus [27]. Commercial phytase increases the productivity of phytate phosphate and zinc. Phytase enhances calcium availability and improves the absorption and bioavailability of Mg, Cu, and Fe [28]. Ultimately, it leads to improved production performance, along with enhanced hematological and biochemical markers. Therefore, this study was conducted to evaluate the impact of phytase enzyme supplementation on growth performance and hemato-biochemical markers as a potential alternative to antibiotic growth promoters in broiler production.
MATERIALS AND METHODS
Ethical statement
The study was approved by the Animal Experimentation and Ethics Committee of Sylhet Agricultural University, Bangladesh, under approval number [#AUP2022020].
Study site and experimental design
This study was conducted at the Department of Pharmacology and Toxicology, Sylhet Agricultural University, Bangladesh. In a randomized control trial (RCT), a total of 300 chicks (Day old chick) of Arbor Acres broiler chickens (purchased from Nourish Poultry and Hatchery Ltd., Bangladesh) were used which were divided into 5 treatments (T0, T1, T2, T3, T4) having 3 replicates in such a way that each replicates contained 20 chicks. Chicks were reared for up to 42 days (6 weeks), where the chickens were fed a feeding program that fulfilled the growth requirements of Arbor Acres broiler chickens. Broiler groups, feeding patterns, and phytase supplementation timeline are shown in Table 1.
Table 1. Broiler groups, feeding patterns, and Phytase supplementation timeline.
Acclimatization and feeding management of birds
The experimental chickens were maintained on uniform feeding and management practices. Chickens were fed only commercial ration for 6 days before the trial to adapt them to shed conditions. All chicks received commercially available feed for 42 days, with feed distributed three times daily, and chicks in all treatment groups (Table 2).
Chicks were reared in floor pens with a space of 1000 cm2/chicken and offered fresh and dry rice husk bedding aids at a depth of approximately 3–4 inches. Water was always available, normal, fresh, and pure. To keep the temperature and humidity inside the shed, all the curtains were opened during the day, while at night, electric bulbs were used as a source of heat. Three times daily temperature (0C) and humidity (%) were determined with a dry and wet bulb thermometer, respectively.
During the trial period, vaccination was kept for chickens to protect them from common diseases. The chickens were vaccinated with RaniVax Plus Vet (Incepta Pharmaceuticals Ltd., Bangladesh) against Newcastle Disease (ND) and Infectious Bronchitis (IB). The first dose was given on day 1, and the booster was given on day 21. RaniVax Plus Vet is a live, freeze-dried vaccine composed of NDV F strain and IBV Massachusetts strain to protect both ND and IB. The Infectious Bursal Disease (IBD) vaccine, GumboMed Plus Vet (Incepta Pharmaceuticals Ltd., Bangladesh), was applied in drinking water at the 10th day with a boost of the same vaccine on the 17th day. The disease prevention method in the experimentation phase was biosecurity and good sanitary practices. Chicken care and management were performed according to accepted guidelines (The ARRIVE guidelines 2.0).
Table 2. Nutrient composition of supplied feed up to 42 days.
Experimental diet and enzyme preparation
The complete experimental trial was conducted using commercially available poultry feed (Nourish Poultry and Hatchery Ltd., Bangladesh) (Table 1). The chickens were fed with Broiler Pre-starter from 0 to 10 days of broiler starter from day 11 to day 20 and broiler grower from day 21 to day 30, and broiler finisher from day 31 to the rest of the days. From the 7th day onward, Natuphos® E was given just after each weighing of chicken. The experimental therapy was given four days a week. Phytase was provided in a mix with commercial feed two times daily.
Data collection
Chicks’ weights were recorded at the beginning of the experiment on days 7, and just after weights were taken, they initiated phytase treatment on the same day. Then the measurements were recorded weekly until the end of the 42-day period. Chickens were weighed before the morning feeding sessions using a weighing scale. At the end of each week, the feed intake (FI) of the experimental chickens through different replications within each treatment group was weighed, and weekly refusals were also recorded. The refusal was determined by collecting and weighing left over feed in all treatment groups at the end of week. Feed consumed (feed intake) was then determined by subtracting refusals from total feed offered. This was useful information to track the weekly feed consumption of the chickens during the experiment. The broiler chick’s livability was periodically monitored, and the differences in livability were determined for each treatment group.
Methods of determining the performance parameters
The growing performance of broilers was evaluated using the broiler performance efficiency factor (BPEF) and broiler farm economy index (BFEI), after laying out the liveability percentage (%) and feed conversion ratio (FCR) as suggested by Hossain et al. [18] and Rahman et al. [29].
- Liveability (%)
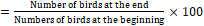
- FCR
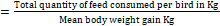
- Average Daily Gain (ADG)
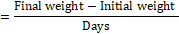
- BPEF
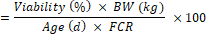
- BFEI
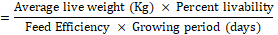
Hematology and biochemical parameters analysis
Blood was collected from the wing vein on day 42 of the experiment for complete blood count (CBC) analysis using a hematology analyzer. A blood test related to a complete blood count (CBC) includes erythrocytes (red blood cells), hemoglobin, and leukocytes (white blood cells). Different biochemical tests, such as total protein (TP), albumin (Alb) alanine transaminase (ALT), aspartate aminotransferase (AST), serum cholesterol (CHL), serum triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), were assessed after serum separation.
Antibiotic growth promoter
Renamycin® vet is a product of Renata Limited from Bangladesh and contains Oxytetracycline, a broad-spectrum antibiotic active against many Gram-positive and Gram-negative bacteria. Commonly utilized in poultry farming, particularly in the case of broilers, it treats bacterial infections in the respiratory, digestive, and urinary tracts. Oxytetracycline prevents bacterial protein synthesis, resulting in improved chicken health, feed efficiency, growth, and performance. Renamycin® vet is available in packs of 100 g, 1 kg and is preferably mixed with feed for easy administration. It is an undoubtedly potent antibiotic, but it is also one of the most widely used in therapy and livestock farming despite increasing concerns about antibiotic resistance.
Natuphos® E
Natuphos® E (imported and distributed by Synergy Bioscience Bangladesh Ltd.), a commercial phytase enzyme product from Bandische Anilin- und Sodafabrik (BASF), enhances phosphorus availability in poultry diets, improving growth and efficiency in broilers. Containing a potent 6-phytase enzyme, it is available in various potencies (5,000, 10,000, and 50,000 FTU/g). Used primarily in broiler farming, it breaks down phytates in plant-based feed, boosting phosphorus and mineral absorption for better bone development and growth. The recommended inclusion rate ranges from 500 to 1,000 FTU/kg of feed, improving nutrient digestibility, feed conversion, and growth performance in broilers.
Statistical analysis
Collected and calculated data were analyzed using the R program. ANOVA was performed, followed by post-hoc Duncan tests to determine significant differences and compare parameters between treatments. Data were visualized through a graphical representation in R using the ‘ggplot2’ and ‘readxl’ packages.
RESULTS
Effect of phytase supplementation on growth performance in broilers
The growth performance of broilers under different dietary treatments is summarized in Table 3 and Figure 1. Body weight showed significant variations among the groups from day 14 onwards (p < 0.05). While no significant differences were observed on day 7, broilers in the phytase-supplemented groups (T2, T3, and T4) exhibited improved growth performance compared to the control (T0) and antibiotic-treated (T1) groups from day 14 to day 42.
On day 21, the highest body weight was recorded in T4 (1082.49 g), followed by T1 (1051.65 g) and T3 (1049.34 g), whereas T0 had the lowest (953.95 g). Similar trends were observed on days 28, 35, and 42, with T4 achieving the highest body weight at the final stage (2915.14 g), followed by T3 (2830.81 g), T1 (2799.82 g), and T2 (2765.27 g). The control group (T0) exhibited the lowest body weight at all stages. FCR also improved significantly with phytase supplementation, with T4 demonstrating the best efficiency, indicating enhanced nutrient utilization. The statistical analysis revealed a highly significant effect of dietary treatments on body weight (p < 0.001), confirming the beneficial impact of phytase supplementation in broiler production.

Table 3. Effects of treatments on body weight and FCR in broiler production.
Effect of phytase supplementation on feed conversion ratio in broilers
The feed intake, weight gain, and FCR at 42 days of age are presented in Table 4. The results indicate significant differences (p < 0.05) in feed intake, weight gain, and FCR among the treatment groups. The highest feed intake was observed in the control group (T0) (4832.20 g), followed by T2 (4673.30 g), T1 (4619.70 g), and T3 (4614.31 g), while the lowest feed intake was recorded in T4 (4430.95 g). Despite consuming the least amount of feed, broilers in T4 exhibited the highest weight gain (2915.14 g), followed by T3 (2830.81 g), T1 (2799.82 g), and T2 (2765.27 g), whereas T0 had the lowest weight gain (2714.72 g).
Table 4. Feed conversion ratio at the age of 42nd Day of the experimental trial in broilers.
Effect of phytase supplementation on growth performance efficiency indicators in broilers
The performance of broiler production in terms of livability (%), BPEF, BFEI, and ADG of the treatments (T0 to T4) is presented in Figure 2. The best livability percentage was found 100% in T4 because T4 chickens received a higher dose of phytase (1000 FTU/kg), which greatly improved nutrient absorption, bone strength, and immune health, leading to 100% livability, BPEF (155.57), BFEI (4.51), and ADG (68.58 g) were noted on T4. Next, T3 comes with a livability of 93.65%, BPEF of 129.64, BFEI of 3.49, and ADG of 66.98 g, making this a strong performer but less efficient compared to T4. Moderate results were found from T1 of 99% livability, 124.25 BPEF, 3.31 BFEI, and 65.34g ADG, and T2 of 91% livability, 123.63 BPEF, 3.08 BFEI, and 64.82g ADG.

Effect of phytase supplementation on hematological parameters and serum biochemistry in broilers
The effects of different treatments on hematological parameters are summarized in Table 5. Erythrocyte count was significantly higher in T1 2.87 × 1012/mL) and T4 (2.79 × 1012/mL) compared to T0 (2.36 × 1012/mL) and T2 (2.27 × 1012/mL), with T1 showing the highest value. Hemoglobin levels were also significantly higher in T1 (105.15 ± 26 g/mL) and T3 (104.71 ± 41 g/mL) compared to T0 (102.45 ± 73 g/mL) and T2 (101.91 ± 37 g/mL). In terms of leukocytes, T4 (35.17 ± 0.15 × 109/mL) showed the highest count, followed by T3 (32.38 ± 1.15 × 109/mL) and T1 (31.56 ± 0.94 × 109/mL). These results indicated that phytase supplementation, particularly at higher doses (T1 and T4), positively impacts erythrocyte count, hemoglobin levels, and leukocyte count, indicating improved overall health and immune function in broilers.
The effects of different treatments on biochemical parameters are presented in Table 6. Total protein (TP) levels were highest in T4 (4.26 ± 75 g/dL), followed by T1 (3.96 ± 72 g/dL), while the lowest levels were observed in T0 (3.09 ± 19 g/dL). Albumin (Alb) levels were significantly higher in T4 (2.63 ± 39 g/dL) compared to T0 (1.93 ± 73 g/dL), with T1 (2.38 ± 52 g/dL) also showing an improvement. AST and ALT levels were lowest in T4, with AST at 135 ± 75 U/L and ALT at 3.12 ± 13 U/L, indicating better liver function in this group. Cholesterol levels were significantly reduced in T4 (117 ± 27 mg/dL), compared to T0 (159 ± 64 mg/dL), while HDL levels were highest in T4 (69 ± 64 mg/dL), followed by T1 (67 ± 91 mg/dL). LDL and triglyceride levels were lowest in T4 (59 ± 85 mg/dL and 81 ± 83 mg/dL, respectively), suggesting improved lipid metabolism. Overall, these results indicate that phytase supplementation, particularly at higher doses, positively influences biochemical markers related to protein metabolism, liver function, and lipid profiles in broilers.
Table 5. Effects of different treatments on hematological parameters in broiler production.
Table 6. Effects of different treatments on biochemical parameters in broiler production.
DISCUSSION
The results of this study demonstrated that phytase supplementation in broiler diets, particularly at higher doses (1000 FTU/Kg), significantly enhances growth performance, feed efficiency, and overall health status of the chickens. These findings are consistent with numerous studies that highlight the importance of enzyme supplementation in improving the bioavailability of essential nutrients, particularly phosphorus, which is largely unavailable due to the presence of phytate in plant-based feeds [25,26]. Phytase supplementation has been shown to effectively degrade phytate, thereby enhancing the absorption of key minerals such as calcium, magnesium, zinc, and iron, which are critical for optimal growth and bone development [30,31]. In this study, the T4 group, which received the highest phytase dose, exhibited the highest body weight, FCR, and the BFEI, reflecting enhanced nutrient utilization and metabolic health. This observation supports the research conducted by Derakhshan et al. [31], who reported improved growth performance and metabolic efficiency in poultry with higher doses of phytase.
This study examines the impact of different treatments on feed conversion efficiency in broiler production. T0 (control) showed the highest feed intake (4832.20 g) and poorest FCR (1.78), consistent with Khan et al. [32], who found that excessive feed intake without corresponding weight gain leads to inefficiency. T1 (antibiotic growth promoter) improved FCR to 1.65, supporting El-Fateh et al. [25] who noted that antibiotics enhance feed utilization. T2, T3, and T4 (phytase enzyme supplementation) showed progressively better FCR, with T4 performing the best (1.53), reflecting the findings of Derakhshan et al. [31]. Overall, the results suggest that both antibiotic growth promoters and phytase enzyme supplementation improve feed conversion efficiency. Phytase supplementation in broiler diets has also been shown to reduce feed intake. This decrease may be attributed to the enhanced availability of phosphorus from the feed used in the study, which improves nutrient utilization efficiency and subsequently reduces feed consumption for growth. Mechanics of phytase improved the digestibility of phytate-bound phosphorus because of the phytase action, enabling the chickens to achieve their nutritional requirements more efficiently and thus had less need for excess feed to meet the same [33].
Livability was also significantly higher in T4 (100%), suggesting that higher phytase supplementation contributes to improved overall health, potentially through its role in enhancing immune function. This finding aligns with the work of Haque et al. [28] and Gholami-Ahangaran et al. [8], who found that phytase not only promotes growth but also strengthens immune responses, which is essential for disease resistance and survival rates in poultry. The increased BPEF and ADG observed in T4 further support the positive impact of phytase on growth performance. Similar improvements in these parameters have been reported in other studies evaluating phytase supplementation [25, 26].
In addition to growth performance, hematological parameters such as erythrocyte count, hemoglobin levels, and leukocyte count were positively influenced by phytase supplementation, particularly at higher doses. The increased erythrocyte and hemoglobin levels observed in T4 suggest an enhancement in the oxygen-carrying capacity of blood, which is essential for supporting high metabolic activity and promoting muscle growth. This is consistent with studies that have shown that phytase supplementation improves mineral absorption, leading to better blood health and growth performance [8, 30]. Additionally, the higher leukocyte count in T4 indicates a stronger immune response, which could explain the increased livability and resistance to diseases in this group.
The biochemical parameters also revealed significant improvements with higher phytase supplementation. Total protein, albumin, and the reduction in liver enzymes (AST and ALT) in T4 suggest that phytase supplementation enhances liver function and overall metabolic health. These findings are supported by previous research showing that phytase supplementation can improve protein metabolism and reduce liver stress [25,26]. Furthermore, the reduction in cholesterol and triglyceride levels in T4 indicates improved lipid metabolism, which is beneficial for cardiovascular health and overall performance. These findings are in line with the work of Derakhshan et al. [31], who observed similar improvements in lipid profiles with phytase supplementation.
This study was conducted under controlled experimental conditions, which may not fully represent commercial broiler farm environments. The long-term effects of phytase supplementation on gut microbiota and immune function were not assessed. Additionally, the economic feasibility and cost-effectiveness of phytase inclusion require further evaluation.
CONCLUSIONS
Phytase supplementation proved to be a promising strategy for enhancing broiler productivity by optimizing nutrient utilization and improving physiological health. The observed improvements in growth performance, hematological, and biochemical parameters suggest that phytase can serve as a viable alternative to antibiotic growth promoters. By increasing phosphorus bioavailability and promoting overall well-being, phytase supplementation offers a sustainable approach to improving broiler production efficiency while mitigating environmental concerns associated with phosphorus excretion.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to the Department of Pharmacology and Toxicology, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Bangladesh, for their support and collaboration in the successful completion of this research.
AUTHOR CONTRIBUTIONS
MI and HH – Conceptualization, Methodology, Data Curation, Software, Formal Analysis, Investigation, Writing – Original Draft, Writing – Review and Editing; FSP, DK, MSH, SA, PRD, MAR, MAIS – Data Curation, Investigation, Formal Analysis, Writing – Original Draft; Writing-reviewing and editing; MMR, MSI and MMR – Conceptualization, Methodology, Data Curation, Software, Validation, Visualization, Resources, Project Administration, Supervision, Writing – Original Draft, Writing – Review and Editing.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [2]Chowdhury EU, Morey A. Intelligent Packaging for Poultry Industry. J Appl Poult Res. 2019; 28:791–800.
- [3]Tanni FY, Rahman Chowdhury MS, et al. Prevalence and antimicrobial resistance of extended spectrum beta-lactamase (ESBL) producing Klebsiella spp. in poultry meat. Heliyon. 2025;11: e41748.
- [4]Raihan S, Mahmud N. Trade and Poverty Linkages A Case Study of the Poultry Industry in Bangladesh 2008.
- [5]Division RPP and H. Good practices in planning and management of integrated commercial poultry production in South Asia 2003.
- [6]Hussain J, Rabbani I, et al. An overview of poultry industry in Pakistan. Worlds Poult Sci J. 2015; 71:689–700.
- [7]Kamruzzaman M, Islam S, et al. Financial and factor demand analysis of broiler production in Bangladesh. Heliyon. 2021;7: e07152.
- [8]Gholami-Ahangaran M, Ahmadi-Dastgerdi A, et al. Thymol and carvacrol supplementation in poultry health and performance. Vet Med Sci. 2021; 8:267.
- [9]Liza NA, Hossain H, et al. Molecular Epidemiology and Antimicrobial Resistance of Extended-Spectrum β-Lactamase (ESBL)-Producing Klebsiella pneumoniae in Retail Cattle Meat. Vet Med Int. 2024;3952504.
- [10]Rahman MdM, Hossain H, et al. Molecular Characterization of Multidrug-Resistant and Extended-Spectrum β-Lactamases-Producing Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Raw Meat in Retail Markets. Antibiotics. 2024; 13:586.
- [11]Haque MH, Sarker S, et al. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology (Basel) 2020; 9:411.
- [12]Selaledi LA, Hassan ZM, et al. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics (Basel) 2020; 9:1–18.
- [13]Paintsil EK, Ofori LA, et al. Antimicrobial Usage in Commercial and Domestic Poultry Farming in Two Communities in the Ashanti Region of Ghana. Antibiotics. 2021; 10:800.
- [14]Rahman M, Parvin M, et al. Effects of growth promoter and multivitamin-mineral premix supplementation on body weight gain in broiler chickens. J Bangladesh Agril Univ. 2013; 10:245–8.
- [15]Peric L, Zikic D, et al. Application of alternative growth promoters in broiler production. Biotechnology in Animal Husbandry 2009; 25:387–97.
- [16]Nhung NT, Chansiripornchai N, et al. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front Vet Sci. 2017; 4:126.
- [17]Manyi-Loh C, Mamphweli S, et al. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules. 2018; 23:795.
- [18]Hossain H, Nuradji H, et al. Impact of synbiotic on growth performance, histo-architectural modulation of lymphoid organ, hematology, blood biochemistry and humoral immune response in naked neck chicken. Trop Anim Health Prod. 2025; 57:1–16.
- [19]Carvalho IT, Santos L. Antibiotics in the aquatic environments: A review of the European scenario. Environ Int. 2016; 94:736–57.
- [20]Gonzalez Ronquillo M, Angeles Hernandez JC. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control. 2017; 72:255–67.
- [21]Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005; 84:634–43.
- [22]Melaku M, Zhong R, et al. Butyric and Citric Acids and Their Salts in Poultry Nutrition: Effects on Gut Health and Intestinal Microbiota. Int J Mol Sci. 2021; 22:10392.
- [23]Diarra MS, Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front Microbiol. 2014;5.
- [24]Khan RU, Naz S, et al. Chromium: Pharmacological applications in heat-stressed poultry. International Journal of Pharmacology. 2014; 10:213–7.
- [25]El-Fateh M, Bilal M, et al. Effect of antibiotic growth promoters (AGPs) on feed conversion ratio (FCR) of broiler chickens: A meta-analysis. Poult Sci. 2024; 103:104472.
- [26]Singh PK. Significance of phytic acid and supplemental phytase in chicken nutrition: A review. Worlds Poult Sci J. 2008; 64:553–80.
- [27]Rizwanuddin S, Kumar V, et al. Microbial phytase: Their sources, production, and role in the enhancement of nutritional aspects of food and feed additives. J Agric Food Res. 2023; 12:100559.
- [28]Haque N, Hossain A. Phytase: Their Biochemistry, Physiology and Application in Poultry. Int J Livestock Res. 2012; 2:30.
- [29]Rahman M, Hossain H, et al. Selection of efficient broiler strain for productive performances and immunity under local farming system in Bangladesh. J Adv Biotechnol Exp Ther. 2025; 8:218.
- [30]Cowieson AJ, Bedford MR. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? Worlds Poult Sci J. 2009; 65:609–24.
- [31]Derakhshan M, Ghasemian SO, et al. The effects of probiotic and phytase on growth performance, biochemical parameters and antioxidant capacity in broiler chickens. Vet Med Sci. 2023; 9:860–6.
- [32]Nawaz H, Rasheed S, et al. Effects of different fat sources on performance parameters and carcass characteristics of broiler chicks. Indian J Anim Sci. 2015; 85:414–8.
- [33]Wang W, Wang Z, Yang H, Cao Y, Zhu X. Effects of phytase supplementation on growth performance, slaughter performance, growth of internal organs and small intestine, and serum biochemical parameters of broilers. Open J Anim Sci. 2013; 03:236–41.
- []Raquib A, Uddin A, et al. Seroprevalence of Mycoplasma gallisepticum infection in layer chickens of Bangladesh. Iraqi J Vet Sci. 2022; 36:9–13.