Screening of phytochemicals, antioxidant activity, and in vivo safety profile of the hydroethanolic peel extract of Musa sapientum
Abstract
Banana peels can be utilized in many ways such as nutraceuticals to prevent or cure diseases. Therefore, it is important to understand the phytochemical composition, antioxidant capacity, and safety profile of Musa sapientum variety Muraru peels. The study identified the chemical composition of unripe and ripe banana (Muraru) peels of the Musa sapientum, along with their antioxidant capacity, and evaluated the oral acute and sub-acute toxicity of the hydroethanolic extracts. Qualitative and quantitative phytochemical analyses were performed, and GC-MS was used in the identification of bioactive compounds present. The DPPH total antioxidant activity was used to evaluate the scavenging percentage and IC50. Acute and sub-acute toxicity tests were done for the hydroethanolic peel extract. Haematology, biochemistry, and relative organ weight were analyzed, and the sub-acute group organs were further analyzed for histopathology. Flavonoids, tannins, and phenols were found abundant in both unripe and ripe banana peels. GC-MS showed that the peels were rich with lipids, fatty acids, and terpenoids. The unripe peels showed significant total antioxidant capacity. No significant difference (p> 0.05) was observed in haematology, biochemistry, or relative organ weight compared to the normal controls. Histopathological examination of the liver, lung, heart, kidney, and spleen showed normal tissue orientation in all treated groups. Unripe and ripe banana peels both contained rich secondary metabolites, which greatly influence their antioxidant capacity. The toxicity profile indicated that the hydroethanolic peel extract is nontoxic.
INTRODUCTION
Banana fruit plays a crucial role in human nutrition around the world, and it is good for digestive and heart health. Consuming bananas helps boost potassium, magnesium, vitamin B6, and they are rich in serotine and dopamine, which are neurotransmitters that help improve mood and reduce stress. The plant belongs to the Musaceae family, genus Musa, this fruit category encompasses around 70–75 species globally [1], which includes over 1,000 distinct banana varieties [2]. Remarkably, only a handful of these varieties gain recognition in health circles. Notably, Musa sapientum variety Muraru (local name in Maragua, Kenya), unlike the familiar golden yellow color bananas, it remains green or green-yellow when ripe [3]. Additionally, Muraru banana exhibit thicker peels and a firmer texture compared to Pisang Mas [3]. Although Onyango et al. [3] acknowledged its similarities to Cavendish and Gros Michel, Muraru peels remain unexplored in terms of the potential that could provide additional benefits to human health.
Research has shown that banana peels have a higher abundance of bioactive compounds compared to the pulp [4]. Phytochemicals are natural compounds found in plants that have various biological effects on the human body. A previous study showed that banana peels are rich in phenolic compounds, carotenoids, flavonoids, tannins, terpenes, alkaloids, glycosides, and phytosterols, all of which offer significant dietary benefits with positive effects on human health and well-being [5]. Endogenous antioxidants are molecules produced naturally within the body that help neutralize harmful free radicals and protect cells from oxidative damage. Exogenous antioxidants help fix this imbalance by lowering free radicals, promoting the growth of healthy cells, protecting cells from premature and abnormal ageing, fighting age-related molecular degeneration, and boosting the immune system, which prevents or manages chronic diseases [6]. Bioactive compounds have various mechanisms and properties to function as antioxidants, anti-inflammatory, anticancer, and antidiabetic agents and strengthen the body's defenses against various illnesses [5].
However, bioactive compounds classified as secondary metabolites, while known for their pharmacological effects, can also exhibit toxicological properties in both humans and animals [7-9]. It is important to evaluate the toxicological properties of medicinal plants before using them to make new drugs or improve the effectiveness of treatments that are already in use [7]. Studies have demonstrated that non-nutritive parts of the banana plant, such as the flower, pseudo-stem, and leaf, are non-toxic [10]. Nevertheless, the toxicity profile of banana peels remains inadequately elucidated. Therefore, it is imperative to elucidate the safety profile of Muraru peels.
The abundant presence of bioactive compounds in banana peels positions them as promising candidates for nutraceutical applications. However, it is crucial to elucidate both the antioxidant capacity and toxicological profile of banana peels. This study aims to assess the acute and subacute toxicity of Muraru peel hydro-ethanol extract, as well as its antioxidant activity and phytoconstituents.
MATERIALS AND METHODS
Plant material and preparation
M. sapientum variety ‘Muraru’ bananas were bought from a local farm in Maragua, Kenya. Verification of the banana species was done at the Jomo Kenyatta University of Agriculture and Technology (JKUAT) Botany Department, and the herbarium accession number is MCM- JKUATBH/001/2023. The bananas were acquired at their mature unripe stage, and unripe and ripe stages 1 and 4, respectively [11], where used in this study. The peels were prepared for lyophilization according to [12]. After lyophilization, peels were blended into powder and stored in zip-lock bags wrapped with aluminum foil in a -200C refrigerator for further studies.
Sample extraction
The cold maceration extraction method was employed for both unripe and ripe peels using two different solvents: absolute methanol and 70% ethanol (hydro-ethanolic), following the procedures outlined by Oyeyinka and Afolayan [13] and Siddique et al. [14]. The ratio of solid to solvent used was 1:10, and the mixtures were soaked for 72 hours at 21°C in a shaker rotating at 121 rpm. Afterward, they were filtered using a 0.1 Whatman filter paper.
Phytochemical characterization and screening
Qualitative phytochemical screening
To identify the presence of phytochemical derivatives in methanolic and hydroethanolic extracts, standard phytochemical screening was conducted. The detection of flavonoids, tannins, saponins, steroids, alkaloids, glycosides, phytosterols, phenols, and terpenoids was detected according to the protocol by Kibria & Kar [15].
Quantitative phytochemical screening
The quantitative phytochemical analysis of total phenolic content (TPC), total tannin content (TTC), and total flavonoid content (TFC) were determined according to the standard protocols of Oyeyinka and Afolayan [13], Aboul-enein et al. [16], and Santhosh and Suriyanarayanan [17].
Gas chromatography mass spectrometry
The Shimadzu GC-MS (Qp2010SE, Japan) was utilized, and the National Institute of Standards and Technology (NIST) library was employed to predict the names of scanned compounds based on the mass-to-charge ratios of the compounds.
Total antioxidant capacity
The DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was conducted according to Chaudhary et al. [18]. The absorbance was measured at 517 nm using a spectrophotometer (UV 1800, Shimadzu).
Scavenging ability (%)= Abs(control) – Abs(sample)/Abs(control) x 100
where Abs (control) indicates the absorbance of DPPH radical + methanol, and Abs (sample) is the absorbance of DPPH radical + sample extract or standard.
Experimental animals
Albino rats of the Wistar strain, weighing between 150 to 190 g and aged between 7-8 weeks, were obtained from the Small Animal Facility for Research and Innovation (SAFARI) of the Jomo Kenyatta University of Science and Technology (JKUAT). Twenty-one female animals were housed under standard laboratory conditions of 22 ± 3 °C, a relative humidity of 30%, and a 12 h light and dark cycle. The rats had free access to a standard pellet diet (Unga Feeds™) and water ad libitum. Animal bedding, comprising shredded paper, was changed twice a week.
In accordance with ethical standards, the Animal Use and Care Ethics Review Committee at Jomo Kenyatta University of Science, Agriculture, and Technology, Kenya (JKU/ISERC/02316/1014) granted ethical approval. The conducted experiments were designed to minimize animal suffering, and the experimental protocols strictly followed the guidelines outlined in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals [19].
Analysis of acute toxicity
The acute toxicity of the hydroethanolic extract was tested to determine the safety of the agent following the guidelines set by the Organization for Economic Cooperation and Development (OECD) No. 423 [20]. Twelve healthy females in four groups (n = 3) were used, receiving 2000 mg/kg, 1000 mg/kg, 500 mg/kg, and the normal control group received 0.5% Tween 80 dissolved in distilled water, used as a vehicle, with a dissolving volume of 1 ml/100 g. The animals were allowed to acclimatize for one week before experimentation. Prior to the toxicity test, animals were fasted overnight but provided with water. After oral administration of the hydroethanolic peel extract, with the use of oral gavage, animals were observed for the first 30 min, 4 h, and 24 h for signs of morbidity, mortality and once daily over a period of 14 days [20-21]. On the 15th day, all the animals were fasted for 12 h and then euthanized using chloroform. No sign of morbidity or mortality was observed, therefore, the approximate lethal dose (LD50) of the peel hydroethanolic extract was estimated to be higher than 2000 mg/kg.
Analysis of subacute toxicity
The subacute toxicity analysis of the hydroethanolic extract was assessed on nine healthy female animals (n = 3). The normal control group received Tween 80 (0.5%) dissolved in distilled water; two groups received 500 mg/kg and 250 mg/kg of the hydroethanolic peel extract, dissolved in 1 m/100 g, administered orally once every day for a period of 28 days. Animals were observed daily for any sign of morbidity. On the 29th day, all the animals were fasted for 12 h and then euthanized [20].
Preclinical evaluation
The general behavior, body weight, and feed-water intake of the rats were observed during the acclimatization period. After the administration of the hydroethanolic peel extract, each rat was continuously monitored [22].
Hematology analysis
Blood samples were obtained via cardiac puncture using a syringe and needle, and they were deposited into EDTA-containing tubes. Hematological parameters were conducted using the automated Mindray BC-5000 hematological analyzer from Shenzhen, China [23].
Biochemical analysis
Blood samples for biochemical analysis were placed into non-anticoagulant tubes for sera. An automated Roche-Reflotron dry chemistry analyzer (Roche Diagnostics, Germany) was employed for liver and kidney damage markers, alanine transaminase (ALT), aspartate transaminase (AST), creatinine, and urea, respectively [24].
Histopathology analysis
After euthanasia, the liver, lung, kidney, spleen, and heart organs were surgically removed, examined for abnormalities, and weighed. Relative organ weight (ROW) was calculated using the following equation as described [25].
Relative organ weight = Absolute organ weight (g)/ Body weight (g) of rats on final day × 100
The subacute-treated group organs were taken for histopathological analysis, according to Kamsu et al. [26]. Stained tissue sections were photographed using photomicrographs and documented for subsequent analysis by a pathologist.
Statistical analyses
All the data was expressed as mean ± standard error of the mean (SEM). The difference between treated and control groups of acute and subacute toxicity tests were determined by one-way analysis of variance (ANOVA). Values were considered significant at p < 0.05. All statistical analysis was done using GraphPad Prism (student) version 8.4.3 for Windows.
RESULTS
Qualitative and quantitative phytochemical profile
The preliminary qualitative phytochemical profile of Musa sapientum var Muraru showed unripe peels had a significant number of compounds in the hydroethanolic extracts. While the ripe peel showed more compounds in methanolic extracts. Flavonoids, tannins, phytosterols, phenols, and terpenoids secondary metabolites were present across all the peel extracts (Table 1). The quantitative phytochemical profile showed that unripe and ripe Muraru peels are rich in phenolics, tannins, and flavonoids (the most abundant) (Table 1).
Table 1. Qualitative and quantitative phytochemical profile for unripe and ripe Muraru peels.
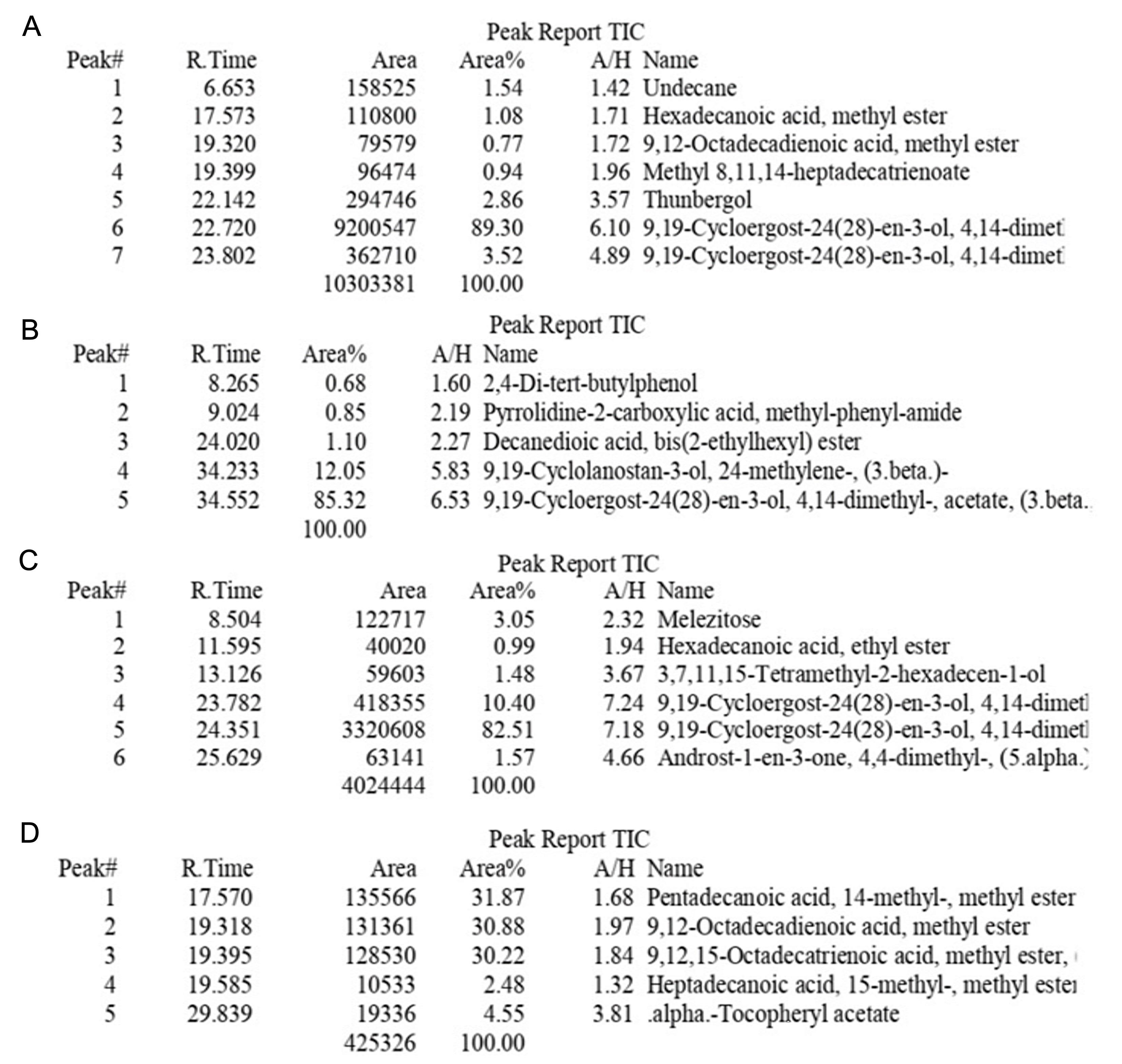
Gas chromatography- mass spectrophotometry of Muraru peels
The chromatograms of unripe and ripe peels confirmed the presence of unsaturated fatty acids, saturated fatty acids, triterpenoids, terpenoids, fatty acids, steroids, and vitamin E (Figure 1). Unripe ethanolic peel extracts revealed seven compounds, with terpenoids (92.82%) being the most abundant. The unripe methanolic extract had five compounds, comprising terpenoids (85.32%) and triterpenoids (12.05%). The ripe ethanolic extract exhibited six compounds, with terpenoids (92.40%) being the most predominant. The ripe methanolic extract contained five compounds, including fatty acids (31.87%), unsaturated fatty acids (30.88%), and lipids (30.22%).
Effect of Muraru peels extract on antioxidant activity
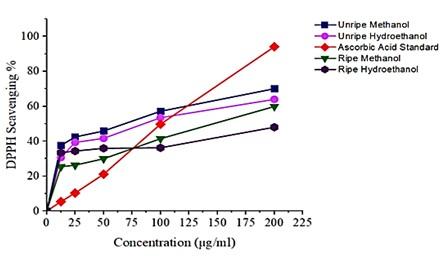
Unripe peel extracts showed significant total antioxidant capacity compared to the ripe peel extracts (Figure 2). Further, the effect of the activity was seen in the Inhibitory Concentration at 50%, where the unripe peel extracts showed a lower IC50 compared to the ripe peel extracts. Unripe methanolic and hydroethanolic extracts have an IC50 of 73.70 μg/ml; 102.77 μg/ml respectively. While the IC50 for methanol and hydroethanolic ripe peel extracts were 147.99 μg/ml; 243.45 μg/ml respectively. The lower the IC50 value, the higher the antioxidant capacity.
Effect of Muraru peels extract on acute toxicity
Hydroethanolic extracts were used to assess the toxicity of the peels. During the acute toxicity of the peel, no sign of morbidity was noted, and no motility was incurred throughout the doses used in the 14-day period of observation. All animals displayed normal clinical signs and behavior. The weight of the animals during the acute toxicity assessment showed a gradual increase from the initial stage to the end of the treatment (Figure 3A).
Hemogram analysis showed that all doses of Muraru peel hydroethanolic extract did not induce significant changes (p > 0.05) in all the measured parameters (Table 2). Furthermore, there was no alteration in kidney and liver enzymes. Creatinine, urea, ALT, and AST values showed no significant (p > 0.05) changes among the groups (Table 2).
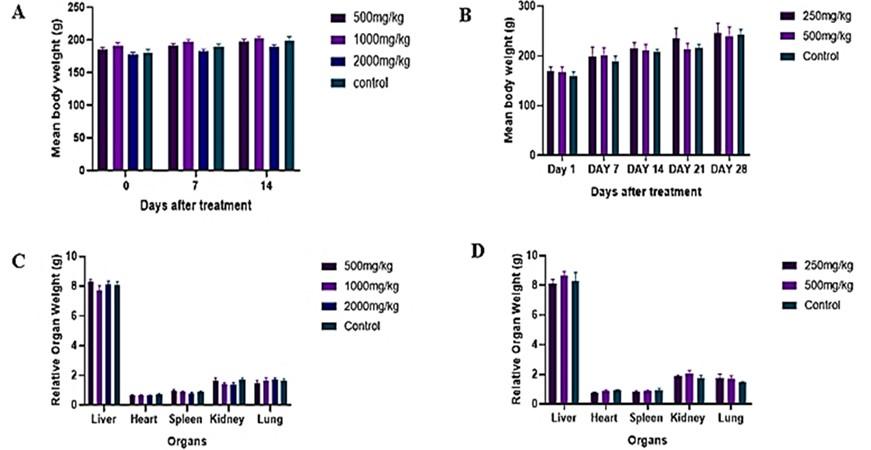
Table 2. Effects of Muraru peel ethanol extract on blood parameters in the acute toxicity study.
Effect of Muraru peels extract on subacute toxicity
The subacute toxicity of the hydroethanolic peel extract showed no sign of morbidity in all the doses, and no mortality occurred throughout the study. There was significant weight gain, which was noticed over the 28 days of subacute toxicity testing in all the groups (Figure 3B). The hemogram assay showed no significant difference (p > 0.05) between the treatment groups and the control group (Table 3). Serum biochemistry of the liver and kidney enzymes showed no significant difference (p > 0.05) from the control group (Table 3). The relative organ weight of the treatment groups showed no significant differences (p > 0.05) among the groups (Figure 3D).
The histopathology of the lung, liver, heart, spleen, and kidney was conducted for the subacute toxicity test, where administration of the hydroethanolic extract was done orally for 28 days. When the histology of the lung, liver, heart, spleen, and kidney from the treated animals was examined, normal morphological features were observed (Figure 4).
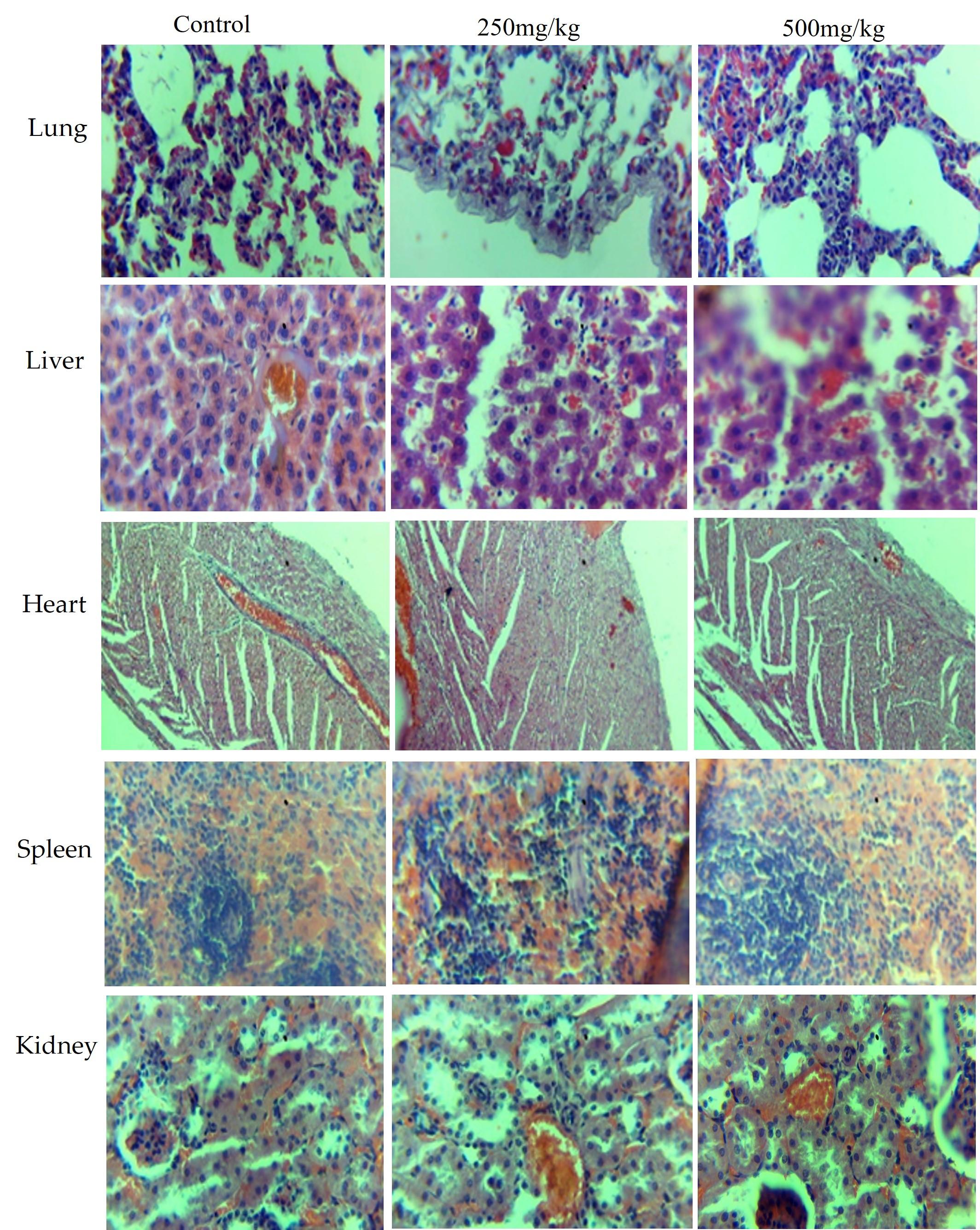
Table 3. Effects of Muraru peel hydroethanolic extract on blood parameters in the subacute toxicity.
DISCUSSION
Over centuries, people have been using plant materials to improve health, treat, and manage various diseases due to the presence of secondary metabolites. This study explored the qualitative phytochemicals of unripe and ripe peels. Both unripe and ripe peels showed the presences of flavonoids, tannins, phytosterols, phenols, alkaloids, and terpenoids. The study results aligned with those by Kibria and Kar [15] and were consistent with research on unripe Kepok and Kultuk peels conducted by Agung et al. [27]. The qualitative results showed the effects of solvent polarity in phytochemical extraction as they varied in number. These results were similar to those of González-Montelongo et al. [28] and Sundaram and Anjum [29]. A study by Ehiowemwenguan et al. [30], showed that ethanol solvent was the best for the extraction of phytochemicals in M. sapientum peels.
The quantitative profile revealed that both unripe and ripe peels are rich in phenolics, tannins, and flavonoids (most abundant). The findings of this study are consistent with previous studies on the phytochemical composition of banana peels, including Kepok and Kultuk varieties [27], and extraction methods utilizing ethanol solvent [30], notable differences were observed. For instance, the quantitative profile of Muraru peels revealed a higher abundance of flavonoids compared to total tannins, differing from the ratios reported in M. sinensis peels [13]. Additionally, chromatographic analysis highlighted the presence of terpenoids, triterpenoids, lipids, and fatty acids, consistent with prior studies on M. sapientum peels [31-32] albeit with variations in compound diversity.
Further, the chromatogram of the peels showed an abundance of terpenoids, triterpenoids, lipids, and fatty acids. Puraikalan [32] study revealed that M. sapientum peels were rich in terpenoids and triterpenoids, although their varieties showed more compounds than the current study. The study by Mordi et al. [31] also revealed the presence of fatty acids, palmitic acid, linoleic acids, and methyl esters. However, a difference was noted, as their study showed more compounds than the current study. The extracts showed significant antioxidant activity with unripe peel, with higher activity compared to ripe peel in both methanolic and hydroethanolic extracts. Furthermore, unripe peel extract showed the lowest IC50 compared to the ripe peels. Mature unripe peel extracts showed increased antioxidant activity compared to mature ripe peel extracts; these results correspond with the study of Sandaram et al. [29]. Note that the current study’s lower IC50 value indicates high antioxidant activity in the sample. The banana extracts showed strong and moderate antioxidant activity, affirming their efficacy in combating oxidative stress [33].
Secondary metabolites are produced in nature to serve survival functions for plants and these have also played an important role in the improvement of the health of humans. When comparing the secondary metabolites found in Muraru peels, a variation was seen compared with other studies. This greatly influenced the qualitative, quantitative, GC-MS, and antioxidant capacity of Muraru peels. This is attributed to differences in banana varieties used, the extraction methods employed, the state and quality of the solvent, and variances in geographical locations. Geographical factors such as soil type, climate, altitude, and sunlight exposure can influence the composition of phytochemicals. This diverse array of phytochemicals contributes to the richness and uniqueness of the plant's bioactive compounds.
Muraru peels contain flavonoids, phenols, and tannins that can help with inflammation, diabetes mellitus, and cancer. They can also lower cholesterol and bad cholesterol, and they can eliminate bacteria [34-35], which makes them useful for herbal medicine and nutraceutical use. The most abundant compounds, terpenoids, and triterpenoids, in M. sapientum var. Muraru peels exert multifaceted effects on tumor development, inhibiting initiation, promoting apoptosis, and suppressing angiogenesis. These compounds also offer diverse biological activities, including antioxidant, anti-inflammatory, and cardiovascular protective effects [36-37]. The peel's rich plant oils, containing lipids and therapeutic components like fatty acids and steroids, contribute to its potential to manage diseases. Secondary metabolites such as flavonoids, alkaloids, terpenoids, tannins, and phenols, found in abundance, greatly influenced the antioxidant activity of Muraru peels. Several studies [38-39] have found these compounds to play a significant role as antioxidants. Oxidative stress deregulates cellular functions, leading to neurodegenerative diseases, gastro-duodenal pathogenesis, cancer, premature aging, inflammation, cardiovascular, and endocrine/metabolic dysfunction [40-41]. Therefore, exogenous antioxidants like those found in Muraru peels can balance reactive oxygen species, preventing their occurrence and enhancing the management of chronic diseases and their complications.
In our current study, the peels did not exhibit any signs of toxicity in acute toxicity tests, with an LD50 greater than the limit dose of 2000 mg/kg. According to the classification by the Ministry of Agriculture, Food and Fisheries [42], substances with an LD50 above 2000 mg/kg are considered non-toxic. Furthermore, repeated doses of Muraru peel hydroethanolic extract administered over 28 days showed no signs of morbidity and no mortality. Furthermore, the rats' behavior patterns, skin, fur, eyes, salivation, and occurrence of diarrhea showed no discernible changes. Throughout the acute and subacute toxicity studies, the animals consistently demonstrated weight gain. Weight loss is recognized as a prominent signal for health issues and excessive toxicity [43]. Importantly, the peel hydroethanolic extract induced no significant effects on the relative organ weights of the treated groups compared to the control group. The assessment of relative organ weights is crucial for identifying potential organ damage due to exposure to toxic substances. The extent of toxicity and the ratio of body weight would alter the weight of a damaged organ [44]. These reflect the overall safety profile of banana peel hydroethanolic extract.
The hemopoietin system functions as a sensitive target for toxic compounds and serves as a key indicator of physiological and pathological status in both humans and animals. During both acute and subacute tests, the banana peel hydroethanolic extract did not significantly alter the red blood cell (RBC) indices, suggesting that it has no effect on erythropoiesis, red blood cell morphology, or osmotic fragility. White blood cells (WBCs) play a crucial role in defending against infectious agents, tissue injury, or inflammation. Importantly, no significant changes were noted in neutrophils, lymphocytes, and monocytes in response to the banana peel extract, suggesting that the Muraru peel extract did not challenge the immune system of the animals in both acute and subacute tests. All of these results show that the hydroethanolic extract of the Muraru peel has a calming effect on hematological parameters, indicating that it may be safe for blood-related functions. Given that the analysis of hematological parameters provides valuable insights into blood-related functions and aids in diagnosing conditions such as anemia, infections, acute hemorrhagic states, allergies, and immune deficiencies [45].
Assessing liver and kidney function is crucial to evaluating the potential toxicity of herbal formulations and drugs [46]. Urea serves as an indicator of kidney function, while creatinine is employed as a marker for the glomerular filtration rate. ALT is a sensitive marker for detecting liver cell damage, and AST is present in RBCs, heart, kidney, and skeletal muscles [46]. These parameters were examined to determine potential alterations induced by the peel hydroethanolic extract. The absence of significant changes in ALT, AST, creatinine, and urea levels, recognized indicators of liver and kidney functions, suggests that the acute and subacute administration of the extract did not adversely affect hepatocytes, kidneys, or the normal metabolism of the rats. The safety of the extract is further evident in the morphology of the organs, which displayed no alterations or lesions.
Hence, the wealth of bioactive compounds found in M. sapientum var. Muraru peels, coupled with their high antioxidant capacity and safety profile, positions them as valuable candidates for nutraceutical applications. These versatile peels could potentially be utilized in various functional foods, dietary supplements, or natural health products aimed at promoting wellness and preventing oxidative stress-related diseases.
CONCLUSION
M. sapientum var Muraru unripe and ripe peels are rich in alkaloids, phenols, terpenoids, flavonoids, tannins, and phytosterols. The peels exhibit significant antioxidant activity, supported by an IC50 indicating moderate to strong activity. The results of acute and subacute oral toxicity studies on M. sapientum var Muraru peel hydroethanolic extract indicate its non-toxic nature across all tested groups, with no observable morbidity. These findings suggest that the peels could serve as a valuable source of bioactive compounds and can be efficiently utilized, minimizing waste. These findings position Muraru peels as promising candidates for pharmacological and therapeutic applications as nutraceuticals in improving oxidative stress.
ACKNOWLEDGEMENTS
The authors appreciate Mr. John Kumau (Gok-Botany Lab) for plant identification, the GoK Chemistry Laboratory at JKUAT for assisting in the phytochemical analysis, and the Analytical Laboratory for the GC-MS. Special thanks are extended to Patricia Karanja, the Muraru banana farmer, and Mr. Perminus Kimathi of the Small Animal Facility for Research and Innovation (SAFARI) at JKUAT for their assistance with animal handling. Heartfelt gratitude is extended to Dr. Ifeoluwa Gbala, who provided valuable insights, critical feedback, and dedicated time to help shape the content. A huge thank you to the African Union for funding the research project through the Pan African University scholarship.
AUTHOR CONTRIBUTIONS
MCM: worked on methodology, data curation, conceptualization visualization, validation, writing the original draft, writing the review, and editing. AK: visualization, validation, methodology, review, and editing. RW: supervision, conceptualization, methodology, review, and editing. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
There is no conflict of interest among the authors.
References
- [1]Bhavani M, Morya S, et al. Nutraceutical applications of banana peel, International Journal of Food Properties. 2023; 26(1):1277–1289.
- [2]Jenkins W, Tucker ME, et al. Routledge handbook of religion and ecology, Routledge Handbook of Religion and Ecology. 2016.
- [3]Onyango M, Karamura D, et al. Morphological Characterisation of East African AAB and AA Dessert Bananas (Musa spp), Proc. Int’l ISHS-ProMusa Symp. 2011.
- [4]Ishak NA, Yusuf M, et al. Antidiabetic and Antioxidant Capacities of Local Banana Peels Extract by Using Subcritical Water Extraction Technique, The Malaysian Journal of Islamic. 2019; 26.
- [5]Singh B, Singh JP, et al. Bioactive compounds in banana and their associated health benefits: A review, Food Chemistry. 2016; (16): 1-11.
- [6]Avcil M. Role of Antioxidants and its Functions in Human Body, Oxidants and Antioxidants in Medical Science. 2022; 11(7): 1-2.
- [7]Ohiagu OF, Chikezie P, et al. Toxicological Significance of Bioactive Compounds of Plant Origin, Pharmacogn. Commn. 2021;11(2):67-77.
- [8]Guaadaoui A, Benaicha N, et al. What is Bioactive Compound? A combined definition for a preliminary consensus. International Journal of Nutrition and Food Sciences. 2014; 3(3): 174-179.
- [9]Bernhoft A. A brief review on bioactive compounds in plants, Bioactive compounds in plants- benefits and risks for man and animals. 2010: 11–17.
- [10]Mondal A, Banerjee, S. et al. Cancer Preventive and Therapeutic Potential of Banana and Its Bioactive Constituents: A Systematic, Comprehensive, and Mechanistic Review, Frontiers in Oncology. 2021; 11: 1-19.
- [11]Soltani Firouz M, Alimardani R, et al. Evaluating banana ripening status from measuring dielectric properties, Journal of Food Engineering. 2011; 105: 625–631.
- [12]Toupal S, Coşansu S. Antioxidant and antimicrobial properties of freeze-dried banana and watermelon peel powders, Food and Humanity. 2023; (1)607–613.
- [13]Oyeyinka BO, Afolayan AJ. Potentials of Musa Species Fruits against Oxidative Factors and Dietary Secondary. 2020; 25, 5036.
- [14]Siddique S, Nawaz S, et al. Phytochemical screening and in-vitro evaluation of pharmacological activities of peels of Musa sapientum and Carica papaya fruit, Natural Product Research, 2018; 6419.
- [15]Kibria AA, Kar A. Extraction and Evaluation of Phytochemicals from Banana Peels (Musa Sapientum) And Banana Plants (Musa paradisiaca), Malaysian Journal of Halal Research Journal. 2019; 2(1): 22–26.
- [16]Aboul-enein AM, Salama ZA. et al. Research Article Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. 2016; 8(4): 46–55.
- [17]Santhosh R, Suriyanarayanan B. Plants: A Source for New Antimycobacterial Drugs, Planta medica. 2013; 80.
- [18]Chaudhary P, Varshney N. et al. Analysis of pharmacognostical standardization, antioxidant capacity and separation of phytocompounds from five different vegetable peels using different solvents, Environment Conservation Journal. 2022; 23: 247–259.
- [19]Council N. R. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th edition Washington (DC). NAP: 2011.
- [20]Organisation for Economic Co-operation and Development (OECD). Chapter IV, Guidelines for Toxicity Tests, 2008; 5–7.
- [21]Navghare VV, Dhawale SC. Alexandria University Faculty of Medicine In vitro antioxidant, hypoglycemic and oral glucose tolerance test of banana peels, Alexandria Journal of Medicine, 2017; 53(3): 237–243.
- [22]Pariyani R, Ismali IS. et al. Phytochemical Screening and Acute Oral Toxicity Study of Java Tea Leaf Extracts, BioMed Research International. 2015; 742420.
- [23]Erhabor O, Muhammad H, et al. Interpretation of Full Blood Count Parameters in Health and Disease. 2021; 5(1): 00180.
- [24]Dosseh K, Tchamdja E, et al. Anti-Fatigue Fatigue and Ergogenic Effect of Rourea Coccinea Schum. And Thonn. (Connaraceae) Ethanolic Extract in Rats. 2022; 14(11): 22759-22763.
- [25]Shamaki B, Sandabe UK, et al. Toxicity studies and body weights changes in Wistar rats following oral administration of methanol extract from indigenous ganoderma sp. in Nigeria. 2017; 1(130): 138–141.
- [26]Kamsu GT, Chuisseu D. et al. Toxicological Profile of the Aqueous Extract of Tectona grandis L. F. (Verbenaceae) Leaves: A Medicinal Plant Used in the Treatment of Typhoid Fever in Traditional Cameroonian Medicine, Journal of Toxicology. 2021; Article ID 6646771.
- [27]Agung SFK, Febrianti M, et al. Comparison of Unripe Banana Peel of Kepok (Musa paradisiaca L.) and Klutuk (Musa balbisiana Colla): Phytochemical and Anti- dysenteriae Activity. 2020; 10(4): 911-914.
- [28]González-Montelongo R, Lobo G, et al. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds, Food Chemistry. 2010; 119: 1030–1039.
- [29]Sundaram S, Anjum S. Antioxidant Activity and Protective effect of Banana Peel against Oxidative Haemolysis of Human Erythrocyte at Different Stages of Ripening, 2011; 1192–1206.
- [30]Ehiowemwenguan G, Emoghene A, et al. Antibacterial and phytochemical analysis of Banana fruit peel. IOSR Journal of Pharmacy (IOSRPHR), 4, 18–25. https://doi.org/10.9790/3013-0408018025
- [31]Mordi R, Fadiaro T, et al. Identification by GC-MS of the Components of Oils of Banana Peels Extract, Phytochemical and Antimicrobial Analyses, Research Journal of Phytochemistry, 2016; (10):39–44.
- [32]Puraikalan, Y, Current Research in Nutrition and Food Science Characterization of Proximate, Phytochemical and Antioxidant Analysis of Banana (Musa sapientum) Peels / Skins and Objective Evaluation of Ready to Eat / Cook Product made with Banana Peels, 2018; 06(2):362-391.
- [33]Fidrianny I, Rizki R, et al. In vitro antioxidant activities from various extracts of banana peels using abts, Dpph assays and correlation with phenolic, Flavonoid, Carotenoid content, International Journal of Pharmacy and Pharmaceutical Sciences. 2014; 6: 299–303.
- [34]Oriakhi K, Uadia P, Hypolipidemic Activity of Tetracarpidium conophorum (African walnut) Seed Oil and Its Mechanism of Action, Planta Med Int Open 2020; 7: 170–178.
- [35]Harcourt P, Eedee F, et al. Antimicrobial Assessment of Fresh Ripe and Dry Ripe Musa sapientum L. Peels against Selected Isolates Associated with Urinary Tract Infection in, Port Harcourt. Journal of Medical Science and Clinical Research. 2018; 6(12): 420-430.
- [36]Masyita A, Sari RM, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chemistry: X, 2022; (13): 100217.
- [37]Yang W, Chen X, et al. Advances in Pharmacological Activities of Terpenoids, Natural Product Communications. 2020; 15(3): 1–13.
- [38]Hikal WM Said-AL A, et al. Banana Peels: A Waste Treasure for Human Being. Evidence-Based Complementary and Alternative Medicine. 2022; Article ID 7616452.
- [39]Madhavan M, Joy S. Antioxidant Activity of Fruit Peel Extracts of Musa paradisiaca L Antioxidant Activity of Fruit Peel Extracts of Musa paradisiaca L, Biological Forum International Journal. 2023; 15 (5): 278-282.
- [40]Bennett V, Saliu IO. Exploring the Biochemical Components of Fresh Peels of Three Varieties of Musa sapientum (Banana). 2022; 10(4): 115-119.
- [41]Sharifi-rad M, Kumar N. et al. Lifestyle, Oxidative Stress and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020; 11:(694): 1–21.
- [42]Ministry of Agriculture, Food and Fisheries, Pesticide Toxicity and Hazard, 2022; 1–9.
- [43]Berlo V, Wouterson M. et al. 10 % Body weight (gain) change as criterion for the maximum tolerated dose: A critical analysis. 2021; 134: 105235.
- [44]Ghauri AO, Mohiuddin E, et al. Acute and subacute toxicity studies of a poly herbal formulation used for diabetes. 2022; 38(6), 1668–1673.
- [45]Celkan TT. What does a hemogram say to us? Turk pediatri arsivi. 2020; 55(2): 103–116.
- [46]Ghauri AO, Mohiuddin E, et al. Acute and subacute toxicity studies of a poly herbal formulation used for diabetes., Pakistan journal of medical sciences, 2022; 38(6): 1668–1673.