Antibacterial and cytotoxic activity of seeds of white hyacinth bean (Lablab purpureus L. sweet ‘white’)
Abstract
Seeds of white hyacinth bean available in Khulna, Bangladesh were investigated to assess bioactivities such as antibacterial and cytotoxic properties. For the evaluation of bioactivities solvent extraction was performed by using 50% ethanol and 50% methanol. In the present study, in vitro antibacterial screening was done by the method known as disc diffusion assay. Additionally, the cytotoxic activity was screened by using brine shrimp lethality bioassay. Both the extracts exhibited excellent activity against both gram positive and gram negative bacteria. For ethanolic extract of white hyacinth bean, maximum zone of inhibition was found 17.75 mm at 1000 μg/disc against Staphylococcus epidermidis and 16.75 mm against Staphylococcus aureus. On the other hand, for methanolic extract the maximum zone of inhibition was found as 17.25 mm for 1000 μg/disc against Escherichia coli and 16.75 mm against Pseudomonas aeruginosa. In comparison to ethanolic extract, methanolic extract was found to be more active against all tested microorganisms. Compared to vincristine sulphate (with LC50 of 0.99 μg/ml) both ethanolic and methanolic extracts of hyacinth bean seeds showed toxicity lower than 100 μg/ml and they were 34.67 μg/ml and 45.5 μg/ml, respectively. The LC50 values suggest moderate cytotoxicity of the tested samples. The experimental findings could be correlated with the traditional medicinal uses of the seeds of this plant and showed the rational for further investigation which would be required for isolating the possible bioactive constituents responsible for such activities.
INTRODUCTION
The wild forms of hyacinth bean (Lablab purpureus L.) which have originated in India or South-East Asia, and is introduced into Africa from Southeast Asia during the eighth century. It was widely distributed to many tropical and subtropical countries [1]. Hyacinth bean also known as Dolichos bean or field bean is one of the most ancient crops among cultivated plants. It is mainly cultivated either as a pure crop or mixed with finger millet, groundnut, castor, corn, bajra or sorghum in Asia and Africa. As it is considered as a multipurpose crop, it is grown for the pulse, vegetable, and forage. The traditional use of this plant includes treatment of worm, treatment of inflammation to the uterus, treatment of cholera and leucorrhoea. The flower and leaf have the antibacterial potentiality against Staphylococcus aureus [2]. Different parts of this plant also have the proof of its potential antioxidant, anti-diabetic, anti-inflammatory, analgesic, anti-fungal, hepatoprotective activity [3-6].
For determination of antibacterial activity of a substance, there are a number of methods available. Majority of the researchers use one of the following in vitro assays: disc diffusion, broth dilution, and agar dilution method to determine antibacterial activity. But the most popular method is disc diffusion [9] method probably due to its easy setup, low cost and requirements of substances are relatively small. Cytotoxicity of a sample can be determined by ‘brine shrimp lethality’ assay [12] which has a good correlation with cytotoxic activity on tumors in the human body [13].
The objective of this study was to find out the antibacterial potentiality and cytotoxic activity of crude seed extracts of Lablab purpureus L.sweet ‘white’.
MATERIALS AND METHODS
Collection of seeds
Seeds of Lablab purpureus were purchased from the local seed market of Daulatpur, Khulna. For hyacinth bean Lablab purpureus L. sweet ‘white’ which is also known as ‘white hyacinth bean’ was chosen.
Preparation of sample/extract
Seeds were washed three to four times rapidly with distilled water. Then seeds were dried 2 to 3 days under sunlight with shades. Dried seeds were powdered by using grinder. Fifty gm of powder sample was soaked into 100 ml of 50% ethanol and similarly, 50 gm of powder sample was soaked into 100 ml of 50% methanol. Contents were then kept in the water bath at 50⁰C for two hours. Then the contents were kept out for a while for cooling down. After that, the contents were filtered through Whatman filter paper. The filtrates obtained were evaporated by rotary evaporator and then air dried. The air-dried extract was weighted and 3.2 gm of hyacinth bean seed extracts were obtained from ethanolic and 2.9gm from methanolic extraction. The crude extracts were then stored in the refrigerator at 4⁰C for further experiment. For the antibacterial assay, sterile filter paper discs of 5 mm were impregnated with 500 μg and 1000 μg of each of the test substances and dried under the aseptic condition to evaporate residual solvent.
Antibacterial screening
Antibacterial screening of crude extracts was tested by the agar disc diffusion method. Seven pathogenic bacterial strain including five gram-negative and two gram-positive bacteria were chosen for testing as they were maintained on nutrient agar media by streak plate method [10]. Standard azithromycin (30 μg/disc) were used as positive control and blank discs were used as negative control. Nutrient agar plate was prepared by pouring 15 ml nutrient agar media into Perti plates (100mm x 15 mm). After solidification of nutrient agar media, the media was inoculated with bacteria, cultured previously on liquid broth. The colony forming unit of tested bacteria was ranged from 1.17 to 2.29×108 CFU/ml. The sample discs, antibiotic discs, negative control discs were gently placed on to bacteria-inoculated nutrient agar plate. The plates were inversely kept in an incubator at 37⁰C for 24 hours. The antibacterial activity was determined by measuring the diameter of the zone of inhibition [11].
Cytotoxic activity testing
The brine shrimp lethality test was used to predict the presence of cytotoxic activity in the extracts [13]. For the experiment 5mg of each extract were dissolved in 1ml of seawater and one drop Tween-80 and adjusted to a final concentration of 5μg/μl. Then 4ml of seawater was given to each of the test tubes. With the help of micropipette specific volumes (5, 10, 20, 40, 80, 160 and 320 μl) of samples were transferred from the stock solution to the test tubes by serial dilution and adjusted to 10 ml with saline water to get the final concentration of 2.5, 5, 10, 20, 40, 80, 160 μg/ml, respectively. Finally, with the help of a Pasteur pipette 10 live brine shrimp nauplii were taken into each of the test tubes [13]. Vincristine sulfate was used as positive control. After 24 hours the test tubes were inspected to count mortality and a graph of % mortality and log concentration was plotted and median lethal concentration (LC50) were calculated by using MS Office 2007. Test of ethanolic and methanolic extracts were done in triplicates to get a reliable result.
RESULTS
Effect on antibacterial activity
Both ethanolic and methanolic extracts of hyacinth bean seeds had shown activity against all tested bacteria. 1000 μg/disc of seed extract was found to be more potent against bacteria as there were some significant differences between the zone of inhibition of the same extract at a different concentration as p-value was less than 0.05 for all the tested bacteria except Pseudomonas aureginosa and Vibrio cholerae for ethanolic extract (Table 1). Both the extracts showed significant zone of inhibition (p<0.05) against Staphylococcus aureus, Staphylococcus epidermidis, E.coli and Proteus vulgaris. At the concentration of 1000 μg/disc ethanolic extract of hyacinth bean showed the maximum zone of inhibition against Staphylococcus epidermidis (17.75 mm) (Figure 1A) and for methanolic extract, it was Escherichia coli (17.25 mm) (Figure 1B). The results for positive control were ranged from 32 to 37 mm for all the tests.
Table 1. Result of significance test (p < 0.05) for mean of zone of inhibition at two different concentration
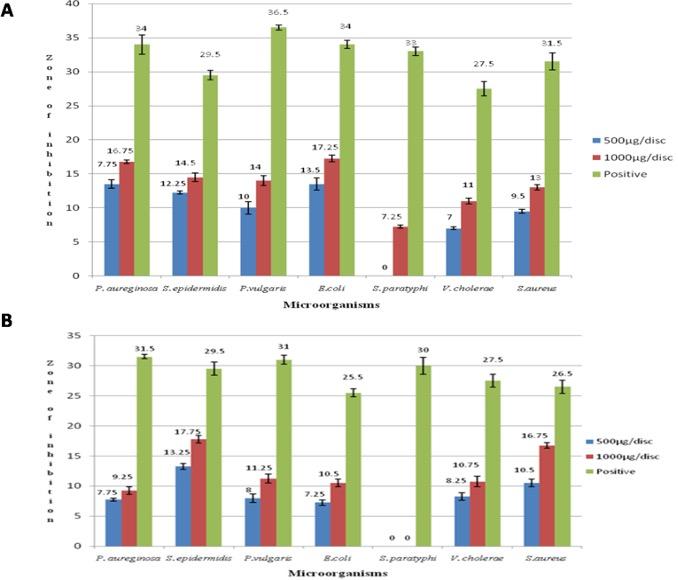
Effect on cytotoxic activity by brine shrimp lethality test
Brine shrimp lethality tests results (Table 2) showed that both the extract of Lablab purpureus L. had moderate toxicity to brine shrimp as the LC50 values were below 100 μg/ml and over 30 μg/ml [13]. The LC50 values were 34.67 μg/ml for ethanolic extract (Figure 2A) and 45.5 μg/ml for methanolic extract (Figure 2B), respectively. For positive control, the LC50 value was 0.997 μg/ml (Table 3).
Table 2. Effect of ethanolic and methanolic extract of hyacinth bean seeds on brine shrimp
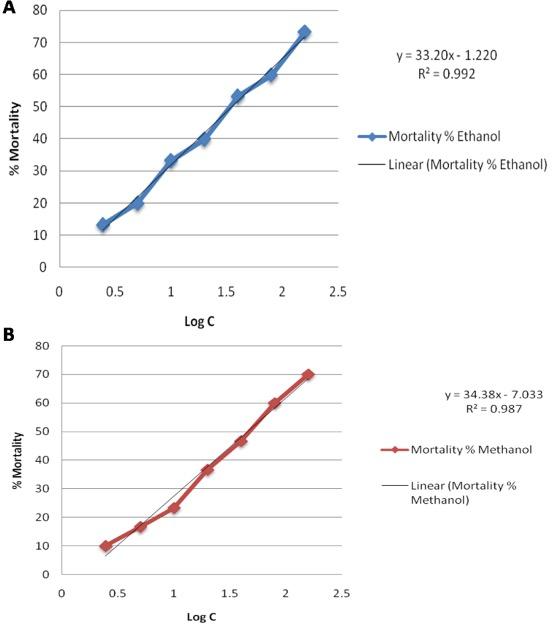
Table 3. Effect of ethanolic and methanolic extract of hyacinth bean seeds on brine shrimp
DISCUSSION
The use of plant derived drugs and search for dietary supplements from plants have advanced in recent years. Traditional healers use plants to prevent or cure infectious diseases. Plant is a rich of secondary metabolites, which have been found to have both in vitro and in vivo antimicrobial activities [14]. For their own protection, plants accumulate an armory of antimicrobial secondary metabolites where some metabolites represent as constitutive chemical barriers to microbial attack and others are inducible antimicrobials [15].The results of the present study clearly indicated that all the extract of seeds showed potent antibacterial activity against tested pathogens. Seeds of hyacinth bean have been traditionally used to treat cholera, diarrhea, poisoning by bacteria [7]. This study provides scientific proof of using seeds of white hyacinth bean as antibacterial agents. The ethanolic extracts showed the maximum zone of inhibition against both the gram-positive bacteria Staphylococcus epidermidis and Staphylococcus aureus. The diameter of the zone of inhibition found against these two microorganisms can be evaluated as the extract showed susceptible result against the growth of these two microorganisms. According to the result, we found it can be told that the extract has potential antibacterial activity against all tested microorganisms [16]. As it showed a good zone of inhibition against gram-negative bacteria, the possible causes of excellent potentiality showed against gram-positive bacteria due to the presence of outer membrane of gram-negative bacteria which act as a barrier against numerous antibiotic molecules and the enzymes of the periplasmic spaces which have the ability to break down foreign molecules [17].
On the other hand methanolic extract showed the maximum zone of inhibition against gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. The diameter of the zone of inhibition found against these two microorganisms can be evaluated as the extract showed susceptible result against the growth of these two microorganisms [16]. The extract also showed good zone of inhibition (p<0.05) against other tested microbes including gram-positive bacteria. The variation of the result may be due to the potentiality to extract out molecules by ethanol and methanol, respectively.Analyzing the result of the antibacterial activity of ethanolic and methanolic extracts of seeds of white hyacinth bean is a clear indication of the potentiality of bean seeds as an antibacterial agent. The seeds can be used to treat disease like the skin infection and the infection after replacement surgery which are caused by S. aureus [18, 19] as there was a significant zone of inhibition against that bacteria. The disease like septicemia and meningitis caused by Pseudomonas aeruginosa [20] can also be treated by using hyacinth bean seeds as extracts of seeds showed a significant zone of inhibition (p < 0.05) against Pseudomonas aeruginosa. The seeds can be a potential agent for treating catheter infection caused by Staphylococcus epidermidis [21]. Thus this study suggests isolation of active ingredients from seeds of hyacinth bean to develop potential antibacterial agents.
Another part of this research was to find out the potential cytotoxic activity of seeds of white hyacinth bean. The brine shrimp lethality bioassay is normally conducted to draw inferences on the safety of the plant extracts and to further depict trends of their biological activities and considered as a useful tool for the preliminary assessment toxicity [21]. The dried seeds extracts showed cytotoxicity at such a level that it could be termed as moderate cytotoxicity compare to positive control [13]. The inhibitory effect of the extracts might be due to the presence of toxic compounds in the active fraction that possess ovicidal properties. The toxicity may be due to the presence of cyanogenic glucosides present in the dried seeds [7]. Considerable interest arose regarding the use of a synthetic cyanogenic glucoside as an alternative anticancer compound [8], so the in vitro cytotoxic effect showed by hyacinth bean seed extract can be an initial indicator of in vivo antitumor and anticancer activity.
Cytotoxic effect can be occurred due to other active compounds because a wide range of phytochemicals have capability to exhibit nonspecific cytotoxicity. There is correlation between cytotoxicity and activity against the brine shrimp nauplii using plant extracts [23], therefore isolation of active compounds and further cell line assay is required to eliminate cytotoxic compounds and to develop potential anticancer agent. The findings of this study also support the previous study which was done to find out the potential cytotoxic activity of two hyacinth bean seeds which were Lablab purpureus L. sweet purple and white and they found the white one as more potent [24]. Again pod of white hyacinth bean has potent cytotoxic activity [24]. So, it is suggested that effective anticancer agent can be developed by combining pods and seeds together.
Here in vitro studies provide scientific footing to enhance confidence in the traditional claims of L.purpureus seeds. The antibacterial screening in this study supports traditional medicinal practices of this plant. Another part of this study was to investigate cytotoxic potentialities of seeds of white hyacinth bean. The result obtained suggests that it has potent cytotoxic activity. The antibacterial and cytotoxic activity of hyacinth bean seed extracts suggests further isolation of active ingredients through bioassays. In-vivo trials would help to sort out active compounds of the seed as pharmaceutical and therapeutic agents.
CONFLICT OF INTEREST
The author declares that no conflict of interest exists.
References
- [1]Murphy, AM, Colucci, PE. A tropical forage solution to poor quality ruminant diets: A review of Lablab purpureus. Livestock Research for Rural Development 1999; 11(2): 96-113.
- [2]Priya, S, Jenifer, S. Antibacterial activity of leaf and flower extract of Lablab purpureus against clinical isolates of Staphylococcus aureus. Research & Reviews: A Journal of Drug Design & Discovery 2014; 1(2), 1–4.
- [3]Habib, MAM, Hasan, R, Nayeem, J, Uddin, N, Rana, S. Anti-inflammatory, antioxidant and cytotoxic potential of methanolic extract of two Bangladeshi bean Lablab purpureus L. sweet white and purple. International journal of pharmaceutical sciences and research 2012; 3(3): 776-781.
- [4]Fakhoury, AM, Woloshuk, CP. Inhibition of growth of Aspergillus flavus and fungal alpha-amylases by a lectin-like protein from Lablab purpureus. Mol Plant Microbe Interact 2001; 14(8):955-961.
- [5]Im, AR, Kim, YH, Lee, HW, Song, KH. Water extract of Dolichos lablab attenuates hepatic lipid accumulation in a cellular nonalcoholic fatty liver disease model. J Med Food 2016; 19(5):495-503.
- [6]Ahmed, M, Trisha, UK, Shaha, SR, Dey, AK, Rahmatullah, M. An initial report on the antihyperglycemic and antinociceptive potential of Lablab purpureus beans. World Journal of Pharmacy and Pharmaceutical Sciences 2015; 4(10): 95-105.
- [7]Al-Snafi, AE. The pharmacology and medical importance of Dolichos lablab (Lablab purpureus)-A review. IOSR Journal of Pharmacy 2017; 7(2), 22-30.
- [8]Bruneton, J. Principles of herbal treatment, Principles and Practice of Phytotherapy 2013.
- [9]Bauer AW, Kirby WM, Sherris JC, Turck, M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1966; 45:493-6.
- [10]Bauman, RW. Microbiology: International Edition 2004.
- [11]Wilkinson JM. Methods for testing the antimicrobial activity of extracts. In: Ahmad, I, Aqil, F, Owais, M, editors. Modern phytomedicine: Turning medicinal plants into drugs. Germany: Wiley-VCH 2007; p. 157-69.
- [12]Michael, AS, Thompson, CG, Abramovitz, M. Artemia salina as a test organism for a bioassay. Science 1956;123:464.
- [13]Meyer, BN, Ferrign, RN, Putnam, JE, Jacobson, LB, Nicholas, DE, McLaughlin, JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 1982; 45:31-34.
- [14]Cowan, MM. Plant products as antimicrobial agents. Clinical microbiology reviews 1999; 12(4), 564-582.
- [15]González-Lamothe, R, Mitchell, G, Gattuso, M, Diarra, MS, Malouin, F, Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. International Journal of Molecular Sciences 2009; 10(8), 3400-3419.
- [16]Johnson, T, Case, C. Chemical Methods of Control, Adapted From Laboratory Experiments in Microbiology, Brief Edition, Redwood City 1995.
- [17]Neidhardt, FC, Ingraham, JL, Schaechter, M. Physiology of the bacterial cell: a molecular approach (Vol. 20). Sunderland, MA: Sinauer Associates 1990.
- [18]Tong, SY, Davis, JS., Eichenberger, E, Holland, TL, Fowler, VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews 2015; 28(3), 603-661.
- [19]Rasmussen, RV, Fowler Jr, VG, Skov, R, Bruun, NE. Future challenges and treatment of Staphylococcus aureus bacteremia with emphasis on MRSA. Future microbiology 2011; 6(1), 43-56.
- [20]Bodey, GP, Bolivar, R, Fainstein, V, Jadeja, L. Infections caused by Pseudomonas aeruginosa. Reviews of infectious diseases 1983; 5(2), 279-313.
- [21]Hedin G. Staphylococcus epidermidis — hospital epidemiology and the detection of methicillin resistance. Scandinavian Journal of Infectious Diseases Supplementum 1993; 90: 1–59.
- [22]Solis, PN, Wright, CW, Anderson, MM, Gupta, MP, Phillipson, JD. A Microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta medica 1993; 59(03), 250-252.
- [23]Manilal, A, Sujith, S, Kiran, GS, Selvin, J, Shakir, C. Cytotoxic potentials of red alga, Laurencia brandenii collected from the Indian coast. Global J Pharmacol 2009; 3(2), 90-94.
- [24]Momin, MAM, Habib, MR, Hasan, MR, Nayeem, J, Uddin, N, Rana, MS. Anti-inflammatory, antioxidant and cytotoxicity potential of methanolic extract of two Bangladeshi bean Lablab purpureus (l.) sweet white and purple. International Journal of Pharmaceutical Sciences and Research. 2012; 3(3), 776.